Abstract
Voltage-gated sodium (Na+) channels are a fundamental target for modulating excitability in neuronal and muscle cells. When depolarized, Na+ channels may gradually enter long-lived, slow-inactivated conformational states, causing a cumulative loss of function. Although the structural motifs that underlie transient, depolarization-induced Na+ channel conformational states are increasingly recognized, the conformational changes responsible for more sustained forms of inactivation are unresolved. Recent studies have shown that slow inactivation components exhibiting a range of kinetic behavior (from tens of milliseconds to seconds) are modified by mutations in the outer pore P-segments. We examined the state-dependent accessibility of an engineered cysteine in the domain III, P-segment (F1236C; rat skeletal muscle) to methanethiosulfonate-ethylammonium (MTSEA) using whole-cell current recordings in HEK 293 cells. F1236C was reactive with MTSEA applied from outside, but not inside the cell, and modification was markedly increased by depolarization. Depolarized F1236C channels exhibited both intermediate (IM; τ ∼ 30 ms) and slower (IS; τ ∼ 2 s) kinetic components of slow inactivation. Trains of brief, 5-ms depolarizations, which did not induce slow inactivation, produced more rapid modification than did longer (100 ms or 6 s) pulse widths, suggesting both the IM and IS kinetic components inhibit depolarization-induced MTSEA accessibility of the cysteine side chain. Lidocaine inhibited the depolarization-dependent sulfhydryl modification induced by sustained (100 ms) depolarizations, but not by brief (5 ms) depolarizations. We conclude that competing forces influence the depolarization-dependent modification of the cysteine side chain: conformational changes associated with brief periods of depolarization enhance accessibility, whereas slow inactivation tends to inhibit the side chain accessibility. The findings suggest that slow Na+ channel inactivation and use-dependent lidocaine action are linked to a structural rearrangement in the outer pore.
Keywords: local anesthetic, gating, cysteine mutagenesis, lidocaine, electrophysiology
INTRODUCTION
Local anesthetic compounds, such as lidocaine, suppress the ionic current through Na+ channels. By attenuating central and peripheral neuronal excitability, these compounds enjoy widespread use in the treatment of epilepsy and the relief of pain. In addition, local anesthetic compounds block Na+ channels in skeletal and cardiac muscle, and are used to treat neuromuscular diseases and cardiac arrhythmias. An essential characteristic of the local anesthetic action is use dependence, which is the sustained loss of excitability lasting many hundreds of milliseconds that is induced only when the drug-exposed channel is depolarized (Courtney 1975). Although studies have identified amino acid residues in the cytoplasmic end of the pore that, when mutagenized, attenuate the action of local anesthetic compounds (Ragsdale et al. 1994; Nau et al. 1999), the structural basis of the sustained, depolarization-dependent loss of excitability associated with use dependence is not understood.
When briefly depolarized, Na+ channels inactivate rapidly within a few milliseconds (fast inactivation), a process mediated by residues situated near the cytoplasmic face of the channel (West et al. 1992; Smith and Goldin 1997; Filatov et al. 1998). With prolonged depolarization, Na+ channels progressively occupy more stable slow-inactivated states (Rudy 1978). These nonconducting states have diverse lifetimes ranging from tens of milliseconds (Kambouris et al. 1998; Benitah et al. 1999) to many seconds (Cummins and Sigworth 1996; Featherstone et al. 1996; Hayward et al. 1997), and are distinct from fast inactivation that typically recovers within 10 ms between stimuli. Importantly, slow inactivation influences cellular excitability, particularly in the pathophysiological conditions associated with prolonged membrane depolarization such as epilepsy (Fleidervish et al. 1996), neuronal or cardiac ischemia (Shander et al. 1995), arrhythmias (Veldkamp et al. 2000), and neuromuscular diseases (Cannon 1996). Amino acid residues from the P-segments (S5-S6 linkers), donated from each of the four homologous domains, line the extracellular aspect of the Na+ channel pore (see Fig. 1). Although the structural basis of slow inactivation gating is not defined, site-directed mutations and chimeric studies suggest a role for the P-segments (Balser et al. 1996a; Benitah et al. 1999; Todt et al. 1999; Vilin et al. 1999). In addition, P-segment mutations and extracellular cation substitutions that reduce slow inactivation (Townsend and Horn 1997) seem to inhibit use-dependent lidocaine action (Zilberter et al. 1991; Kambouris et al. 1998; Chen et al. 2000), suggesting a potential mechanistic linkage between these phenomena.
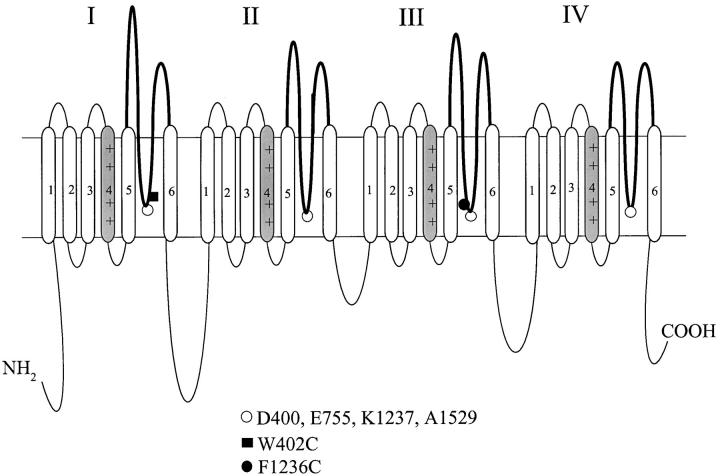
Figure 1.
The Na+ channel P-segments influence slow inactivation. The predicted transmembrane topology of the Na+ channel α subunit is shown, with a bold line in each domain indicating the P-segment region that lines the outer pore. The open circles at the base of each P-segment identify the DEKA ring residues that form a putative selectivity filter (Heinemann et al. 1992). Residues are numbered according to the rat skeletal muscle Na+ channel sequence (Trimmer et al. 1989). The domain III lysine (K1237) is known to influence slow inactivation gating (Benitah et al. 1999; Todt et al. 1999), and is adjacent to the F1236C residue (filled circle). In addition, substitution of the W402 tryptophan (filled square) in domain I alters slow inactivation (Balser et al. 1996a).
Investigating the structural basis of slow inactivation is hampered by its electrical silence, and requires a means to detect relatively slow changes in the conformational architecture of a nonconducting channel. Cysteine substitution of a P-segment residue in domain III (see Fig. 1, F1236C) yields a Na+ channel reactive with the positively charged methanethiosulfonate (MTS) reagent MTS-ethylammonium (MTSEA) applied from outside the cell, yet the residue is entirely resistant to MTSEA applied from inside the cell (Yamagishi et al. 1997). Adjacent to the domain III lysine that contributes to the Na+ channel selectivity filter (see Fig. 1, K1237; Heinemann et al. 1992), this cysteine cannot be modified by larger (MTS-ethyltrimethylammonium [MTSET]) or negatively charged (MTS-ethylsulfonate, MTSES) reagents (Yamagishi et al. 1997). We postulated that sulfhydryl modification of a residue harboring such restrictive access might be sensitive to the voltage-gated conformational state of the ion channel. The results show that modification of F1236C is greatly enhanced by brief depolarizations, but is inhibited by more sustained depolarizations that induce slow inactivation. Moreover, lidocaine inhibited depolarization-induced MTSEA modification during sustained, but not brief, depolarizations. We propose that Na+ channel slow inactivation and use-dependent lidocaine action are linked to a common structural rearrangement involving the outer pore.
MATERIALS AND METHODS
Molecular Biology and Heterologous Expression
For heterologous expression, the wild-type rat skeletal muscle Na+channel (μ1) α subunit was subcloned into the HindIII-XbaI site of the vector GFP-IRS for bicistronic expression of the channel protein and GFP reporter as previously described (Johns et al. 1997). The rat skeletal muscle mutant Na+ channel F1236C was prepared (Yamagishi et al. 1997) using a PCR-based method (QuickChange site-directed mutagenesis kit; Stratagene). In brief, sense and antisense oligonucleotides (30–34 mer) containing the desired mutation were used as primers for amplification with Pfu DNA polymerase. The PCR product was treated with DpnI to select for the mutant plasmid and transformed into Escherichia coli for subsequent isolation and purification. Wild-type and mutant Na+ channels α subunits were transiently transfected into HEK 293 (human embryonic kidney cell line) cells using lipofectamine (GIBCO BRL), and were cultured in MEM medium supplemented with 10% fetal bovine serum and 1% pen-strep in a 5% CO2 incubator at 37°C for 1–3 d. In all cases, the HEK cells were cotransfected with the Na+ channel β1 subunit (provided by Dr. Alfred George, Vanderbilt University). Cells exhibiting green fluorescence were chosen for electrophysiological analysis.
Electrophysiology and Data Analysis
Whole-cell Na+ currents (INa) were recorded (Axopatch 200B; Axon Instruments) using electrodes with resistances of 1–3 MΩ when filled with a pipet solution containing (in mM): 140 NaF, 10 NaCl, 5 EGTA, 10 HEPES, pH 7.40. Replacing the intracellular K+ with Na+ eliminated the time-dependent K+ currents in our HEK cell recordings. Experiments were conducted at room temperature. Current magnitudes were 1–2 nA, and 85% of the series resistance was compensated, yielding a maximum voltage error of ∼1 mV. The bath solution contained (in mM): 150 NaCl, 4.5 KCl, 1.5 CaCl2, 1 MgCl2, 10 HEPES (titrated to pH 7.40 with NaOH). MTSEA, MTSES, and MTSET (Toronto Research Chemicals) were kept at 4°C as high concentration stock solutions and were diluted to 25–100 μΜ in the appropriate bath solution immediately before use. The disulfide reducing agents dithiothreitol (DTT) and glutathione were dissolved directly in the extracellular solution at a concentration of 5 mM (titrated to pH 7.4 with NaOH). Lidocaine HCl (Sigma-Aldrich) or QX-314 (Almone Labs) were diluted from stock solutions to the bath concentrations indicated in the text.
Cells were dialyzed for a 15-min equilibration period before recording data. To avoid junction potentials with solution changes, a 3-M KCl agar bridge was used. Inactivation gating kinetics and use-dependent block were assessed using the voltage-clamp protocols described in the text and figure legends. Whole-cell currents were sampled at 20 kHz (DigiData 1200 A/D converter; Axon Instruments) and low pass–filtered at 5 kHz. The data were acquired and analyzed using pClamp8.0 software (Axon Instruments). The results are expressed as mean ± SEM, and statistical comparisons were made using One-Way ANOVA (Microcal Origin) with P < 0.05 indicating significance. Multiexponential functions were fitted to the data using nonlinear least-squares methods (Origin).
RESULTS
We first examined the accessibility of the F1236C (Fig. 1) cysteine side chain to sulfhydryl modification using 100 μM MTSEA. A 3-min exposure to MTSEA during hyperpolarization (−100 mV; Fig. 2 A, protocol I) reduced F1236C peak INa by 31 ± 3% (after MTSEA washout; summary data Fig. 2 B). This exceeded wild-type modification (13 ± 3%, P < 0.05; Fig. 2A and Fig. B), but was less than that previously seen with F1236C using a much higher concentration of MTSEA (49 ± 15%, 2.5 mM; Yamagishi et al. 1997). However, modification was substantially increased by clamping to −20 mV during MTSEA exposure for F1236C (73 ± 5%, P < 0.0001; Fig. 2A and Fig. B, protocol II) but not wild type (Fig. 2 B), suggesting depolarization increased the accessibility of the cysteine side chain. Identical experiments using the larger, positively charged reagent MTSET, or the negatively charged analogue MTSES, produced reactivity indistinguishable from the wild type, even with sustained (protocol II) depolarization (Fig. 2 B).
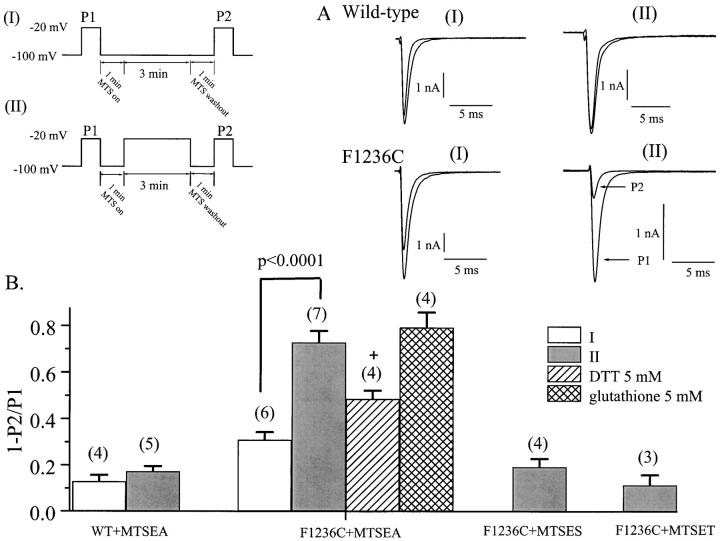
Figure 2.
MTSEA modification of F1236C exhibits voltage dependence. Cells expressing wild-type and F1236C channels were subjected to the voltage-clamp protocols shown (I and II). After an initial pulse (P1) to −20 mV, MTSEA (100 μΜ) was washed into the bath. After a 1-min equilibration period, the membrane was held at −100 mV for 3 min (I), or alternatively was depolarized to −20 mV (II) for the same time period. MTSEA was washed out of the bath (1 min), and INa was reassessed with a brief pulse to −20 mV (P2). (A) Currents through wild-type (top) and F1236C (bottom) channels were recorded using protocol I (left) or II (right). The INa recorded during P1 and P2 are superimposed for comparison. The reduction in peak INa reflects the extent of covalent modification of Na+ channels during MTSEA exposure. (B) Summary data showing the fractional reduction of peak INa because of MTS reagent exposure (1 − P2/P1, INa) under varying conditions. The number of cells recorded in each condition is shown in parentheses. Depolarization potentiated MTSEA modification of F1236C channels (P < 0.0001). This modification was partially reversible (over ∼20 min) with 5 mM DTT (+, P < 0.005, paired comparison with pre-DTT, 3 min MTSEA modification of INa in the same cell), but not 5 mM glutathione. Modification of F1236C by MTSES or MTSET was indistinguishable from wild type.
The additional covalent modification afforded by depolarization was partially reversed by the hydrophobic reducing agent DTT. Fig. 2 B indicates that after 3 min of depolarization-induced MTSEA modification, the fractional reduction in peak INa (relative to pre-MTSEA) after an additional 20 min of DTT exposure is reduced to 49 ± 5% (P < 0.005 versus the 73% pre-DTT value), indicating the INa reduction associated with MTSEA exposure results from formation of a reducible disulfide bond. The prolonged time (20 min) required for only partial DTT reversal suggests the reducing agent accesses the disulfide bond with some difficulty, perhaps through a hydrophobic pathway. We could not achieve reversal using glutathione, a larger, more hydrophilic, and generally less reactive reducing agent (Fig. 2 B). Considered together, the data (Fig. 2) suggest that although depolarization increases the accessibility of the F1236C side chain (allowing enhanced modification by MTSEA), the residue still lies at a relatively inaccessible position in the outer pore, limiting the effects of the larger sulfhydryl modification compounds or reducing agents.
The rate of F1236C INa decay during the depolarizing pulse did not differ from wild type (Fig. 2 A), suggesting the mutant had no marked effect on the fast inactivation gating process. To examine the kinetics of slow inactivation, wild-type and F1236C peak INa were measured after prepulses of incremental duration (Fig. 3, inset, clamp protocol). Each pair of depolarizations was separated by a brief, 20-ms hyperpolarization that allowed channels to recover fully from fast, but not slow, inactivation. Wild-type and F1236C slow inactivation were described by biexponential functions with similar time constants (parameters given in legend), corresponding to intermediate (IM, τ ∼ 30 ms) and slower (IS, τ ∼ 2 s) kinetic components of slow inactivation described in previous studies of μ1 expressed in Xenopus oocytes (Kambouris et al. 1998; Benitah et al. 1999). However, the rate of slow inactivation increased in wild-type channels as the membrane potential increased from −20 to +20 mV (Fig. 3 A) because of an increase in the amplitude of the IM component (from 0.2 to 8%) and a shorter IS time constant (τ2, from 2,519 to 1,255 ms; see Fig. 3 legend). Conversely, the rate of development of slow inactivation in F1236C was similar at −20 mV and +20 mV (Fig. 3 B; amplitude of the IM component was ∼10%). Hence, consistent with previous evidence that P-segment substitutions alter slow inactivation gating processes in Na+ channels (Balser et al. 1996a; Todt et al. 1999), the F1236C mutation did modify (increase) the propensity of the Na+ channels to slow inactivate.
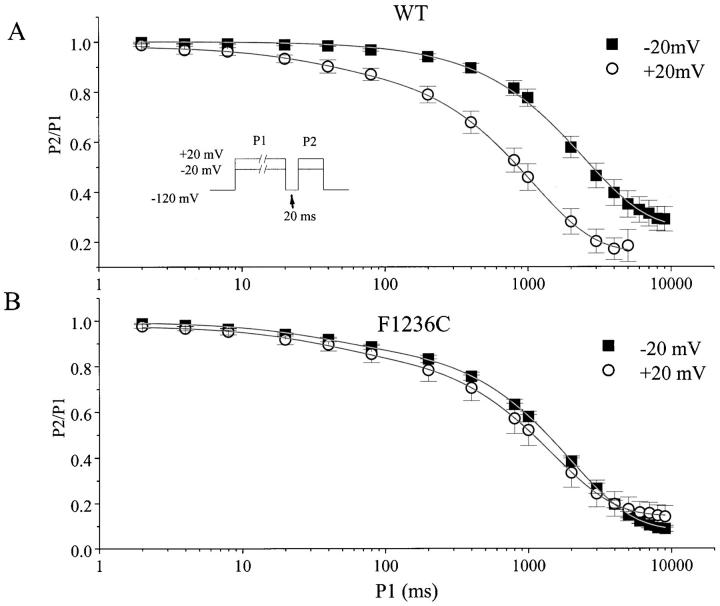
Figure 3.
Time-dependent development of slow inactivation for wild-type (A) and F1236C channels (B). The paired-pulse voltage-clamp protocol is shown (inset). The duration of the initial (P1) pulse was varied, and the extent of recovery after a 20-ms hyperpolarization to −120 mV was assessed using a 50-ms test pulse (P2) to either −20 or + 20 mV. Plotted is the fractional recovery from inactivation (peak INa: P2/P1) as a function of the prepulse duration. The solid lines indicate nonlinear least squares fits of the function y = y0 + A1exp(−t/τ1) + A2exp(−t/τ1) to the data. For wild type at −20 mV (n = 5, τ is in milliseconds): A1 = 0.002 ± 0.0007, τ1 = 44 ± 4.3; and A2 = 0.74 ± 0.04, τ2 = 2519 ± 180. For wild type at +20 mV (n = 7): *A1 = 0.08 ± 0.02, τ1 = 36 ± 5.7; and A2 = 0.77 ± 0.04, τ2 = 1255 ± 188. For F1236C at −20 mV (n = 6): A1 = 0.08 ± 0.01, τ1 = 30 ± 3.4; and A2 = 0.83 ± 0.02, τ2 = 2039 ± 151. For F1236C at +20 mV (n = 5): A1 = 0.11 ± 0.04, τ1 = 38 ± 8.9; and A2 = 0.73 ± 0.04, τ2 = 1558 ± 155. The asterisk indicates that the amplitude of IM (A1) increased with the membrane potential in the wild-type channel (P = 0.002), but did not change for F1236C (P = 0.45).
A meaningful analysis of the depolarization-dependent rate of MTSEA modification of the F1236C side chain must recognize the distinctive kinetic features of slow inactivation, including the biexponential characteristics noted in Fig. 3. Therefore, we used parameters derived from the kinetic analysis of F1236C slow inactivation (−20 mV; Fig. 3 legend) to select depolarization pulse widths that would maximize the intermediate and slow kinetic components, IM (100 ms; ∼ 3 × τM) and IS (6 s; ∼ 3 × τS). In addition, a brief, 5-ms pulse width was used to examine depolarization-induced MTSEA modification in the complete absence of slow inactivation (Fig. 3 B). Notably, while the pulse widths are selected to bias the channel toward distinct kinetic components of slow inactivation, caution is needed when relating these components to occupancy of individual gated states. The inactivated state dwell-times depend on many factors, including the detailed connectivity of the kinetic states involved.
Fig. 4 (A and B) shows the time-dependent reduction of INa due to MTSEA modification during trains of either 5- or 100-ms pulses (−20 mV). In all experiments, MTSEA was allowed to equilibrate in the bath for at least 1 min before application of the first depolarization pulse. For these studies, the MTSEA was lowered to 25 μM (vs. 100 μM in Fig. 2) to minimize tonic, depolarization-independent modification; as such, the reduction in INa during the first pulse after MTSEA addition was consistently ≤5% (Fig. 4A and Fig. B), compared with 31% at the higher concentration (Fig. 2 B). In Fig. 4, currents are plotted from every hundredth pulse (A) or every fifth pulse (B), and the currents shown in the two panels are aligned to reflect matching cumulative depolarization time.
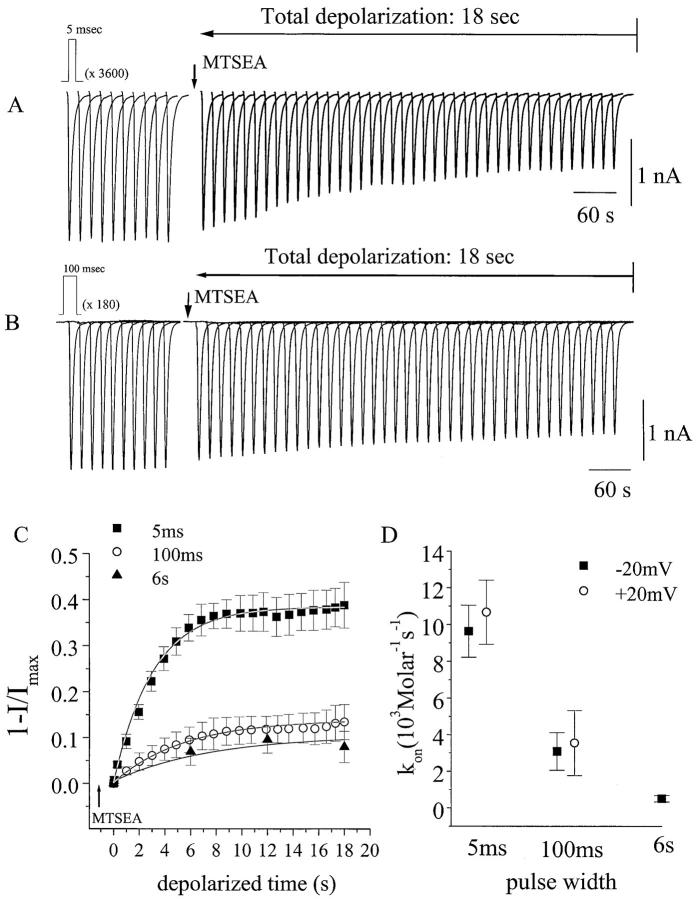
Figure 4.
MTSEA modification of F1236C channels during pulse trains of variable pulse width. (A and B) INa elicited by trains of depolarizing pulses (−20 mV) of either 5 ms (A) or 100 ms (B) duration, with interpulse repolarization intervals (−120 mV) of 200 ms or 4 s, respectively. Currents are plotted from every hundredth pulse (A) or every fifth pulse (B), so that the currents shown in the two panels reflect matching cumulative depolarization time. Each cell was subjected to a 5-min pulse train before MTSEA addition, and cells exhibiting a change in INa magnitude during this control period were discarded. Stable cells were held at −120 mV and exposed to MTSEA for 1 min before reinitiating the pulse train. The depolarization-independent modification (reflected by the magnitude of the first INa recorded in MTSEA) was consistently ≤5%. (C) Plot summarizing the depolarization-dependent MTSEA modification as a function of cumulative depolarization time using pulse widths (at −20 mV) of 5 ms (n = 7), 100 ms (n = 6), and 6 s (n = 4). All current amplitudes are normalized to that of the first depolarization recorded in MTSEA, and are plotted as the fractional reduction in peak INa (1 − I/Imax). The solid lines are the best fit of a single exponential function (Ae−t/τ) to the data (every tenth data point plotted for 100-ms pulses, every two hundredth data point plotted for 5-ms pulses). (D) The rate of MTSEA modification, calculated at −20 mV and +20 mV, assuming an irreversible bimolecular reaction: kon = 1/{τ × [MTSEA]}, where τ is the exponential fitted as shown in C. At −20 mV (× 103 M−1s−1): for 5 ms, kon = 9.6 ± 1.4; for 100 ms, kon = 3.1 ± 1.0 (P = 0.004 vs. 5 ms); for 6 s, kon = 0.5 ± 0.18 (P = 0.001 vs. 5 ms, but not significantly different from 100 ms). At +20 mV: for 5 ms (n = 10), kon = 10.7 ± 1.7; and for 100 ms (n = 5), kon = 3.5 ± 1.8 (P < 0.005 vs. 5 ms).
A comparison of A and B in Fig. 4 reveals that extending the depolarization duration from 5 to 100 ms markedly reduced the rate of modification. Fig. 4 C plots the time-dependent reduction in INa, due to MTSEA modification for a number of cells, as a function of matching (cumulative) depolarization time, allowing direct visual comparison of the MTSEA modification rates for the three pulse widths (5 ms, 100 ms, and 6 s). The repolarization intervals between pulses (200 ms, 4 s, and 240 s, respectively) were sufficiently long to prevent cumulative reduction in the current because of slow inactivation, and also were chosen such that the cumulative depolarization time increased as a function of total experimental time at the same rate for all three pulse widths. At the 6-s pulse width, the total (18 s) depolarization period was generated by only three pulses (Fig. 4 C). Hence, in contrast to the 100-ms pulse train, channels transiently occupy the IM component, but spend a much higher percentage of their total depolarized time in the IS kinetic component. Nonetheless, the rate of depolarization-dependent MTSEA modification is still reduced compared with the brief, 5-s depolarizations (Fig. 4 C), indicating that pulses recruiting IS (like IM) reduce MTSEA accessibility of the cysteine side chain. The data in Fig. 4 C were fitted to an exponential function (solid line) to determine reaction rates (kon; Fig. 4 D, see legend) for each depolarization pulse width; increasing the pulse width to either 100 ms or 6 s significantly reduced kon. Fig. 4 D also indicates that the effects of pulse width (5 and 100 ms) were insensitive to changing the depolarization voltage from −20 to +20 mV, which is consistent with the similar rate of slow inactivation for F1236C (in contrast to wild type) at these two membrane potentials (Fig. 3 B).
The rate of MTSEA modification changes with the depolarization pulse width; the extent of modification also appears to change. Incomplete elimination of the current could partly result from residual current flow through MTSEA-modified channels. Even under conditions where slow inactivation is prevented (i.e., brief 5-ms pulses), the rate of depolarization-dependent modification is still 100-fold slower than in previous studies examining MTS modification of freely accessible cysteinyl groups (i.e., III–IV linker 1304C; Kellenberger et al. 1996; Vedantham and Cannon 1998), which is consistent with restricted access of MTSEA to the 1236C intrapore cysteinyl. Given the even slower rate of MTSEA modification at the longer pulse widths, we may speculate that a slow, competing side reaction may gradually oxidize the 1236 cysteine (while the channel remains Na+ permeable), as proposed in previous studies of intrapore cysteine disulfide formation (Benitah et al. 1997). Moreover, the modification rate, with long pulses at a low MTSEA concentration (25 μM, Fig. 4 C), is quite slow (relative to the practical limitations on experimental time), making it difficult to determine precisely when modification saturates. Hence, the modification rates for 100-ms and 6-s pulses may be even slower than estimated from the fitting procedure.
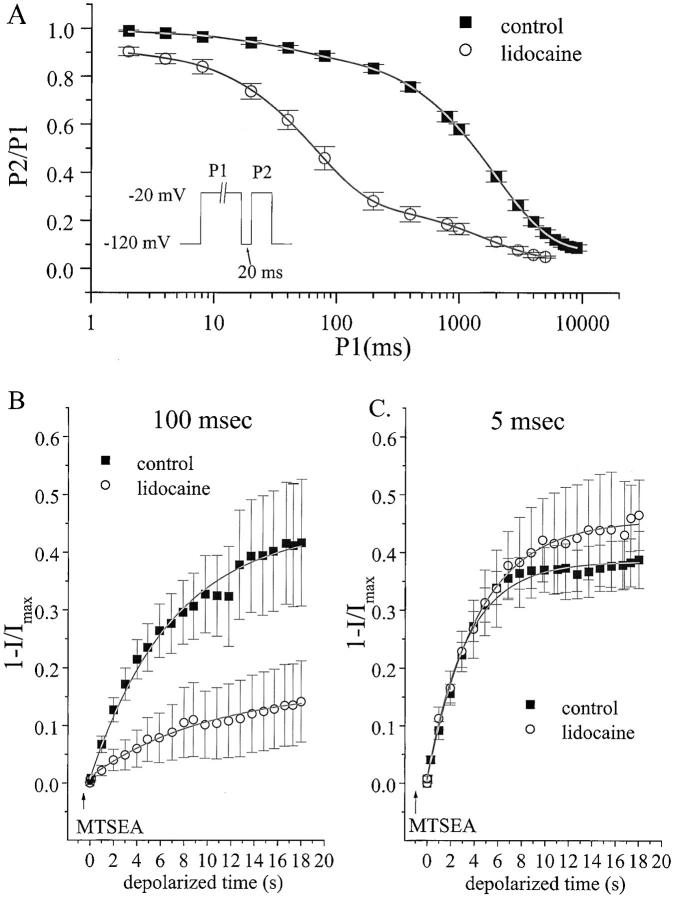
We next considered whether accessibility of F1236C to sulfhydryl modification was modified by use-dependent lidocaine block. Since the F1236C mutation alone modifies slow inactivation under control conditions (Fig. 3), we first established whether lidocaine would induce significant depolarization-dependent INa suppression in this mutant. Bath exposure to 100 μM lidocaine (Fig. 5 A) reduced INa availability mainly by increasing the amplitude of an intermediate (τ = 68 ms) kinetic component (see Fig. 5 legend, lidocaine increased A1 from 0.08 to 0.65). Hence, the mutant channel retains use-dependent lidocaine sensitivity typical for skeletal muscle Na+ channels expressed in HEK cells (Wang et al. 1996).
Figure 5.
Lidocaine modulates F1236C depolarization-dependent MTSEA modification. (A) Time-dependent development of slow inactivation and use-dependent lidocaine suppression of INa in the F1236C channel. Voltage-clamp protocol (inset) was identical to Fig. 3 and was applied in control conditions (n = 6, replotted from Fig. 3 B) and in 100 μM lidocaine (n = 3). The solid lines indicate nonlinear least squares fits of a biexponential function to the data. In 100 μM lidocaine: *A1 = 0.65 ± 0.03, *τ1 = 68 ± 4 ms; and A2 = 0.23 ± 0.05, τ2 = 1949 ± 268. The asterisk indicates that lidocaine increased the amplitude and time constant of the intermediate kinetic component (P < 0.0003 versus drug-free). (B and C) Plots summarizing the depolarization-dependent MTSEA modification (percent change in peak INa) as a function of cumulative depolarization time using pulse widths of either 100 ms (B) or 5 ms (C) in control or lidocaine-containing (100 μM) solutions. Pulse train protocols are identical to Fig. 4, and interpulse recovery intervals are 200 ms for 5-ms pulses and 4 s for 100-ms pulses (both control and lidocaine). Every two hundredth data point is plotted for 5-ms pulses, and every tenth data point is plotted for the 100-ms pulses. A single exponential is fitted to the data (solid lines) to determine the modification rates (kon, see legend Fig. 4 D). In B, the MTSEA concentration was increased to 50 μM, to allow significant modification using the 100-ms pulse width in lidocaine-free conditions. Lidocaine markedly reduced the depolarization-dependent MTSEA modification rate when the pulse duration was 100 ms, but not 5 ms. The kon values (× 103 M−1s−1) are as follows. For 5-ms pulses: control (from Fig. 4 C), kon = 9.6 ± 1.4; 100 μM lidocaine (n = 5), kon = 9.7 ± 1.3. For 100-ms pulses: control (n = 8), kon = 4.4 ± 0.6 (P = 0.004 vs. 5 ms control); 100 μM lidocaine (n = 7), kon = 0.7 ± 0.3 (P = 0.0002 vs. 100 ms control).
To determine whether the effects of lidocaine on use-dependent loss of INa availability could be linked to a structural change in the outer pore, we examined voltage-dependent MTSEA modification during lidocaine exposure. Fig. 5 B shows the rate of MTSEA modification induced by 100- ms depolarizing pulses to −20 mV during MTSEA exposure, alone and with lidocaine superfusion. For these experiments, we raised the MTSEA concentration from 25 to 50 μM to allow significant modification of the cysteinyl side chain using a 100-ms pulse width in lidocaine-free conditions (note, for comparison, only minimal modification using a 100-ms pulse width in Fig. 4 C). Notably, in lidocaine-free solutions, the rate of MTSEA modification (kon, 103 M−1s−1) using 100-ms pulses (Fig. 5 B, 4.4 ± 0.6) was slower than with 5-ms pulses (Fig. 5 C, 9.6 ± 1.4, P = 0.004 vs. 100-ms pulses) even though the MTSEA concentration was raised in Fig. 5 B. This finding supports the results of Fig. 4, indicating a slower rate of depolarization-dependent modification when the pulse duration is lengthened. The data also reveal a marked reduction in MTSEA modification with lidocaine exposure; in Fig. 5 B (100-ms pulse width), kon in lidocaine was as decreased to 0.7 ± 0.3 (P = 0.0002). In contrast, lidocaine exposure had no effect on the rate of MTSEA modification when a brief pulse width was used (Fig. 5 C, 5 ms). Hence, the protection from sulfhydryl modification afforded by lidocaine is use-dependent and requires a sustained depolarization. This pulse width sensitivity suggests the observed lidocaine effect on MTSEA modification was not a nonspecific, gating-unrelated antagonism between the local anesthetic the MTS reagent.
DISCUSSION
Based on evidence that enzymes (Cahalan 1978; Yeh 1978) and mutations (Bennett et al. 1995; Balser et al. 1996b) that remove fast inactivation also attenuate use-dependent suppression of INa by lidocaine and other Na+ channel blockers, a high affinity interaction between lidocaine and the fast-inactivated state has been a candidate structural motif for the sustained inactivation induced by these compounds. However, a recent study suggests that amino acid residues critically involved in fast inactivation (West et al. 1992) are not trapped in the fast-inactivated conformational state by lidocaine (Vedantham and Cannon 1999), raising the possibility that this conformational state may not form a high affinity drug receptor as previously proposed. P-segment mutations that reduce an intermediate kinetic component of slow inactivation (termed IM) also attenuate use-dependent lidocaine block (Kambouris et al. 1998), as do cation substitutions that inhibit this component of slow inactivation (Zilberter et al. 1991; Chen et al. 2000). Although these and earlier studies (Khodorov et al. 1976) postulated a linkage between slow inactivation gating and a use-dependent local anesthetic block, unambiguous interpretation of these data has not been possible because the interventions themselves (mutations and cation substitutions) could alter drug binding through gating-independent mechanisms (Hille 1992). Here, we show that slow inactivation alters the accessibility of an outer pore cysteine to sulfhydryl modification (Fig. 3 and Fig. 4). Moreover, lidocaine attenuates MTSEA modification of the same cysteine when exposed to sustained (100 ms) depolarizations (Fig. 5 B), but not brief, 5-ms depolarizations (Fig. 5 C), suggesting the lidocaine effect to suppress MTSEA modification is specifically linked to gated state(s) associated with sustained depolarization. The findings suggest that the use-dependent lidocaine block and slow inactivation share a common, outer pore structural rearrangement that reduces MTS accessibility.
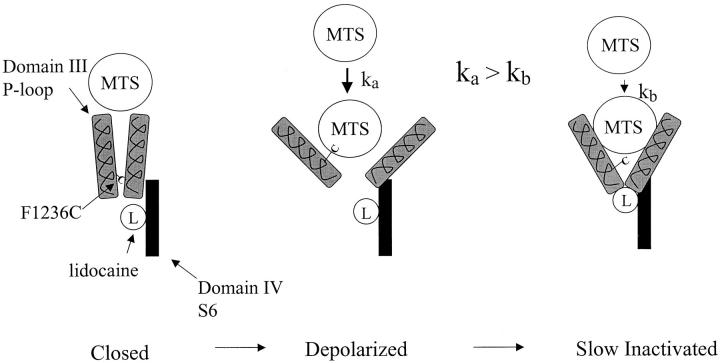
These results do not resolve whether the impeded sulfhydryl modification rate during slow inactivation results from movement of the domain III P-segment to a less accessible position, or rather from movement of other pore structures into positions that somehow protect the F1236C side chain from MTSEA. Nonetheless, a conceptual model based upon recent studies of the Na+ channel pore demonstrating exceptional mobility of the P-segments (Benitah et al. 1997, Benitah et al. 1999; Tsushima et al. 1997) is illustrated schematically in Fig. 6. Whereas the 1236 cysteine side chain is largely inaccessible in the hyperpolarized channel (Fig. 6, left), upon depolarization, we postulate that the P-segments assume positions that increase MTSEA accessibility of the cysteine residue. This would increase the rate of covalent modification (Fig. 6, middle, ka) as we observed (Fig. 2). As the depolarization is prolonged, the accessibility of the 1236C residue is once again compromised (Fig. 6, right, kb < ka), which is consistent with our results (Fig. 4). Hence, Na+ channel gating during sustained depolarization (i.e., slow inactivation) might involve a constriction of the outer pore, analogous to that envisioned for C-type inactivation of potassium channels (Baukrowitz and Yellen 1996; Liu et al. 1996). Our results show that pulse widths sufficient to recruit either the intermediate (IM) or slower (IS) inactivated state kinetic components (Fig. 3) are both effective in reducing depolarization-dependent modification (Fig. 4C and Fig. D), which is consistent with studies linking the P-segments to slow-inactivated states spanning a wide kinetic range (Kambouris et al. 1998; Todt et al. 1999; Vilin et al. 1999). Nonetheless, the marked reduction in MTSEA modification at the 100-ms pulse width is substantial, given the relatively small amplitude of intermediate (IM) inactivation developing by 100 ms (Fig. 3 B). We speculate that a gating motion involving the pore may develop with intermediate kinetics closely related to IM (recognizing 5-ms pulses do not inhibit modification, whereas 100-ms pulses do), which reduces 1236C accessibility but does not suppress Na+ permeation (or else, recovers more rapidly than 20 ms upon hyperpolarization and, therefore, is not visible in Fig. 3 B).
Figure 6.
Hypothesis for P-segment rearrangements during slow inactivation and lidocaine block. The F1236C side chain is relatively inaccessible in the hyperpolarized channel (left), but, upon depolarization, the domain III P-segment changes position in a manner that increases the accessibility of the cysteine residue (middle). In this condition, the rate of sulfhydryl modification (ka) could increase because of reduced steric interference. When the channel slow inactivates, the accessibility of the cysteine residue is once again compromised (right), and the rate of sulfhydryl modification is also reduced (kb < ka). Lidocaine is bound to site(s) in the aqueous pore on the cytoplasmic side of the selectivity filter (Ragsdale et al. 1994, Ragsdale et al. 1996), but in very close proximity to the selectivity filter P-segment residues (Sunami et al. 1997). The model suggests that the P-segment structural rearrangement associated with slow inactivation may move the deepest P-segment domains into positions that stabilize lidocaine binding (right).
The 1236 cysteine side chain is accessible only to modification from outside the cell (Yamagishi et al. 1997), while lidocaine binds to a site in the aqueous pore on the cytoplasmic side (Ragsdale et al. 1994, Ragsdale et al. 1996). Residues forming the P-segment selectivity filter (Fig. 1) of the skeletal muscle Na+ channel act as electrostatic barriers, preventing extracellular access and escape of the local anesthetic (Sunami et al. 1997). Although mutations in the Na+ channel domain IV-S6 segment may create an extracellular access pathway for charged lidocaine derivatives (Ragsdale et al. 1994; Qu et al. 1995), we find that the P-segment F1236C mutation does not make the skeletal muscle Na+ channel sensitive to extracellular QX-314 (1 mM, n = 4, data not shown). Hence, it is unlikely that lidocaine directly inhibits extracellular MTSEA modification of the cysteine residue. At the same time, electrostatic interactions between residue K1237 in domain III (Fig. 1) and lidocaine suggest that drug receptor in domain IV, S6 lies near the P-segment selectivity filter residues (Sunami et al. 1997). Hence, (Fig. 6, right) we postulate that the structural rearrangements associated with slow inactivation may move the Na+ channel P-segments into positions that stabilize the interaction between lidocaine and the pore.
Acknowledgments
We wish to thank Drs. Steve Cannon and Paul Bennett for valuable criticism of the manuscript.
This work was supported by grants from the National Institutes of Health R01 GM56307 (to J.R. Balser) and R01 HL 50411 (to G.F. Tomaselli).
Footnotes
Abbreviations used in this paper: DTT, dithiothreitol; HEK, human embryonic kidney; INa, Na+ channel; MTS, methanethiosulfonate; MTSEA, MTS-ethylammonium; MTSET, MTS-ethyltrimethylammonium.
References
- Balser J.R., Nuss H.B., Chiamvimonvat N., Perez-Garcia M.T., Marban E., Tomaselli G.F. External pore residue mediates slow inactivation in μ1 rat skeletal muscle sodium channels J. Physiol. 494 1996. 431 442a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Balser J.R., Nuss H.B., Orias D.W., Johns D.C., Marban E., Tomaselli G.F., Lawrence J.H. Local anesthetics as effectors of allosteric gatinglidocaine effects on inactivation-deficient rat skeletal muscle Na+ channels J. Clin. Investig. 98 1996. 2874 2886b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz T., Yellen G. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K channel. Science. 1996;271:653–656. doi: 10.1126/science.271.5249.653. [DOI] [PubMed] [Google Scholar]
- Benitah J.P., Ranjan R., Yamagishi T., Janecki M., Tomaselli G.F., Marban E. Molecular motions within the pore of voltage-dependent sodium channels. Biophys. J. 1997;73:603–613. doi: 10.1016/S0006-3495(97)78096-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benitah J.P., Chen Z., Balser J., Tomaselli G.F., Marban E. Molecular dynamics of the sodium channel pore vary with gatinginteractions between P-segment motions and inactivation. J. Neurosci. 1999;19:1577–1585. doi: 10.1523/JNEUROSCI.19-05-01577.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett P.B., Valenzuela C., Li-Qiong C., Kallen R.G. On the molecular nature of the lidocaine receptor of cardiac Na+ channels. Circ. Res. 1995;77:584–592. doi: 10.1161/01.res.77.3.584. [DOI] [PubMed] [Google Scholar]
- Cahalan M.D. Local anesthetic block of sodium channels in normal and pronase- treated squid axons. Biophys. J. 1978;23:285–311. doi: 10.1016/S0006-3495(78)85449-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cannon S.C. Slow inactivation of sodium channelsmore than just a laboratory curiosity. Biophys. J. 1996;71:5–7. doi: 10.1016/S0006-3495(96)79203-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z., Ong B.-H., Kambouris N.G., Marban E., Tomaselli G.F., Balser J.R. Lidocaine induces a slow inactivated state in rat skeletal muscle sodium channels. J. Physiol. 2000;524:37–49. doi: 10.1111/j.1469-7793.2000.t01-1-00037.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Courtney K.R. Mechanism of frequency-dependent inhibition of sodium currents in the frog myelinated nerve by the lidocaine derivative gea 968. J. Pharmacol. Exp. Ther. 1975;195:225–236. [PubMed] [Google Scholar]
- Cummins T.R., Sigworth F.J. Impaired slow inactivation in mutant sodium channels. Biophys. J. 1996;71:227–236. doi: 10.1016/S0006-3495(96)79219-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Featherstone D.E., Richmond J.E., Ruben P.C. Interaction between fast and slow inactivation in Skm1 sodium channels. Biophys. J. 1996;71:3098–3109. doi: 10.1016/S0006-3495(96)79504-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Filatov G.N., Nguyen T.P., Kraner S.D., Barchi R.L. Inactivation and secondary structure in the D4/S4-5 region of the SkM1 sodium channel. J. Gen. Physiol. 1998;111:703–715. doi: 10.1085/jgp.111.6.703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleidervish I.A., Friedman A., Gutnick M.J. Slow inactivation of Na+ current and slow cumulative spike adaptation in mouse and guinea-pig neocortical neurones in slices. J. Physiol. 1996;493:83–97. doi: 10.1113/jphysiol.1996.sp021366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayward L.J., Brown R.H., Cannon S.C. Slow inactivation differs among mutant Na channels associated with myotonia and periodic paralysis. Biophys. J. 1997;72:1204–1219. doi: 10.1016/S0006-3495(97)78768-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heinemann S.H., Terlau H., Stühmer W., Imoto K., Numa S. Calcium channel characteristics conferred on the sodium channel by single mutations. Nature. 1992;356:441–443. doi: 10.1038/356441a0. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1992. Mechanisms of block. Ionic Channels of Excitable Membranes. 2nd ed. Sinauer Associates, Inc., Sunderland, MA. 390–422.
- Johns D.C., Nuss H.B., Marban E. Suppression of neuronal and cardiac transient outward currents by viral gene transfer of dominant negative KV4.2 constructs. J. Biol. Chem. 1997;272:31598–31603. doi: 10.1074/jbc.272.50.31598. [DOI] [PubMed] [Google Scholar]
- Kambouris N., Hastings L., Stepanovic S., Marban E., Tomaselli G.F., Balser J.R. Mechanistic link between local anesthetic action and inactivation gating probed by outer pore mutations in the rat μ1 sodium channel. J. Physiol. 1998;512:693–705. doi: 10.1111/j.1469-7793.1998.693bd.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kellenberger S., Scheuer T., Catterall W.A. Movement of the Na+ channel inactivation gate during inactivation. J. Biol. Chem. 1996;271:30971–30979. doi: 10.1074/jbc.271.48.30971. [DOI] [PubMed] [Google Scholar]
- Khodorov B., Shishkova L., Peganov E., Revenko S. Inhibition of sodium currents in frog Ranvier node treated with local anestheticsrole of slow sodium inactivation. Biochim. Biophys. Acta. 1976;433:409–435. [Google Scholar]
- Liu Y., Jurman M.E., Yellen G. Dynamic rearrangement of the outer mouth of a K+ channel during gating. Neuron. 1996;16:859–867. doi: 10.1016/s0896-6273(00)80106-3. [DOI] [PubMed] [Google Scholar]
- Nau C., Wang S.Y., Strichartz G.R., Wang G.K. Point mutations at N434 in D1-S6 of μ1 Na(+) channels modulate binding affinity and stereoselectivity of local anesthetic enantiomers. Mol. Pharmacol. 1999;56:404–413. doi: 10.1124/mol.56.2.404. [DOI] [PubMed] [Google Scholar]
- Qu Y., Rogers J., Tanada T., Scheuer T., Catterall W.A. Molecular determinants of drug access to the receptor site for antiarrhythmic drugs in the cardiac Na+channel. Proc. Natl. Acad. Sci. USA. 1995;92:11839–11843. doi: 10.1073/pnas.92.25.11839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ragsdale D.S., McPhee J.C., Scheuer T., Catterall W.A. Molecular determinants of state-dependent block of Na+ channels by local anesthetics. Science. 1994;265:1724–1728. doi: 10.1126/science.8085162. [DOI] [PubMed] [Google Scholar]
- Ragsdale D.S., McPhee J.C., Scheuer T., Catterall W.A. Common molecular determinants of local anesthetic, antiarrhythmic, and anticonvulsant block of voltage-gated Na+ channels. Proc. Natl. Acad. Sci. USA. 1996;93:9270–9275. doi: 10.1073/pnas.93.17.9270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudy B. Slow inactivation of the sodium conductance in squid giant axons. Pronase resistance. J. Physiol. 1978;238:1–21. doi: 10.1113/jphysiol.1978.sp012485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shander G.S., Fan Z., Makielski J.C. Slowly recovering cardiac sodium current in rat ventricular myocyteseffects of conditioning duration and recovery potential. J. Cardiovasc. Electrophysiol. 1995;6:786–795. doi: 10.1111/j.1540-8167.1995.tb00355.x. [DOI] [PubMed] [Google Scholar]
- Smith M.R., Goldin A.L. Interaction between the sodium channel inactivation linker and domain III S4-S5. Biophys. J. 1997;73:1885–1895. doi: 10.1016/S0006-3495(97)78219-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sunami A., Dudley S.C., Jr., Fozzard H.A. Sodium channel selectivity filter regulates antiarrhythmic drug binding. Proc. Natl. Acad. Sci. USA. 1997;94:14126–14131. doi: 10.1073/pnas.94.25.14126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todt H., Dudley S.C., Jr., Kyle J.W., French R.J., Fozzard H.A. Ultra-slow inactivation in μ1 Na+ channels is produced by a structural rearrangement of the outer vestibule. Biophys. J. 1999;76:1335–1445. doi: 10.1016/S0006-3495(99)77296-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend C., Horn R. Effect of alkali metal cations on slow inactivation of cardiac Na+ channels. J. Gen. Physiol. 1997;110:23–33. doi: 10.1085/jgp.110.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trimmer J.S., Cooperman S.S., Tomiko S.A., Zhou J., Crean S.M., Boyle M.B., Kallen R.G., Sheng Z., Barchi R.L., Sigworth F.J. Primary structure and functional expression of a mammalian skeletal muscle sodium channel. Neuron. 1989;3:33–49. doi: 10.1016/0896-6273(89)90113-x. [DOI] [PubMed] [Google Scholar]
- Tsushima R.G., Li R.A., Backx P.H. P-loop flexibility in Na+ channel pores revealed by single and double cysteine replacements. J. Gen. Physiol. 1997;110:59–72. doi: 10.1085/jgp.110.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantham V., Cannon S.C. Slow inactivation does not affect movement of the fast inactivation gate in voltage-gated Na+ channels. J. Gen. Physiol. 1998;111:83–93. doi: 10.1085/jgp.111.1.83. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vedantham V., Cannon S.C. The position of the fast-inactivation gate during lidocaine block of voltage-gated Na+ channels. J. Gen. Physiol. 1999;113:7–16. doi: 10.1085/jgp.113.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Veldkamp M.W., Viswanathan P.C., Bezzina C., Baartscheer A., Wilde A.A., Balser J.R. Two distinct congenital arrhythmias evoked by a multidysfunctional Na(+) channel. Circ. Res. 2000;86:E91–E97. doi: 10.1161/01.res.86.9.e91. [DOI] [PubMed] [Google Scholar]
- Vilin Y.Y., Makita N., George A.L., Jr., Ruben P.C. Structural determinants of slow inactivation in human cardiac and skeletal muscle sodium channels. Biophys. J. 1999;77:1384–1393. doi: 10.1016/S0006-3495(99)76987-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang D.W., Nie L., George A.L., Bennett P.B. Distinct local anesthetic affinities in Na+ channel subtypes. Biophys. J. 1996;70:1700–1708. doi: 10.1016/S0006-3495(96)79732-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West J., Patton D., Scheuer T., Wang Y., Goldin A.L., Catterall W.A. A cluster of hydrophobic amino acid residues required for fast Na+-channel inactivation. Proc. Natl. Acad. Sci. USA. 1992;89:10910–10914. doi: 10.1073/pnas.89.22.10910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamagishi T., Janecki M., Marban E., Tomaselli G.F. Topology of the P-segments in the sodium channel pore revealed by cysteine mutagenesis. Biophys. J. 1997;73:195–204. doi: 10.1016/S0006-3495(97)78060-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yeh J.Z. Sodium inactivation mechanism modulates qx-314 block of sodium channels. Biophys. J. 1978;24:569–574. doi: 10.1016/S0006-3495(78)85403-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zilberter Y., Motin L., Sokolova S., Papin A., Khodorov B. Ca-sensitive slow inactivation and lidocaine-induced block of sodium channels in rat cardiac cells. J. Mol. Cell. Cardiol. 1991;23:61–72. doi: 10.1016/0022-2828(91)90025-h. [DOI] [PubMed] [Google Scholar]