Abstract
We have used the patch-clamp technique to study the effects of changing extracellular ATP concentration on the activity of the small-conductance potassium channel (SK) on the apical membrane of the mouse cortical collecting duct. In cell-attached patches, the channel conductance and kinetics were similar to its rat homologue. Addition of ATP to the bathing solution of split-open single cortical collecting ducts inhibited SK activity. The inhibition of the channel by ATP was reversible, concentration dependent (K i = 64 μM), and could be completely prevented by pretreatment with suramin, a specific purinergic receptor (P2) blocker. Ranking of the inhibitory potency of several nucleotides showed strong inhibition by ATP, UTP, and ATP-γ-S, whereas α, β-Me ATP, and 2-Mes ATP failed to affect channel activity. This nucleotide sensitivity is consistent with P2Y2 purinergic receptors mediating the inhibition of SK by ATP. Single channel analysis further demonstrated that the inhibitory effects of ATP could be elicited through activation of apical receptors. Moreover, the observation that fluoride mimicked the inhibitory action of ATP suggests the activation of G proteins during purinergic receptor stimulation. Channel inhibition by ATP was not affected by blocking phospholipase C and protein kinase C. However, whereas cAMP prevented channel blocking by ATP, blocking protein kinase A failed to abolish the inhibitory effects of ATP. The reduction of K channel activity by ATP could be prevented by okadaic acid, an inhibitor of protein phosphatases, and KT5823, an agent that blocks protein kinase G. Moreover, the effect of ATP was mimicked by cGMP and blocked by L-NAME (N G-nitro-l-arginine methyl ester). We conclude that the inhibitory effect of ATP on the apical K channel is mediated by stimulation of P2Y2 receptors and results from increasing dephosphorylation by enhancing PKG-sensitive phosphatase activity.
Keywords: K secretion, purinergic receptors, protein kinase A, phosphatase, protein kinase G
INTRODUCTION
Principal cells of the cortical collecting ducts play a key role in the body's K homeostasis by controlling K secretion in the kidney. This process involves both active and passive processes, including active uptake of K in exchange for sodium by Na-K ATPase activity in the basolateral membrane, followed by passive diffusion along a favorable electrochemical gradient through K channels in the apical membrane (Giebisch 1998). The properties and regulation of the apical K channel in principal cells have been extensively investigated (Wang et al. 1997) and available evidence supports the view that the properties of the secretory K channel share many characteristics with ROMK, the cloned inward-rectifying K channel from the renal medulla (Ho et al. 1993; Palmer et al. 1997; Xu et al. 1997). These similarities include single channel conductance, channel kinetics, pH sensitivity, and dependence on phosphorylation and dephosphorylation processes. The only difference between the cloned and native channels is the lack of sensitivity of ROMK to a sulfonylurea compound (Aguilar-Bryan et al. 1995; Wang et al. 1995). In addition, the intake of K, several hormones, and acid-base factors play an important role in regulating small-conductance potassium channel (Giebisch 1998).
Several studies have shown that purinergic receptors are located along the nephron, including the cortical collecting tubule (Anderson et al. 1991; Firestein et al. 1996; Cha et al. 1998; Chan et al. 1998; Bailey et al. 1999) and that agonists of purinergic receptors modulate the effects of vasopressin on water transport (Kishore et al. 1995; Rouse et al. 1994). Furthermore, activation of purinergic receptors in several epithelia, including pancreas (Dubyak 1999), colon (Leipziger et al. 1997), lung (Mason et al. 1991), cultured canine (Madin-Darby) kidney cells (Gordjani et al. 1997), cortical collecting duct cells (Ecelbarger et al. 1994; Cuffe et al. 2000), as well as perfused mouse cortical collecting ducts (Deetjen et al. 2000), leads to significant modulation of cell calcium levels as well as to changes in Na and Cl transport. Also, ATP has been shown to be secreted into the tubule lumen (Taylor et al. 1998; Wilson et al. 1999), and an important role has been suggested for CFTR, also present in the kidney (Morales et al. 1996; Todd-Turla et al. 1996), to mediate ATP secretion (Devidas and Guggino 1997; Jiang et al. 1998). It is thus possible that ATP, released from tubule cells, may act on purinergic receptors along the nephron. Our study was designed to explore this possibility and to investigate the possible role of purinergic receptors and ATP in the regulation of K channels in principal cells of the cortical collecting tubule.
MATERIALS AND METHODS
Preparation of Mouse Cortical Collecting Duct
C57 mice and Sprague-Dawley rats were obtained from Charles River Laboratories and Taconic Farms, Inc. and kept on normal chow diet (PMI Nutrition International, Inc.) for 7–10 d before the experiments. Their weight varied between 20 and 25 g (mice) and 80 and 100 g (rats). The method for dissection of single mouse tubules was similar to that previously employed (Lu et al. 1997). Animals were killed by cervical dislocation. Their kidneys were removed, and coronary slices were cut and placed in ice-cold dissection solution. The cortical collecting ducts (CCDs) were dissected under microscopic view (Leitz) at room temperature, the tubules were immobilized on a 5 × 5-mm cover glass coated with Cell Tac (Becton Dickinson) and transferred to a chamber mounted on the stage of an inverted microscope (IMT-2; Olympus). The cortical collecting tubules were opened with a sharpened pipette to gain access to the apical membrane. Only principal cells (PCs) were patched in the present study, and PCs were recognized by their morphological characteristics under the microscope. In brief, PCs appear with a large flat surface and their shape is hexagonal, whereas the shape of intercalated cells is irregular. Experiments were performed at room temperature (22–24°C).
Patch-Clamp Technique
Borosilicate glass capillaries (Dagan Corp.) were used with a microelectrode puller (PP-83; Narishige Scientific Instrument Laboratory). The pipette resistance varied between 6 and 8 MΩ when filled with 140 mM KCl. Single channel currents were recorded using an EPC-7 amplifier (List Electronics) and low-pass filtered at 1 kHz by an eight-pole Bessel filter (902 LPF; Frequency Devices, Inc.). Currents were digitized by an Axon Interface (Digidata 1200; Axon Instruments, Inc.) and transferred to a PC (E-3100; Gateway 2000). Data were analyzed using pCLAMP software (6.0.4; Axon Instruments, Inc.). If patches contained more than five channels, we used the NP o analysis program written by Dr. Junliang Sui from Mount Sinai Medical School (available at http://www.axon.com/pub/userware/popen) to calculate open probability. The effect of a particular agent was determined by observing the transition point where the channel open probability changed into a new steady state for at least 30–60 s. The NP o was calculated as follows: NP o = Σ(t 1 + t 2 + t i) where t i is fractional open time spent at each of the observed current levels.
Solutions and Chemicals
Bath and dissection solutions were identical and contained (mM): 140 NaCl, 5 KCl, 1.8 MgCl2, 1.8 CaCl2, and 10 mM HEPES (pH 7.4 with NaOH). The pipette solution contained (mM): 140 KCl, 1.8 MgCl2, and 10 HEPES (pH 7.4 with KOH). Mg-ATP, adenosine 5′-0-3-thiotriphosphate (ATP-γ-S), suramin, sodium fluoride, and indomethacin were purchased from Sigma Chemical Co., H7, H8, H9, H89, and U73122 from Alexis Biochemical Co., forskolin, calphostin C, okadaic acid, KT5823, L-NAME (N G-nitro-l-arginine methyl ester), 8-bromo-cGMP and 8-bromo-cAMP from Calbiochem Novabiochem Co., staurosporine from Boehringer-Mannheim, 2-methylthio ATP (2-Mes ATP), and α,β-methylene ATP (α,β-Me ATP) from Research Biochemicals Intl. All chemicals were prepared as stock solutions and added directly to the perfusion chamber to reach the desired final concentrations.
Statistics
Data are presented as mean value ± SEM. Paired Student's t test was used to perform statistical analysis and significance determined by P < 0.05.
RESULTS
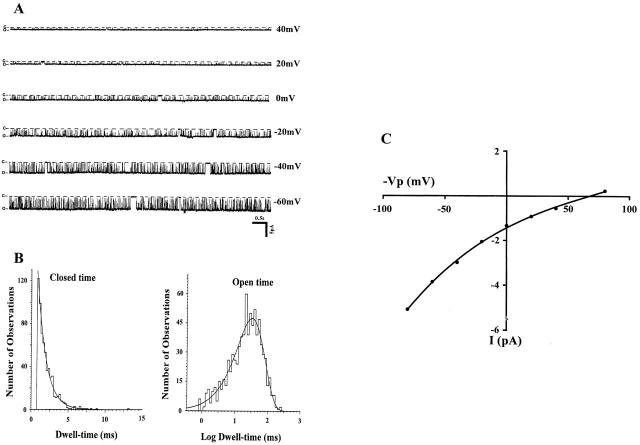
A representative recording of channel activity in a cell-attached patch of the apical membrane of a mouse CCD is shown in Fig. 1 A. It is apparent that the channel has a high open probability (mean NP o = 0.92 ± 0.03, n = 7), similar to the value observed in the rat CCD (NP o = 0.93 ± 0.03, n = 3). Fig. 1 B displays closed- and open-channel histograms, indicating a mean open time of 22.7 ms and a closed time of 1.4 ms. Note that long-lasting closed states can also be observed in the current trace. However, these events were too infrequent to allow appropriate curve fitting. Fig. 1 C is a representative I-V curve yielding a value of 28.4 pS between −20 to +20 mV, a value also quite similar to that derived from data in the rat CCD (28.9 pS). These data are similar to those previously published (Frindt and Palmer 1989; Wang et al. 1990). We conclude that the biophysical single channel characteristics in the mouse closely resemble those of the apical small-conductance K channel in the rat.
Figure 1.
A representative recording showing the kinetics of the apical small-conductance K channel in the mouse CCD. (A) Experiment was carried out in a cell-attached patch with pipette solution (mM): 140 KCl, 1.8 MgCl2, and 10 HEPES; and bath solution (mM): 140 NaCl, 5 KCl, 1.8 MgCl2, 1.8 CaCl2, and 10 HEPES. Different holding potentials (−Vp, from 40 to −60 mV) were applied and are indicated on right side of each trace. Channel closed and open states are indicated by C and O, respectively. (B) The channel open- and closed-time histograms. The mean closed time was 1.4 ± 0.01 ms and mean open time was 22.7 ± 0.03 ms. (C) I-V curve of the apical K channel. The slope conductance was 28.4 pS measured between −20 and 20 mV.
Fig. 2 A shows single channel activity in which the effects of extracellular ATP were investigated. Addition of 100 μM ATP led to a sharp decline and blocked the channel by >90% within 3 min. NP o decreased by 92% ± 5.4 (n = 12). Channel inhibition was reversible (restored channel activity: NP o 98%). Calculation of the dose–response curve yields a value of K i of 64 μm (Fig. 2 B). Recent studies in mammalian epithelia such as cells from mouse cortical collecting tubule have reported that elevation of extracellular ATP stimulated chloride secretion, but inhibited amiloride-sensitive sodium absorption (Cuffe et al. 2000). In another study, extracellular ATP at concentrations similar to those used in our experiments increased cell calcium in perfused microdissected mouse CCD (Deetjen et al. 2000).
Figure 2.
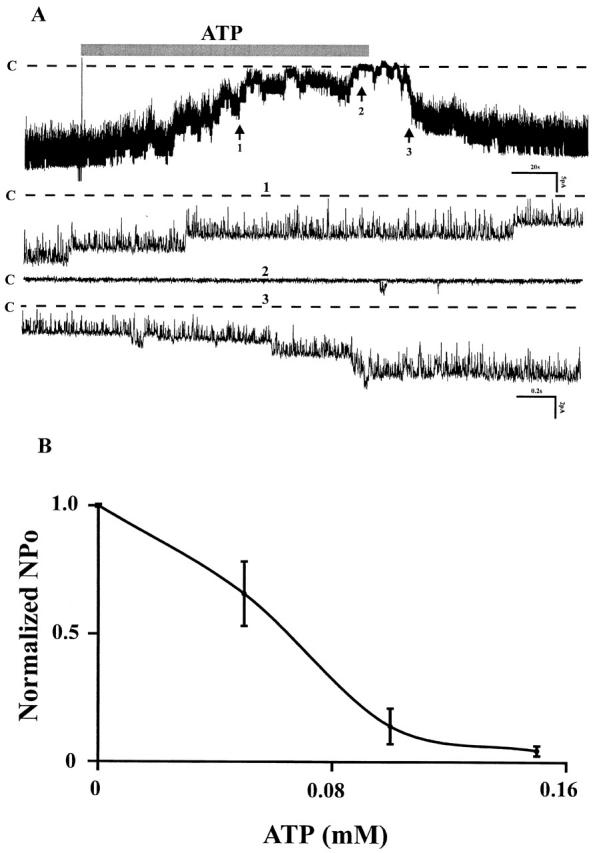
(A) Channel recording showing the affect of external ATP (100 μM) on the apical small-conductance K channel of mouse CCD. Dotted lines and C indicate the channel closed states. The three parts on the bottom (1, 2, and 3) were extended at fast time resolution corresponding to the regions numbered 1, 2, and 3 at top. (B) The dose–response curve of ATP affect on the K channel activities. Experiments were carried out in cell-attached patches. The half maximal inhibitory concentration for ATP was K i = 64 μM.
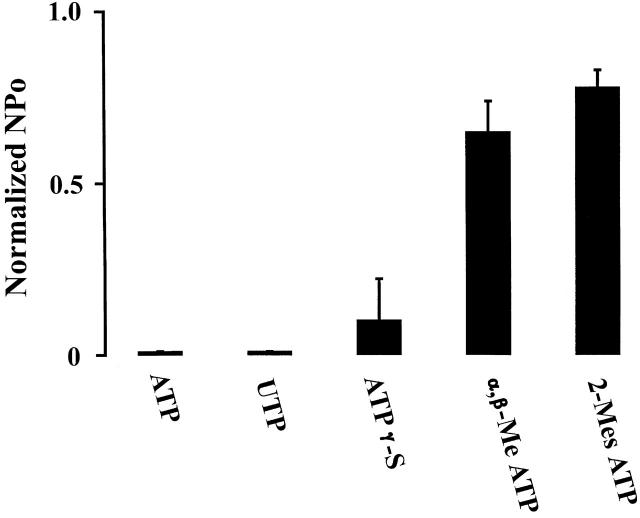
To assess the specificity of ATP-induced channel inhibition, we have examined the effects of several ATP analogs. Fig. 3 summarizes these results. In cell-attached membrane patches, channel activity was sharply reduced by ATP (98%, n = 20), UTP (98%, n = 8), and ATP-γ-S (90%, n = 6). In contrast, addition of α,β-Me ATP and 2-Mes ATP failed to inhibit the channel activity significantly. The sequence of this nucleotide inhibitory potency is consistent with an effect of extracellular ATP on purinergic receptors of the P2Y2 type (King et al. 1998; Ralevic and Burnstock 1998).
Figure 3.
Effects of 200 μM ATP (n = 20), UTP (n = 8), ATP-γ-S (n = 6), α,β-Me ATP(n = 8), and 2-Mes ATP(n = 7) on K channel activities. Experiments were performed in cell-attached patches and nucleotides were added to the bath while channel activity was monitored.
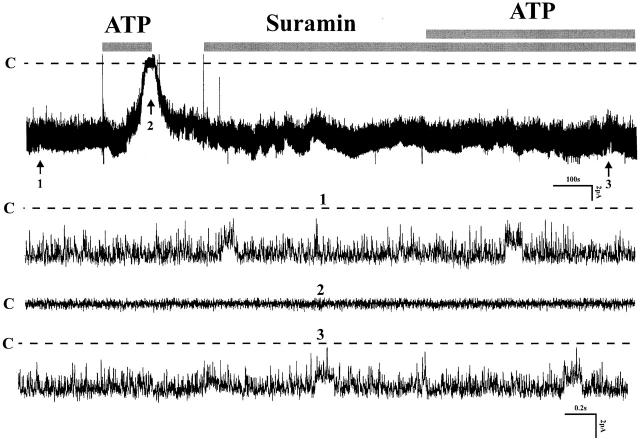
The effects of suramin demonstrated in Fig. 4 further support the involvement of purinergic receptors. Shown are the responses to ATP before and after addition of 100 μM suramin, a potent inhibitor of P2 receptors, to the bath solution. Channel inhibition by ATP is completely abolished by pretreatment of tubules with suramin for 5 min (control NP o: 2.76 ± 0.21; NP o after suramin + ATP: 2.76 ± 0.23, n = 6). These results show that the effects of ATP are dependent on purinergic receptors. They also exclude the possibility that inhibition of K channels could have occurred by direct passage of ATP across the cell membrane since the apical low-conductance K channel is inhibited by elevation of cytosolic ATP (Wang et al. 1997).
Figure 4.
A recording demonstrating the effect of external ATP (200 μM) in the presence of suramin (100 μM). Three parts of the trace were extended to show the channel activity at fast time resolution. C indicates the channel closed state (n = 6).
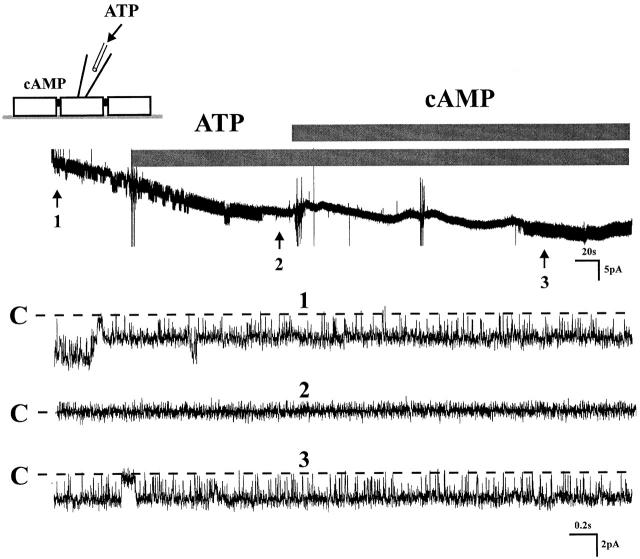
Purinergic receptors have been reported on both apical and basolateral membranes of several epithelia (Leite and Satlin 1996; Ralevic and Burnstock 1998; Bailey et al. 1999). To test whether purinergic receptors are present on the apical membrane of principal tubule cells, channel block was initiated by adding ATP to the patch pipette, a setting that assured that the action of ATP was limited to the domain of the apical membrane. Fig. 5 demonstrates that ATP affected channel block and that addition of 8-bromo-cAMP initiated reactivation of the channel. We conclude from these results the presence of purinergic receptors on the apical membrane. These findings do not exclude that similar receptors on the basolateral membrane may also contribute to the inhibitory effects of ATP.
Figure 5.
A tracing shows that extracellular ATP (100 μM) was applied into the pipette, which means ATP can only block the channel activity through apical membrane, indicating P2 receptors were located on the apical membrane of CCD. 100 μM cAMP was used to restore the channel activity to exclude the possibility of channel run down (n = 4).
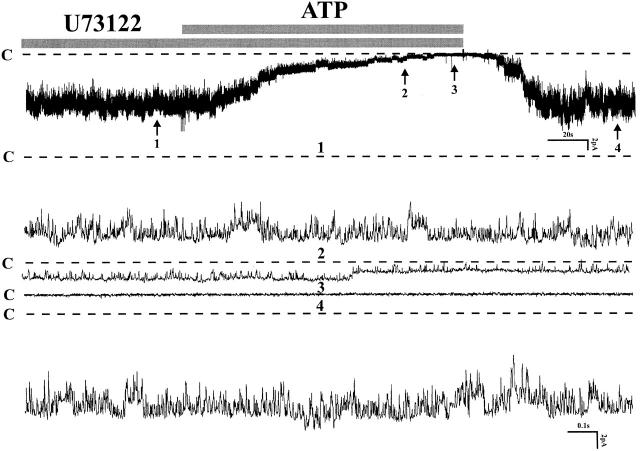
It has been reported that P2Y2 receptors can activate phospholipase C and reduce phosphatidylinositol 4,5-bisphosphate (PIP2) (Fredholm et al. 1997; Baukrowitz et al. 1998). The latter has been shown to activate ROMK, a cloned renal K channel that shares most functional properties with the native small-conductance K channel on the apical membrane of principal cells in the CCD (Huang et al. 1998). We tested the possibility that ATP reduces channel activity by stimulation of phospholipase C and reduction of PIP2. Fig. 6 presents data from an experiment in which the effect of U73122, an inhibitor of phospholipase C, was investigated (NP o before ATP: 6.25 ± 0.31; NP o after ATP: 0.03 ± 0.02, n = 8). Blocking phospholipase C did not abolish the inhibition of channel activity by ATP. We conclude that downregulation of PIP2 does not mediate the ATP effect.
Figure 6.
A channel recording demonstrating the effect of external ATP (200 μM) on channel activity after incubating the cell with 2 μM U73122, a specific PLC inhibitor. Experiments were performed in a cell-attached patch. Four parts of the trace were extended at fast time resolution. C indicates the channel closed state (n = 8).
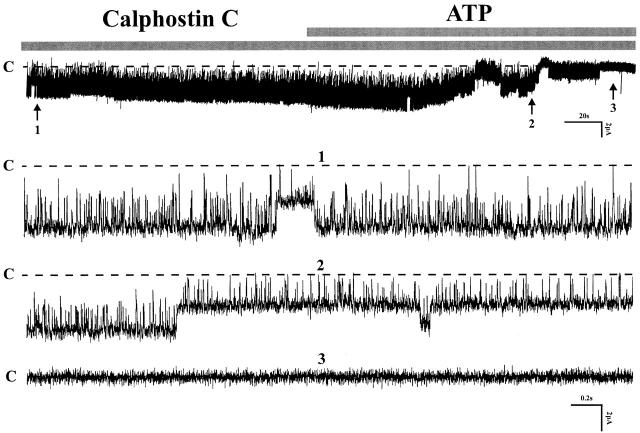
Several previous studies have shown that stimulation of protein kinase C inhibits the native small-conductance K channel in the CCD and thick ascending limb (Wang and Giebisch 1991b), as well as the cloned ROMK2 channel expressed in oocytes (Macica et al. 1998). Furthermore, stimulation of PKC via purinergic receptors has been reported (Koster et al. 1996). To test whether the inhibition by ATP is mediated by activation of PKC, we investigated the effect of several inhibitors of PKC such as calphostin C, H7, and staurosporine. Fig. 7 shows the effects of ATP in the presence of one of these agents, 1 μM calphostin C. Inspection of the results demonstrates that inhibition of PKC failed to prevent channel activity blocked by ATP (calphostin C: NP o 1.86 ± 0.11; after ATP: 0.03 ± 0.02, n = 9). These findings and similar results with H7 (n = 3) and staurosporine (n = 5, data not shown) exclude an important role of PKC mediating the effects of ATP.
Figure 7.
The effect of 1 μM calphostin C on the 200-μM ATP-induced inhibition on channel activity. The experiments were performed in cell-attached patches. Three parts of the recording indicated by numbers 1, 2, and 3 were extended to show the detail of channel activity. Channel closed state is indicated by C (n = 9).
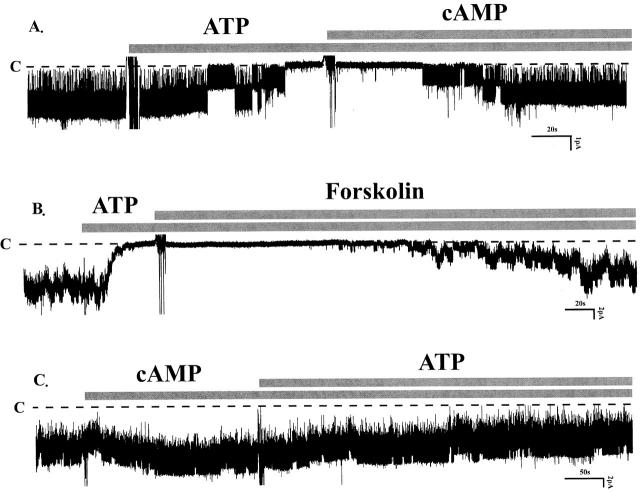
We further investigated the possibility that stimulation of P2Y2 receptors blocks the small-conductance K channel by decreasing the intracellular cAMP concentration. Such a mechanism is suggested by observations that extracellular ATP inhibits adenylate cyclase in renal LLC-PK1 cells through activation of P2 receptors (Anderson et al. 1991). Since PKA stimulates native and cloned K channels (Wang and Giebisch 1991a,Wang and Giebisch 1991b; McNicholas et al. 1994; Xu et al. 1996), a fall in cAMP could block channel activity. Fig. 8 A shows a typical channel recording from an experiment in which the effects of a membrane-permeable analogue of cyclic AMP, 8-bromo-cAMP, were examined. Addition of this agent fully restored channel activity after it had been completely inhibited by ATP (control: NP o 1.84 ± 0.03; after ATP: NP o 0.02 ± 0.01; after ATP + cAMP: NP o 1.84 ± 0.02, n = 12). The effects of 8-bromo-cAMP could be mimicked by forskolin, an agent that increases the cytosolic level of cAMP by stimulating adenylate cyclase (Fig. 8 B, n = 6). Moreover, in the presence of 8-bromo-cAMP, subsequent exposures to ATP failed to inhibit channel activity (Fig. 8 C, n = 6). These experiments demonstrate that stimulation of cAMP-dependent phosphorylation relieves the ATP-induced inhibition of channel activity.
Figure 8.
(A) A single-channel recording showing that addition of 100 μM 8-bromo-cAMP reversed the ATP-induced inhibition (n = 12). (B) A channel recording illustrating that 2 μM Forskolin restored the channel activity inhibited by external ATP (n = 6). (C) A channel recording showing that in the presence of cAMP (100 μM), the effect of ATP on channel activity was abolished (n = 6). All experiments were performed in cell-attached patches and channel closed states are indicated by dotted lines and C. The concentration of ATP was 200 μM.
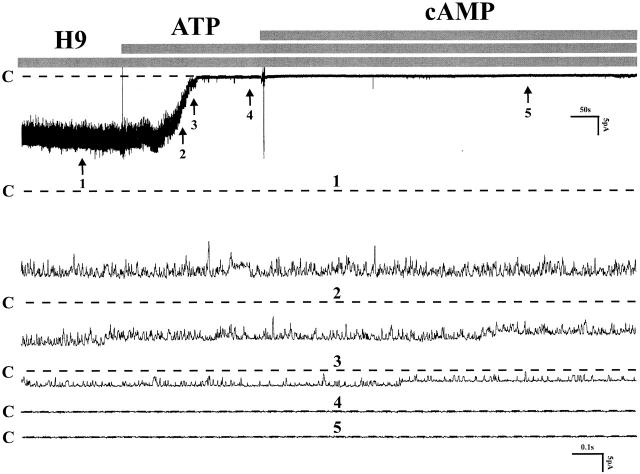
To test the possibility that the effect of ATP results from inhibition of PKA, we examined the effects of several blockers of PKA (H8, H89, and H9). Fig. 9 summarizes the effects of H9, an inhibitor of PKA. H9 had no affect on channel activity before the addition of ATP to the bath solution, and it also fails to abolish the affect of ATP (control: NP o 12.8 ± 0.21; after ATP: NP o 0.02 ± 0.01, n = 6). Note that the membrane-permeable 8-bromo-cAMP now failed to relieve channel block by ATP, although it had been effective in the absence of the PKA inhibitor. Similar maneuvers were also observed with H8 (n = 6) and H89 (n = 4, data not shown), which means that the effect of ATP is not the result of inhibition of PKA. Rather, it suggests that enhanced dephosphorylation could be the mechanism of channel inhibition and that the effect of cAMP consists of antagonizing the inhibitory effect of phosphatases. This conclusion is based on observations that the activity of the native small-conductance K channel as well as that of the cloned ROMK channel depends both on the kinase-mediated phosphorylation of several serine and threonine sites on the channel, and on dephosphorylation by several membrane phosphatases (Wang and Giebisch 1991a,Wang and Giebisch 1991b; McNicholas et al. 1994; Kubokawa et al. 1995a,Kubokawa et al. 1995b).
Figure 9.
A channel recording showing the effect of ATP in the presence of H9 and cAMP on cell-attached patches. Pretreatment of the tubule with 4 μM H9 could not prevent the inhibitory effect of ATP (200 μM) and 200 μM cAMP failed to restore the channel activity. Dotted lines indicated by C were channel closed states (n = 6).
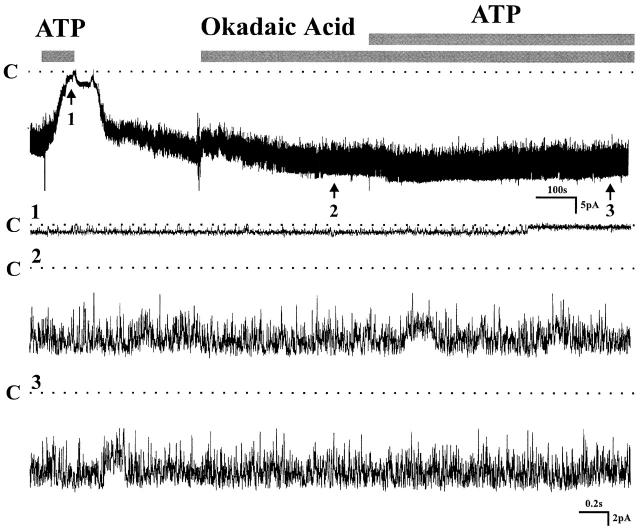
To test the possibility that an increase in phosphatase activity could mediate the inhibitory effect of ATP, experiments were carried out in the presence of a potent phosphatase inhibitor. Fig. 10 shows an experiment in which the effects of okadaic acid were investigated. The ATP block is completely prevented by the inhibitor of phosphatase activity (okadaic acid: NP o 10.1 ± 0.21; after ATP: NP o 11.04 ± 0.29, n = 12). These results strongly support the view that activation of phosphatases mediates the effects of extracellular ATP on the small-conductance K channel.
Figure 10.
Effect of okadaic acid on channel activity in cell-attached patches. Channel activity was first reduced by addition of 200 μM ATP in bath solution, which could be prevented by pretreatment of the tubule with 2 μM okadaic acid. Three parts on the trace indicated by arrows were extended at fast time resolution. Channel closed states are indicated by dotted lines and C (n = 12).
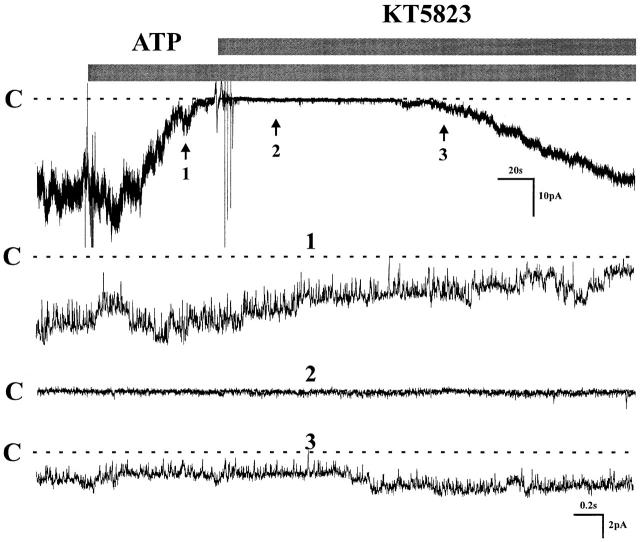
Previous studies from our laboratory had shown that several phosphatases, including PP2A and PP2C, participate in the regulation of both the native K channel in principal cells (Kubokawa et al. 1995a,Kubokawa et al. 1995b) and the cloned channel (ROMK1) expressed in oocytes (McNicholas et al. 1994). Furthermore, it has been demonstrated that protein kinase G can stimulate PP2A in smooth muscle cells (Zhou et al. 1996), suggesting a possible link between purinergic receptors, PKG, and PP2A. To investigate the possible role of PKG in mediating inhibition by ATP, the channel was first blocked by ATP and KT 5823 was subsequently added to the bath solution. Fig. 11 shows that the PKG inhibitor restored channel activity after the block by ATP (control: NP o 9.2 ± 0.31; after ATP: NP o 0.52 ± 0.42; after ATP + KT 5823: NP o 8.28 ± 0.42, n = 6). This finding is consistent with the involvement of PKG in the inhibitory sequence of events that links purinergic receptors on the cell membrane to inhibition of K channels. The inference was further supported by incubation of the tubule with KT 5823 before exposure to ATP, a maneuver that effectively prevented the inhibitory effect of ATP (data not shown).
Figure 11.
Effect of KT 5823 on channel activity after channel was inhibited by ATP. Experiment was carried out in a cell-attached patch. 1 μM KT 5823 reactivated the channel activity that was blocked by 200 μM ATP. Three parts of the trace were extended at fast time resolution. C indicates the channel closed state (n = 6).
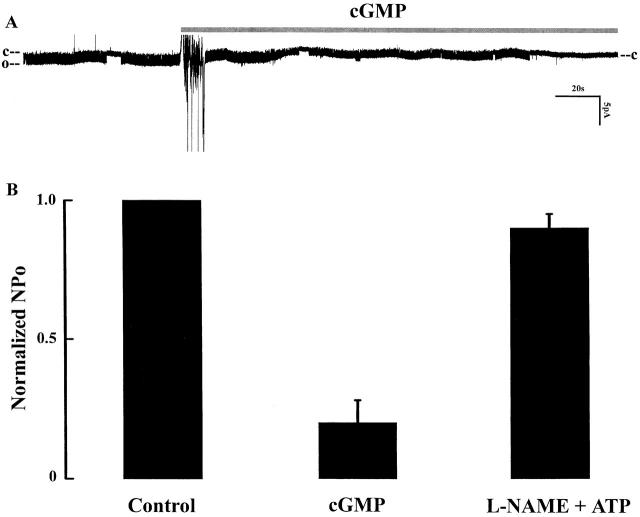
Finally, the role of cGMP in modulating the inhibitory effect of extracellular ATP was directly demonstrated in experiments in which exposure to membrane-permeant cGMP (100 μM) mimicked the effects of ATP (Fig. 12 A). Fig. 12 B summarizes results of 17 experiments showing that channel activity reversibly decreased by cGMP to 20% ± 8.7 of the control value. Furthermore, an important role of NO is suggested by our observations that pretreatment of the tubule for 20 min with 100 μM L-NAME, an inhibitor of NOS, abolished the inhibitory effect of ATP (Fig. 12 B).
Figure 12.
(A) A recording showing the effect of 100 μM 8-bromo-cGMP on channel activity. C and O indicate channel closed and open states. (B) Summary of the effects of 100 μM 8-bromo-cGMP (n = 17) and of 200 μΜ L-NAME + ATP on normalized channel activities (n = 4). All experiments were performed in cell-attached patches.
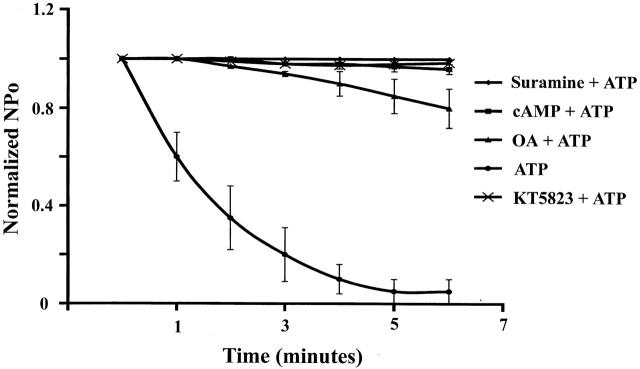
Fig. 13 provides the summary of various maneuvers designed to test the effect of several agents known to modulate small-conductance K channel activity. Since suramin, an inhibitor of P2, abolished the inhibitory effect of ATP and pretreatment of the tubules with cAMP, okadaic acid, and KT 5823 prevented the ATP inhibition on channel activity, it is strongly suggested that the effect of extracellular ATP was involved in channel phosphorylation and dephosphorylation processes.
Figure 13.
Effect of external ATP on channel activity in the presence of suramin, cAMP, okadaic acid, and KT 5823. The tubules were incubated with suramin (100 μM), cAMP (200 μM), okadaic acid (2 μM), and KT 5823 (1 μM) for 10 min before addition of 200 μm ATP.
DISCUSSION
The main finding of the present study is that extracellular ATP inhibits apical small-conductance K channels in principal tubule cells by mechanisms involving purinergic receptor activation and changes in channel dephosphorylation. The finding that purinergic receptors are present in the nephron (Cha et al. 1998; Bailey et al. 1999; McCoy et al. 1999) and that ATP can be released from tubule cells (Taylor et al. 1998; Wilson et al. 1999) raises the possibility that extracellular ATP may serve as a local regulator of tubule function, including the secretion of potassium. There is evidence that external ATP has significant effects on transport in several epithelia, including renal epithelial cell culture preparations (Gordjani et al. 1997), colon (Leipziger et al. 1997), pancreas (Dubyak 1999), and alveolar tissue (Kishore et al. 2000).
Previous studies on excised membrane patches have shown that millimolar concentrations of ATP in the cytosolic medium inhibit the small-conductance K channel (Wang and Giebisch 1991a,Wang and Giebisch 1991b), and similar results have been obtained with ROMK2 expressed in oocytes (McNicholas et al. 1996). The possibility that ATP could have penetrated the apical membrane and acted from the cytosolic compartment is excluded in the present experiments for the following reasons. First, channel block by ATP could be completely prevented by the inhibition of purinergic receptors as well as by phosphatase inhibitors. Second, the potency order of agonists of the purinergic receptor was that typically observed for P2Y2 receptors, a finding inconsistent with a direct action on the channel. Third, the effective extracellular concentration of ATP necessary to block channel activity was much lower than that necessary for channel inhibition from the inside of the membrane (Wang and Giebisch 1991a,Wang and Giebisch 1991b).
Purinergic receptors have been identified in numerous cells including renal epithelium (Anderson et al. 1991; Ecelbarger et al. 1994; Firestein et al. 1996; Cha et al. 1998; Chan et al. 1998; Bailey et al. 1999; Cuffe et al. 2000; Deetjen et al. 2000), colonic (Leipziger et al. 1997), pancreatic (Dubyak 1999), and airway cells (Mason et al. 1991). According to the pharmacological characteristics, intracellular transduction mechanisms and the results of expression cloning studies, the purinergic receptors are currently divided into two major subclasses: P2X and P2Y. P2X receptors are ATP-gated cation channels. P2Y receptors are G protein–coupled receptors linked to second messenger systems (PLC, IP3, Ca2+). At least seven ionotropic P2X receptors (P2X1–7) and six G protein–coupled P2Y receptors (P2Y1–4, 6, 11) have been cloned and characterized pharmacologically (Ralevic and Burnstock 1998). Recent studies from dissected rat CCD using immunohistochemical localization methods show that P2Y2 receptors are present on the apical membrane of principal tubule cells (Kishore et al. 2000). That G protein–linked purinergic receptors of the P2Y2 type are involved in the inhibition of K channels in the present study is also supported by our observation that 5 mM sodium fluoride, known to stimulate G proteins (Staub et al. 1998), mimicked the inhibitory effects of ATP [channel activity was reduced to 1% of control (n = 6) and the effects were partly reversible].
P2 receptors couple to several signaling pathways and their activation has been shown to modulate the activity of phospholipase C (Yamada et al. 1992; Fredholm et al. 1994), to alter the rate of production of cAMP (Anderson et al. 1991; Yamada et al. 1992), to involve PGE2 (Post et al. 1996, Post et al. 1998), and to change cytosolic calcium (Suko et al. 1997; Cuffe et al. 2000; Deetjen et al. 2000). The present study does not support the role of phospholipase C and PKC in mediating the effect of external ATP because application of inhibitors of phospholipase C and PKC failed to abolish the inhibitory effect of external ATP. It is possible that P2Y2 may couple to different second messengers or an U73122-insensitive phospholipase C-pathway in the mouse CCD. Moreover, PGE2-dependent pathways also do not play a significant role in the inhibition of apical small-conductance K channels by ATP. The latter conclusion is based on our observation that indomethacin, a potent inhibitor of PGE2 production, did not interfere with the inhibitory action of ATP (NP o before ATP: 2.77 ± 0.12; after ATP: 0.03 ± 0.02, n = 10). Since channel blockade by ATP could be either prevented or reversed by cAMP or by inhibition of phosphatases, it is most likely that changes in phosphorylation play a key role in the mediating the effects of ATP. Phosphorylation and dephosphorylation processes have significant effects on the activity of the small-conductance K channel (Kubokawa et al. 1995a; Wang et al. 1997). Whereas cAMP-dependent phosphorylation stimulates the K channel (McNicholas et al. 1994), increased phosphatase activity has the opposite effect (Kubokawa et al. 1995a). Channel inhibition by ATP, in our studies, was not mediated by a decrease in PKA-dependent channel phosphorylation, as evidenced by the failure of PKA inhibition to prevent channel block.
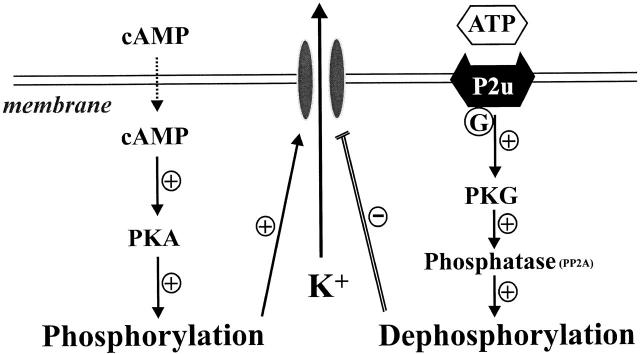
Fig. 14 shows a membrane model including the main factors proposed to mediate the effects of ATP. Shown is the cAMP-dependent pathway responsible for channel phosphorylation and the dephosphorylation pathway acting through the phosphatases such as PP2A and PP2C, both of which have been identified in apical membrane patches of principal cells (Kubokawa et al. 1995a,Kubokawa et al. 1995b). It is of interest that cGMP-dependent protein kinase G has been demonstrated to stimulate protein phosphatase 2A in several tissues such as tracheal smooth muscle, Chinese hamster ovary, pituitary tumor cells, and hippocampal neuron cells (White et al. 1993; Furukawa et al. 1996; Zhou et al. 1996). Our observation that ATP-effects could be abolished by inhibition of cGMP supports the view that the link between purinergic receptor activation and channel effects is through a pathway involving activation of cGMP. A possible sequence of events after stimulation of P2 receptors could involve Ca release from tissue stores (Ecelbarger et al. 1994; Suko et al. 1997; Deetjen et al. 2000), an increase in NO and the subsequent activation of cGMP (Lu and Wang 1998; Wang et al. 1998), PKG and, through the latter, activation of phosphatase PP2A. Previous studies have demonstrated the presence of Ca-dependent NO synthase in principal cells (Wang et al. 1998) and identified participation of cGMP in the regulation of basolateral K channels (Lu and Wang 1996; Lu et al. 1997). The role of NO in mediating the effect of external ATP is strongly indicated by the observation that inhibition of NOS completely suppressed the effect of ATP.
Figure 14.
Scheme illustrating the small-conductance K channel in the apical membrane of CCD was regulated by two different mechanisms: phosphorylation and dephosphorylation processes. Extracellular ATP stimulates G protein–coupled P2Y2 receptors, which potentiate the activity of PKG and increase the channel dephosphorylation through phosphatase (PP2A). Increasing channel phosphorylation processes can reverse the inhibitory effect of extracellular ATP on channel activity via activation of cAMP-dependent PKA.
Distinct effects of extracellular ATP on sodium chloride transport were reported in cultured kidney cells of cortical collecting tubules (Cuffe et al. 2000) and inner medulla of mouse cortical collecting duct cells (McCoy et al. 1999). There is agreement that extracellular ATP, via activation of purinergic receptors, stimulates chloride secretion and inhibits sodium reabsorption (Cuffe et al. 2000). Several studies have also shown that ATP initiates a transient increase in the concentration of calcium (Ecelbarger et al. 1994; Paulais et al. 1995; Bouyer et al. 1998; Deetjen et al. 2000), although inhibition of sodium and calcium absorption by activation of P2Y2 receptors in cultured connecting tubule and cortical collecting duct cells does not require changes in cell calcium concentration (Koster et al. 1996). Preliminary experiments in perfused rabbit cortical collecting tubules in which ATP was added to the tubule perfusion solution (100 μM) have shown significant inhibition of net K secretion (L.M. Satlin, personal communication), findings that support the observations of the present study, in which inhibition of secretory K channels was observed.
One uncertainty concerns the concentration of ATP in the tubule fluid since no measurements are available under free-flow conditions in either native or perfused tubules in either physiological or pathophysiological conditions. Information is presently limited to tubule cells in culture in which the range of extracellular ATP varied between 0.5 and 10 μM (Wilson et al. 1999). Our studies also do not provide information on possible effects that were mediated by purinergic receptors on the basolateral membrane of principal cells. Such receptors have been identified in several epithelia including renal tubules (Deetjen et al. 2000; Kishore et al. 2000). Although we have directly demonstrated significant effects of ATP added to the lumen, we cannot exclude the possibility that ATP may have reached the basolateral membrane by diffusion through the paracellular shunt pathway and acted on basolateral receptors, even though the relatively high resistance of collecting ducts makes this mode of action unlikely.
The physiological role of extracellular ATP in the regulation of tubule function, especially with respect to the process of distal potassium secretion, is incompletely understood. Extracellular ATP signaling has been implicated in several epithelial transport processes, for instance in the control of cell volume during exposure to hypotonic media (Taylor et al. 1998), and release of ATP has been observed in primary cultures of epithelia from cysts of polycystic kidneys (Wilson et al. 1999). A high external concentration of ATP may also result from ischemic damage in which extensive release of K from injured kidney cells has been observed (Reeves and Shah 1994). Under such conditions, release of ATP from damaged cells may limit loss of K. Evidence is also available to suggest that release of ATP in epithelia may be triggered by mechanical stimulation (Lazarowski et al. 1997; Hamada et al. 1998). This raises the possibility that ATP may be moving from tubule cells into the lumen at elevated tubule flow rates. A role of CFTR in the release of ATP from epithelial cells has been demonstrated (Devidas and Guggino 1997; Jiang et al. 1998), and the presence of CFTR in cells of collecting ducts (Todd-Turla et al. 1996) suggests that such mechanisms may be involved in the transport of ATP into the tubule lumen.
In conclusion, we have observed that ATP has significant inhibitory actions on secretory K channels in principal cells of the mouse cortical collecting tubule. These effects can be shown to be mediated by purinergic P2Y2 receptors and are best explained by activation of membrane-bound phosphatases through a cGMP-dependent pathway. It is possible that local release of ATP, through its luminal actions on the small-conductance K channel in the apical membrane of principal cells, plays a role in autocrine or paracrine regulation of K secretion.
Acknowledgments
This work was supported by an interactive research grant (DK 54998) from the National Institutes of Health (NIH). Dr. MacGregor is supported by the National Kidney Foundation. Dr. W.H. Wang is supported by NIH grant DK 47402.
Footnotes
Abbreviation used in this paper: CCD, cortical collecting duct.
References
- Aguilar-Bryan L., Nichols C.G., Wechsler S.W., Clement J.P., IV, Boyd A.E., III, Gonzalez G., Herrera-Sosa H., Nguy K., Bryan J., Nelson D.A. Cloning of the β cell high-affinity sulfonylurea receptora regulator of insulin secretion. Science. 1995;268:423–426. doi: 10.1126/science.7716547. [DOI] [PubMed] [Google Scholar]
- Anderson R.J., Breckon R., Dixon B.S. ATP receptor regulation of adenylate cyclase and protein kinase C activity in cultured renal LLC-PK1 cells. J. Clin. Invest. 1991;87:1732–1738. doi: 10.1172/JCI115191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bailey M.A., Imbert-Teboul M., Burnstock G., Unwin R.J. P2-purinoceptors in the rat outer medullary collecting ductevidence for two receptor subtypes. J. Physiol. 1999;517:78P–79P. [Google Scholar]
- Baukrowitz T., Schulte U., Oliver D., Herlitze S., Krauter T., Tucker S.J., Ruppersberg J.P., Fakler B. PIP2 and PIP as determinants for ATP inhibition of KATP channels. Science. 1998;282:1141–1144. doi: 10.1126/science.282.5391.1141. [DOI] [PubMed] [Google Scholar]
- Bouyer P., Paulais M., Cougnon M., Hulin P., Anagnostopoulos T., Planelles G. Extracellular ATP raises cytosolic calcium and activates basolateral chloride conductance in Necturus proximal tubule. J. Physiol. 1998;510:535–548. doi: 10.1111/j.1469-7793.1998.535bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cha S.H., Sekine T., Endou H. P2 purinoceptor localization along rat nephron and evidence suggesting existence of subtypes P2Y1 and P2Y2 . Am. J. Physiol. Renal Physiol. 1998;274:F1006–F1014. doi: 10.1152/ajprenal.1998.274.6.F1006. [DOI] [PubMed] [Google Scholar]
- Chan C.M., Unwin R.J., Burnstock G. Potential functional roles of extracellular ATP in kidney and urinary tract. Exp. Nephrol. 1998;6:200–207. doi: 10.1159/000020524. [DOI] [PubMed] [Google Scholar]
- Cuffe J.E., Bielfeld-Ackermann A., Thomas J., Leipziger J., Korbmacher C. ATP stimulates Cl seceretion and reduces amiloride-sensitive Na absorption in M-1 mouse cortical collecting duct cells. J. Physiol. 2000;524:77–90. doi: 10.1111/j.1469-7793.2000.00077.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deetjen P., Thomas J., Lehrmann H., Kim S.J., Leipziger J. The luminal P2Y receptor in the isolated perfused mouse cortical collecting duct. J. Am. Soc. Nephrol. 2000;In press doi: 10.1681/ASN.V11101798. [DOI] [PubMed] [Google Scholar]
- Devidas S., Guggino W.B. The cystic fibrosis transmembrane conductance regulator and ATP. Curr. Opin. Cell Biol. 1997;9:547–552. doi: 10.1016/s0955-0674(97)80032-4. [DOI] [PubMed] [Google Scholar]
- Dubyak G.R. Focus on multiple functional P2X and P2Y receptors in the luminal and basolateral membranes of pancreatic duct cells. Am. J. Physiol. Cell Physiol. 1999;277:C202–C204. doi: 10.1152/ajpcell.1999.277.2.C202. [DOI] [PubMed] [Google Scholar]
- Ecelbarger C.A., Maeda Y., Gibson C.C., Knepper M.A. Extracellular ATP increases intracellular calcium in rat terminal collecting duct via a nucleotide receptor. Am. J. Physiol. Renal Physiol. 1994;267:F998–F1006. doi: 10.1152/ajprenal.1994.267.6.F998. [DOI] [PubMed] [Google Scholar]
- Firestein B., Xing M., Hughes R.J., Corvera C.U., Insel P.A. Heterogeneity of P2u- and P2y-purinergic receptor regulation of phospholipases in MDCK cells. Am. J. Physiol. Renal Physiol. 1996;271:F610–F618. doi: 10.1152/ajprenal.1996.271.3.F610. [DOI] [PubMed] [Google Scholar]
- Fredholm B.B., Abbracchio M.P., Burnstock G., Daly J.W., Harden T.K., Jacobson K.A., Leff P., Williams M. Nomenclature and classification of purinoceptors. Pharmacol. Rev. 1994;46:143–156. [PMC free article] [PubMed] [Google Scholar]
- Fredholm B.B., Abbracchio M.P., Burnstock G., Dubyak G.R., Harden T.K., Jacobson K.A., Schwabe U., Williams M. Towards a revised nomenclature for P1 and P2 receptors. Trends Pharmacol. Sci. 1997;18:79–82. doi: 10.1016/s0165-6147(96)01038-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frindt G., Palmer L.G. Low-conductance K channels in apical membrane of rat cortical collecting tubule. Am. J. Physiol. Renal Physiol. 1989;256:F143–F151. doi: 10.1152/ajprenal.1989.256.1.F143. [DOI] [PubMed] [Google Scholar]
- Furukawa K., Barger S.W., Blalock E.M., Mattson M.P. Activation of K+ channels and suppression of neuronal activity by secreted β-amyloid-precursor protein. Nature. 1996;379:74–78. doi: 10.1038/379074a0. [DOI] [PubMed] [Google Scholar]
- Giebisch G. Renal potassium transportmechanisms and regulation. Am. J. Physiol. Renal Physiol. 1998;274:F817–F833. doi: 10.1152/ajprenal.1998.274.5.F817. [DOI] [PubMed] [Google Scholar]
- Gordjani N., Nitschke R., Greger R., Leipziger J. Capacitative Ca2+ entry (CCE) induced by luminal and basolateral ATP in polarized MDCK-C7 cells is restricted to the basolateral membrane. Cell Calc. 1997;22:121–128. doi: 10.1016/s0143-4160(97)90112-3. [DOI] [PubMed] [Google Scholar]
- Ho K., Nichols C.G., Lederer W.J., Lytton J., Vassilev P.M., Kanazirska M.V., Hebert S.C. Cloning and expression of an inwardly rectifying ATP-regulated potassium channel. Nature. 1993;362:31–38. doi: 10.1038/362031a0. [DOI] [PubMed] [Google Scholar]
- Hamada K., Takuwa N., Yokoyama K., Takuwa Y. Stretch activates Jun N-terminal kinase/stress-activated protein kinase in vascular smooth muscle cells through mechanisms involving autocrine ATP stimulation of purinoceptors. J. Biol. Chem. 1998;273:6334–6340. doi: 10.1074/jbc.273.11.6334. [DOI] [PubMed] [Google Scholar]
- Huang C.L., Feng S., Hilgemann D.W. Direct activation of inward rectifier potassium channels by PIP2 and its stabilization by Gβγ. Nature. 1998;391:803–806. doi: 10.1038/35882. [DOI] [PubMed] [Google Scholar]
- Jiang Q., Mak D., Devidas S., Schwiebert E.M., Bragin A., Zhang Y., Skach W.R., Guggino W.B., Foskett J.K., Engelhardt J.F. Cystic fibrosis transmembrane conductance regulator-associated ATP release is controlled by a chloride sensor. J. Cell. Biol. 1998;143:645–657. doi: 10.1083/jcb.143.3.645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- King B.F., Townsend-Nicholson A., Burnstock G. Metabotropic receptors for ATP and UTPexploring the correspondence between native and recombinant nucleotide receptors. Trends Pharmacol. Sci. 1998;19:506–514. doi: 10.1016/s0165-6147(98)01271-1. [DOI] [PubMed] [Google Scholar]
- Kishore B.K., Chou C.-L., Knepper M.A. Extracellular nucleotide receptor inhibits AVP-stimulated water permeability in inner medullary collecting duct. Am. J. Physiol. Renal Physiol. 1995;269:F863–F869. doi: 10.1152/ajprenal.1995.269.6.F863. [DOI] [PubMed] [Google Scholar]
- Kishore B.K., Ginns S.M., Krane C.M., Nielsen S., Knepper M.A. Cellular localization of P2Y2 purinoceptor in rat renal inner medulla and lung. Am. J. Physiol. Renal Physiol. 2000;278:F43–F51. doi: 10.1152/ajprenal.2000.278.1.F43. [DOI] [PubMed] [Google Scholar]
- Koster H.P., Hartog A., Van Os C.H., Bindels R.J. Inhibition of Na+ and Ca2+ reabsorption by P2U purinoceptors requires PKC but not Ca2+ signaling. Am. J. Physiol. Renal Physiol. 1996;270:F53–F60. doi: 10.1152/ajprenal.1996.270.1.F53. [DOI] [PubMed] [Google Scholar]
- Kubokawa M., McNicholas C.M., Higgins M.A., Wang W.H., Giebisch G. Regulation of renal ATP-sensitive K channel by membrane-bound protein phosphatases in rat principal tubule cell Am. J. Physiol. Renal Physiol 269 1995. F355 F362a [DOI] [PubMed] [Google Scholar]
- Kubokawa M., Wang W.H., McNicholas C.M., Giebisch G. Role of Ca/ CaNK II in Ca-induced K channel inhibition in rat CCD principal cell Am. J. Physiol. Renal Physiol. 268 1995. F211 F219b [DOI] [PubMed] [Google Scholar]
- Lazarowski E.R., Homolya L., Boucher R.C., Harden T.K. Direct demonstration of mechanically induced release of cellular UTP and its implication for uridine nucleotide receptor activation. J. Biol. Chem. 1997;272:24348–24354. doi: 10.1074/jbc.272.39.24348. [DOI] [PubMed] [Google Scholar]
- Leipziger J., Kerstan D., Nitschke R., Greger R. ATP increases [Ca2+]i and ion secretion via a basolateral P2Y-receptor in rat distal colonic mucosa. Pflügers Arch. 1997;434:77–83. doi: 10.1007/pl00008079. [DOI] [PubMed] [Google Scholar]
- Leite M., Jr., Satlin L.M. Apical purinergic receptors are present in the rabbit cortical collecting duct (CCD) J. Am. Soc. Nephrol 7 1996. A2169(Abstr.) [Google Scholar]
- Lu M., Giebisch G., Wang W.H. Nitric oxide links the apical Na+ transport to the basolateral K+ conductance in rat CCD. J. Gen. Physiol. 1997;110:717–726. doi: 10.1085/jgp.110.6.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu M., Wang W.H. Nitric oxide regulates the low-conductance K channel in the basolateral membrane of the cortical collecting duct. Am. J. Physiol. Cell Physiol. 1996;270:C1338–C1342. doi: 10.1152/ajpcell.1996.270.5.C1336. [DOI] [PubMed] [Google Scholar]
- Lu M., Wang W.H. Reaction of nitric oxide with superoxide inhibits basolateral K channels in the rat CCD. Am. J. Physiol. Cell Physiol. 1998;275:C309–C316. doi: 10.1152/ajpcell.1998.275.1.C309. [DOI] [PubMed] [Google Scholar]
- Macica C.M., Yang Y., Lerea K., Hebert S.C., Wang W. Role of the NH2 terminus of the cloned renal K+ channel, ROMK1, in arachidonic acid-mediated inhibition. Am. J. Physiol. Renal Physiol. 1998;274:F175–F181. doi: 10.1152/ajprenal.1998.274.1.F175. [DOI] [PubMed] [Google Scholar]
- Mason S.J., Paradiso A.M., Boucher R.C. Regulation of transepithelial ion transport and intracellular calcium by extracellular ATP in human normal and cystic fibrosis airway epithelium. Br. J. Pharmacol. 1991;103:1649–1656. doi: 10.1111/j.1476-5381.1991.tb09842.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCoy D.E., Taylor A., Kudlow B.A., Karlson K., Slattery M.J., Schwiebert L.M., Schwiebert E.M., Stanton B.A. Nucleotides regulate NaCl transport in mIMCD-K2 cells via P2X and P2Y purinergic receptors. Am. J. Physiol. Renal Physiol. 1999;277:F552–F559. doi: 10.1152/ajprenal.1999.277.4.F552. [DOI] [PubMed] [Google Scholar]
- McNicholas C.M., Yang Y., Giebisch G., Hebert S.C. Molecular site for nucleotide binding on an ATP-sensitive renal K+ channel (ROMK2) Am. J. Physiol. Renal Physiol. 1996;271:F275–F285. doi: 10.1152/ajprenal.1996.271.2.F275. [DOI] [PubMed] [Google Scholar]
- McNicholas C.M., Wang W., Ho K., Hebert S.C., Giebisch G. Regulation of ROMK1 K channel activity involves phosphorylation processes. Proc. Natl. Acad. Sci. USA. 1994;91:8077–8081. doi: 10.1073/pnas.91.17.8077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morales M.M., Carroll T.P., Morita T., Schwiebert E.M., Devuyst O., Wilson P.D., Lopes A.G., Stanton B.A., Dietz H.C., Cutting G.R., Guggino W.B. Both the wild type and a functional isoform of CFTR are expressed in kidney. Am. J. Physiol. Renal Physiol. 1996;270:F1038–F1048. doi: 10.1152/ajprenal.1996.270.6.F1038. [DOI] [PubMed] [Google Scholar]
- Palmer L.G., Choe H., Frindt G. Is the secretory K channel in the rat CCT ROMK? Am. J. Physiol. Renal Physiol. 1997;273:F404–F410. doi: 10.1152/ajprenal.1997.273.3.F404. [DOI] [PubMed] [Google Scholar]
- Paulais M., Baudouim-Legros M., Teulon J. Extracellular ATP and UTP trigger calcium entry in mouse cortical thick ascending limbs. Am. J. Physiol. Renal Physiol. 1995;268:F496–F502. doi: 10.1152/ajprenal.1995.268.3.F496. [DOI] [PubMed] [Google Scholar]
- Post S.R., Jacobson J.P., Insel P.A. P2 purinergic receptor agonists enhance cAMP production in Madin-Darby canine kidney epithelial cells via an autocrine/paracrine mechanism. J. Biol. Chem. 1996;271:2029–2032. doi: 10.1074/jbc.271.4.2029. [DOI] [PubMed] [Google Scholar]
- Post S.R., Rump L.C., Zambon A., Hughes R.J., Buda M.D., Jacobson J.P., Kao C.C., Insel P.A. ATP activates cAMP production via multiple purinergic receptors in MDCK-D1 epithelial cells. J. Biol. Chem. 1998;273:23093–23097. doi: 10.1074/jbc.273.36.23093. [DOI] [PubMed] [Google Scholar]
- Ralevic V., Burnstock G. Receptors for purines and pyrimidines. Pharmacol. Rev. 1998;50:413–450. [PubMed] [Google Scholar]
- Reeves W.B., Shah S.V. Activation of potassium channels contributes to hypoxic injury in proximal tubules. J. Clin. Invest. 1994;94:2289–2294. doi: 10.1172/JCI117592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rouse D., Leite M., Suki W.N. ATP inhibits the hydrosmotic effect of AVP in rabbit CCTevidence of a nucleotide P2u receptor. Am. J. Physiol. Renal Physiol. 1994;267:F289–F295. doi: 10.1152/ajprenal.1994.267.2.F289. [DOI] [PubMed] [Google Scholar]
- Staub M., Csapo Z., Spasokukotskaja T., Sasvari-Szekely M. Deoxycytidine kinase can be also potentiated by the G-protein activator NaF in cells. Adv. Exp. Med. Biol. 1998;431:425–428. doi: 10.1007/978-1-4615-5381-6_83. [DOI] [PubMed] [Google Scholar]
- Suko Y., Kawahara K., Fukuda Y., Masuda Y. Nuclear and cytosolic calcium signaling induced by extracellular ATP in rat kidney inner medullary collecting duct cells. Biochem. Biophys. Res. Commun. 1997;234:224–229. doi: 10.1006/bbrc.1997.6488. [DOI] [PubMed] [Google Scholar]
- Taylor A.L., Kudlow B.A., Marrs K.L., Gruenert D.C., Guggino W.B., Schwiebert E.M. Bioluminescence detection of ATP release mechanisms in epithelia. Am. J. Physiol. Cell Physiol. 1998;275:C1391–C1406. doi: 10.1152/ajpcell.1998.275.5.C1391. [DOI] [PubMed] [Google Scholar]
- Todd-Turla K.M., Rusvai E., Naray-Fejes-Toth A., Fejes-Toth G. CFTR expression in cortical collecting duct cells. Am. J. Physiol. Renal Physiol. 1996;270:F237–F244. doi: 10.1152/ajprenal.1996.270.1.F237. [DOI] [PubMed] [Google Scholar]
- Wang T., Wang W.H., Klein-Robbenhaar G., Giebisch G. Effects of glyburide on renal tubule transport and potassium-channel activity. Renal Physiol. Biochem. 1995;18:169–182. doi: 10.1159/000173914. [DOI] [PubMed] [Google Scholar]
- Wang W.H., Schwab A., Giebisch G. Regulation of small-conductance K channel in apical membrane of rat cortical collecting tubule. Am. J. Physiol. Renal Physiol. 1990;259:F494–F502. doi: 10.1152/ajprenal.1990.259.3.F494. [DOI] [PubMed] [Google Scholar]
- Wang W., Giebisch G. Dual effect of adenosine triphosphate on the apical small conductance K channel of the rat cortical collecting duct J. Gen. Physiol 98 1991. 35 61a [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W., Giebisch G. Dual modulation of renal ATP-sensitive K-channel by protein kinase A and C Proc. Natl. Acad. Sci. USA 88 1991. 9722 9725b [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang W.H., Hebert S.C., Giebisch G. Renal K channelsstructure and function. Annu. Rev. Physiol. 1997;59:413–436. doi: 10.1146/annurev.physiol.59.1.413. [DOI] [PubMed] [Google Scholar]
- Wang X.H., Lu M., Gao Y., Papapetropoulos A., Sessa W.C., Wang W.H. Neuronal nitric oxide synthase is expressed in principal cell of collecting duct. Am. J. Physiol. Renal Physiol. 1998;275:F395–F399. doi: 10.1152/ajprenal.1998.275.3.F395. [DOI] [PubMed] [Google Scholar]
- Wilson P.D., Hovater J.S., Casey C.C., Fortenberry J.A., Schwiebert E.M. ATP release mechanisms in primary cultures of epithelia derived from the cysts of polycystic kidneys. J. Am. Soc. Nephrol. 1999;10:218–229. doi: 10.1681/ASN.V102218. [DOI] [PubMed] [Google Scholar]
- White R.E., Lee A.B., Shcherbatko A.D., Lincoln T.M., Schonbrunn A., Armstrong D.L. Potassium channel stimulation by natriuretic peptides through cGMP-dependent dephosphorylation. Nature. 1993;361:263–266. doi: 10.1038/361263a0. [DOI] [PubMed] [Google Scholar]
- Xu J.Z., Hall A.E., Peterson L.N., Bienkowski M.J., Eessalu T.E., Hebert S.C. Localization of the ROMK protein on apical membranes of rat kidney nephron segments. Am. J. Physiol. Renal Physiol. 1997;273:F739–F748. doi: 10.1152/ajprenal.1997.273.5.F739. [DOI] [PubMed] [Google Scholar]
- Xu Z.C., Yang Y., Hebert S.C. Phosphorylation of the ATP-sensitive, inwardly rectifying K channel, ROMK, by cyclic AMP-dependent protein kinase. J. Biol. Chem. 1996;271:9313–9319. doi: 10.1074/jbc.271.16.9313. [DOI] [PubMed] [Google Scholar]
- Yamada M., Hamamori Y., Akita H., Yokoyama M. P2-purinoceptor activation stimulates phosphoinositide hydrolysis and inhibits accumulation of cAMP in cultured ventricular myocytes. Circ. Res. 1992;70:477–485. doi: 10.1161/01.res.70.3.477. [DOI] [PubMed] [Google Scholar]
- Zhou X.B., Ruth P., Schlossmann J., Hofmann F., Korth M. Protein phosphatase 2A is essential for the activation of Ca2+-activated K+ currents by cGMP-dependent protein kinase in tracheal smooth muscle and Chinese hamster ovary cells. J. Biol. Chem. 1996;271:19760–19767. doi: 10.1074/jbc.271.33.19760. [DOI] [PubMed] [Google Scholar]