Abstract
Activator protein-1 (AP-1) is a transcription factor that consists of either a Jun-Jun homodimer or a Jun-Fos heterodimer. Transactivation of AP-1 is required for tumor promoter-induced transformation in mouse epidermal JB6 cells and for progression in mouse and human keratinocytes. Until now, the question of whether AP-1 transactivation is required for carcinogenesis in vivo has remained unanswered, as has the issue of functionally significant target genes. To address these issues we have generated a transgenic mouse in which transactivation mutant c-jun (TAM67), under the control of the human keratin-14 promoter, is expressed specifically in the basal cells of the epidermis where tumor induction is initiated. The keratin-14–TAM67 transgene was expressed in the epidermis, tongue, and cervix, with no apparent abnormalities in any tissue or organ. TAM67 expression blocked 12-O-tetradecanoylphorbol 13-acetate (TPA, phorbol 12-tetradecanoate 13-acetate) induction of the AP-1-regulated luciferase in AP-1 luciferase/TAM67 mice, but did not inhibit induction of candidate AP-1 target genes, collagenase-1 or stromelysin-3. More interestingly, TAM67 expression did not inhibit TPA-induced hyperproliferation. In two-stage skin carcinogenesis experiments, the transgenic animals showed a dramatic inhibition of papilloma induction. We conclude that transactivation of a subset of AP-1-dependent genes is required for tumor promotion and may be targeted for cancer prevention.
Carcinogenesis is a multistage process that encompasses multiple genetic and epigenetic events (reviewed in refs. 1–3). These events can be divided into three independent stages: initiation, promotion, and progression. Initiation is rapid and irreversible and occurs at a high frequency, whereas promotion is a long-term process that requires chronic exposure to a tumor promoter. The rate-limiting steps in multistage carcinogenesis now are understood to occur during tumor promotion and tumor progression. Understanding the molecular basis of these steps is important for prevention of carcinogenesis. Studies in our laboratory (4–6) and others (7–9) using cell culture models have implicated transactivation of the transcription factor activator protein-1 (AP-1) as important in traversing tumor promotion and/or progression stages. AP-1 is composed of heterodimers of Jun (cJun, Jun B, and Jun D) and Fos (cFos, Fos B, Fra 1, and Fra 2) or homodimers of Jun/Jun (10). AP-1 regulates the transcription of a number of genes, some of which may mediate neoplastic transformation (11, 12).
The mouse epidermal JB6 cells have proven to be valuable in studying tumor promotion because they include variants stably trapped in a promotable stage (4, 13, 14). In transformation-sensitive (P+) but not transformation-resistant (P−) JB6 cell lines, tumor promoters such as phorbol esters or growth factors induce AP-1 activity and neoplastic transformation. The AP-1 inhibitor TAM67 (a transactivation domain deletion mutant of cJun) acts to sequester Jun and Fos family proteins in low activity complexes (5, 15). In JB6 cells, inhibition of AP-1 induction by TAM67 or by AP-1 transrepressing retinoids blocks 12-O-tetradecanoylphorbol 13-acetate (TPA, phorbol 12-tetradecanoate 13-acetate)- and epidermal growth factor-induced AP-1 transactivation and cell transformation (5, 16). In mouse keratinocytes, the expression of TAM67 under the control of the human keratin 14 (hK14) promoter (K14-TAM67) inhibited TPA-induced AP-1-dependent transcriptional activity, as well as TPA-induced Matrigel invasion (17). Others also have reported findings implicating AP-1 activation in invasion (9). Similarly, in HPV18-E6E7/v-fos- transformed human keratinocytes, K14-TAM67 expression inhibited elevated AP-1 activation and suppressed anchorage-independent growth (6). K14-TAM67 expression also inhibited TPA-induced or constitutively elevated NFκB transactivation in mouse and human keratinocytes (6, 17).
In vivo models of multistage mouse skin carcinogenesis have proven useful in elucidating the molecular events that occur during tumor induction (3). Studies with mouse models have suggested that c-fos expression is involved in benign-to-malignant tumor progression (18). Furthermore, in c-fos null mice initiated by v-H-ras, papillomas were induced by phorbol ester treatment, but these morphologically unusual tumors did not convert to carcinomas, suggesting that whereas a cFos function is required for the progression of tumors from benign papillomas to malignant carcinomas, it is not required for papillomagenesis (19). The effects on carcinogenesis of c-jun knockout mice could not be assessed as c-jun null embryos were not viable (20). Because cFos can contribute to transcription factor complexes other than AP-1 (21), because activated forms of AP-1 do not necessarily contain cFos, and because expression of matrix metalloproteinases (MMPs) that contain AP-1 binding sites can be insensitive to AP-1 inhibitors (17), the question heretofore has remained unresolved as to whether AP-1 transactivation is required for tumor promotion or tumor progression in vivo.
To more precisely define the role of AP-1 transactivation in tumor promotion and/or progression, we used a K14-TAM67 construct to direct expression of dominant negative c-jun to the basal squamous cells of the epidermis in transgenic mice. We report here that dominant negative c-jun-expressing transgenic mice are inhibited for tumor promoter-induced AP-1 transactivation and are protected against skin tumor promotion without evidence of skin anomalies. These results support the hypothesis that AP-1-dependent gene expression is required during the tumor promotion step of skin carcinogenesis and can be targeted for cancer prevention.
MATERIALS AND METHODS
Construction and Genotyping of Transgenic Mice.
All animals were obtained from the Frederick Cancer Research and Development Center animal processing area maintained by Charles River Labs. The construction of the K14-TAM67-hGH vector (hGH, human growth hormone) has been described (17). Briefly, TAM67 isolated from pMexMTH-neoTAM67 (15) was inserted downstream of the hK14 promoter (22). A 5.8-kb fragment containing the K14-TAM67-hGH gene was isolated and microinjected into B6D2/F1 one-cell embryos as described (23). K14-TAM67 transgenic mice were backcrossed with DBA/2 mice. C57B6/AP-1 luciferase (AP-1-luc) mice (24) crossed with heterozygous K14-TAM67 mice to produce TAM67-positive and TAM67-negative B6D2/AP-1-luc mice.
Offspring carrying the K14-TAM67 transgene were identified by PCR analysis of tail DNA. Reaction containing 20 pmol of hK14 primers (GenBank accession no. U11076) bp 1693–1717 and 2205–2181, glyceraldehyde-3-phosphate dehydrogenase primers (M32599) bp 255–274 and 637–618, or luciferase primers GCGGAATACTTCGAAATGTCC and CCTTAGGTAACCCAGTAGATCC, were amplified by using a GemAmp PCR kit (Perkin–Elmer) according to the manufacturer’s recommendations. PCR products were separated on a 1.2% agarose gel. The DNA copy number of the transgene was determined by Southern analysis of PstI-digested DNA extracted from liver and probed with a TAM67-specific DNA probe.
Reverse Transcription (RT)-PCR and Detection of mRNA Expression.
Tissues for RNA expression were harvested from animals after cervical dislocation. Ears, liver, kidney, cervix, tongue, and full thickness dorsal skin were snap-frozen in liquid nitrogen. Epidermis was separated from dermis as described (25). All tissue was pulverized in liquid nitrogen with a mortar and pestle and immediately placed in quanidium isothiocyanate, homogenized, and extracted with phenol-chloroform by using a Rapid RNA isolation kit from 5 Prime → 3 Prime.
RNA expression of the transgene, mK14, ornithine decarboxylase (ODC), collagenase-1, and stromelysin-3 were analyzed by RT-PCR with the gene amplification kit from Perkin–Elmer according to the manufacturer’s recommendation. Gene-specific antisense primers (for TAM-hGH and mK14) or random hexamer primers (for 18s) were used to prime the RT reaction. For PCR amplification of cDNA the following primers were used: for TAM-hGH, c-jun (GenBank accession no. J04111) bp 2076–2097 and hGH (M13438) bp 908–889; for mK14 (M13806) bp 146–165 and 445–423; for collagenase-1 (X66473) bp 589–627 and 1316–1286; for stromelysin-3 (Z12604) bp 1463–1485 and 1805–1782; for ODC (M10624) bp 865–893 and 1252–1225; and for β-actin (X03765) bp 282–301 and 656–636. 18S primers were from Ambion’s Quantum RNA kit. For quantitative PCR analysis, poly(A)dT was used to prime RT reactions and 10%, 20%, or 40% of the cDNA product was amplified by 20 cycles of PCR in the presence of 2 μCi of [33P]dCTP. PCR products then were separated on 10% polyacrylamide gel. The amount of radiolabel incorporated was quantified with a Molecular Dynamics PhosphorImager.
TPA Induction of Hyperproliferation and AP-1 Activation.
Animals were shaved with surgical clippers 2 days before treatment. Either a single dose or four doses administered twice a week of acetone or TPA (10 nmol in 0.2 ml of acetone) were painted on dorsal skin. Alternatively, 10 nmol of TPA in 0.1 ml of acetone was applied to the left ear and 0.1 ml of acetone to the right. For RNA expression, tissue was collected 6 hr after TPA treatment. For histological analysis, samples were collected at 24 or 48 hr after the last treatment, fixed overnight in 4% paraformaldehyde, and then embedded in paraffin. Five-micrometer sections were stained with hematoxylin/eosin (H&E). TPA-induced hyperproliferation was determined from H&E-stained slides. Four images from each sample were analyzed by confocal laser scanning microscopy from random regions on the slides. Hyperproliferation was determined by measuring the thickness of the epidermis by using the Optimas 6.2 Image Analysis Program (Media Cybernetics, Silver Spring, MD). Four measurements were made at random from the surface of the epidermis starting from the top of the basement membrane to the bottom of the strateum corneum.
Assays of AP-1-Luc Activity.
AP-1-luc activity was measured in AP-1-luc/TAM67 mice and in their TAM67-negative siblings. Mice were treated with 100 μl of acetone on the right ear and 100 μl of TPA (10 nmol) on the left ear. After 16 hr, ear punches of 2 mm were collected and lysed in 100 μl of lysis buffer. Twenty microliters of lysate was added to 100 μl of luciferase substrate (Promega), and luciferase activity was determined in a Monolight 2010 luminometer (Analytical Luminescence Laboratory, San Diego).
cDNA Array and RNase Protection Assays of Gene Expression.
Gene expression from total RNA isolated from dorsal epidermis 6 hr after exposure to TPA or acetone was examined by cDNA array using CLONTECH’s Atlas mouse cDNA expression array according to the manufacturer’s recommendation. Gene expression was determined by scanning with a Molecular Dynamics PhosphorImager.
Two-Stage Skin Carcinogenesis.
Dorsal skins of 8-week-old mice were shaved, and 2 days later a single dose of 400 nmol 7,12-dimethylbenz[a]anthracene (DMBA) in 200 μl of acetone was applied. Ten days after initiation, mice were treated with 10 nmol of TPA in 200 μl of acetone twice a week for 20 weeks. The number of papillomas detected by palpation was recorded once a week. One week after TPA was stopped, three mice from each group were sacrificed, and skin tissues were snap-frozen in liquid nitrogen and/or fixed in freshly prepared 4% paraformaldehyde followed by paraffin embedding. The remaining mice were maintained an additional 20 weeks after TPA was discontinued at which time the mice were sacrificed and tumors were isolated and fixed in 4% paraformaldehyde embedded in paraffin.
RESULTS
Tissue-Specific Expression of K14-TAM67 in Transgenic Mice.
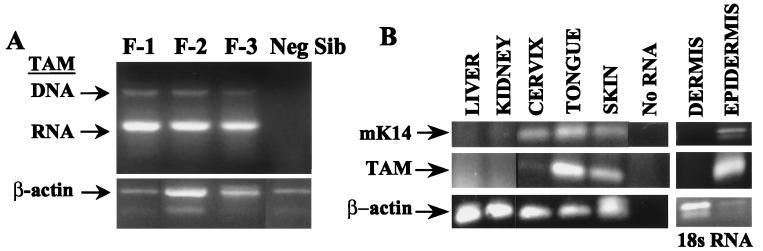
The K14-TAM67 transgenic mice were created in tumor promotion-sensitive strain B6D2/F1. Four K14-TAM67-positive animals originally were identified by PCR analysis of tail DNA using primers that hybridize to the hK14 promoter (data not shown). TAM67 was expressed in three founder colonies (Fig. 1A). The copy number of the transgene in founders 1 and 2 was determined by Southern blot analysis to be approximately 5.
Figure 1.
Expression of K14-TAM67 in transgenic mice. (A) Expression of TAM67 was determined by RT-PCR analysis of RNA isolated from skin. Founders 1, 2, and 3 (lanes 1–3), negative littermate (lane 4). RNA indicates the TAM67 PCR product of the cDNA generated by RT. DNA indicates the DNA product from PCR of the genomic DNA contaminating the RNA samples. β-actin is the RT-PCR product from the mouse β-actin gene. (B) TAM is expressed in epidermis, tongue, and cervix, but not dermis, liver, or kidney. RNA was subjected to RT-PCR using primers to amplify mouse K14 (mK14), TAM-hGH, β-actin mRNA, or 18s RNA.
The hK14 promoter was chosen to direct expression of TAM67 to the epidermis. Tissue-specific expression from the hK14 has been well characterized (26). Expression from the hK14 promoter has been detected in the tongue, oral epithelium, esophagus, and cornea, and to a lesser extent in the cervix and mammary epithelium (26–28). Tissue-specific expression of the K14-TAM67 transgene was analyzed by RT-PCR of total RNA isolated from liver, kidney, dorsal skin, tongue, and cervix (Fig. 1B). TAM-hGH spliced mRNA was detected in dorsal skin and tongue and at low levels in the cervix, but not in liver or kidney. The same pattern of expression was seen for the mouse K14 mRNA. Thus, whereas the hK14 promoter was chosen to direct expression of the dominant negative c-jun to the skin, the K14-TAM67 transgenic mice also may be useful in determining the role of AP-1-regulated genes or of other TAM67 targets in oral and/or cervical carcinogenesis.
Cutaneous expression of K14 is restricted to the basal layer of the epidermis, where tumor induction is initiated, and is down-regulated as the basal layer differentiates. K14 expression has not been detected in the dermis (29). To confirm that K14-TAM67 was expressed specifically in the epidermis, the epidermis of dorsal skin from transgenic mice was separated from dermal tissue, and total RNA was isolated and subjected to RT-PCR analysis (Fig. 1B). K14-driven expression of TAM67 was similar to that of the endogenous mK14 gene; both were detected in the epidermis and not in the dermis of the transgenic animals. 18S rRNA was detected in all samples as a positive control for RT-PCR.
Expression of TAM67 Has No Apparent Effect on the Morphology of the Skin.
There appears to be no gross morphological effect associated with expression of TAM67 in the skin. The basal layer, spinous layer, granular layer, and stratum corneum all appear similar to that of the TAM67-negative sibling. The epidermal thickness was also similar in transgenic and control mice, thus indicating neither a hypo- nor a hyperproliferation response to the transgene expression. Furthermore, no abnormalities were seen in the dermis or any other tissue examined by histopathology analysis. TAM67 mice have normal weights when compared with negative siblings, and there was no apparent morphological change associated with aging in 18-month-old animals. The lack of any detectable morphological defects associated with K14-TAM67 suggests that the basal squamous cell-specific expression of TAM67 had no gross effects on development or growth.
Expression of TAM67 Blocks TPA-Induced AP-1 Transactivation.
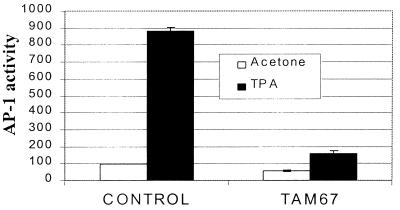
To determine the effects of TAM67 expression on phorbol ester-induced AP-1 activation, K14-TAM67 transgenic were crossed with AP-1-luc reporter transgenic mice (24). Luciferase activity from ear punch biopsies from transgenic mice treated with acetone (right ear) or TPA (left ear) showed that the basal level of AP-1 transcriptional activity was somewhat lower in TAM67 double transgenic mice than in the AP-1-luc mice (Fig. 2). In contrast, TPA-induced AP-1 activation was dramatically inhibited by more than 80% in the TAM67 mice compared with their negative siblings. Repetition of these assays under conditions that allowed more complete lysis and more sensitive measurement of luciferase activity showed greater than 80% inhibition of TPA-induced AP-1-luc activity, without inhibition of basal levels (data not shown). Thus, expression of TAM67 in skin markedly reduced TPA-induced but not basal AP-1 transactivation.
Figure 2.
TAM67 blocks TPA-induced AP-1 activation. The ears from two B6D2/N1/AP-1Luc/TAM67 mice and two TAM67-negative siblings were treated for 16 hr with acetone (right ear) or with 10 nmol of TPA (left ear). Tissue was isolated and lysed overnight, and luciferase activity was determined. The mean range for samples from two mice per group are shown. Similar results were seen on repetition of this experiment.
Analysis of TPA-Induced Gene Expression.
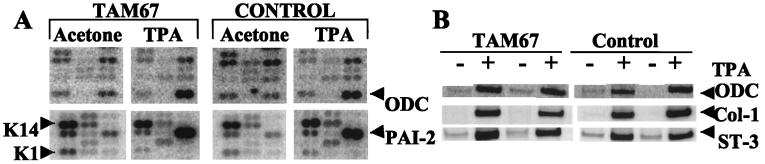
To determine which endogenous AP-1-dependent target genes may be affected by TAM67 expression, it was first necessary to determine the appropriate time after TPA treatment at which to measure AP-1-dependent gene expression. To this end, TPA-induced luciferase activity was determined from ear biopsies at various time intervals. AP-1 activity was not detected at 4 hr but was readily detected at 6 hr after the application of TPA (data not shown). This finding is in agreement with others who have shown TPA induction of candidate AP-1 target genes in the skin at 6 hr after treatment (30, 31). Therefore, dorsal skin of TAM67 mice and their negative siblings was treated with a single application of TPA or acetone, and 6 hr later RNA was isolated from the epidermis of the treated skin. Gene expression was analyzed by DNA array blotting using the mouse Atlas array blot from CLONTECH. Expression of 60% of the 588 genes was clearly detectable by this method. Of these, 46 were up- or down-regulated after TPA exposure (Fig. 3A shows representative grids). Two genes known to be up-regulated by TPA, ODC (30, 31) and plasminogen activator inhibitor-2 (PAI-2) (32), showed a similar induction by TPA in the transgenic and control mice (5- and 4-fold for ODC, 5- and 8-fold for PAI-2). On the other hand, the level of the differentiation-specific keratin-1 (K-1) (29) message was down-regulated similarly in the TPA-treated animals (6- and 4-fold in the TAM67 and control mice, respectively), reflecting reduced differentiation relative to proliferation in the TPA-treated epidermis. The level of the K14 mRNA did not change significantly in either set of mice with TPA treatment. Of the 350 genes whose expression was detectable, none appeared to be differentially regulated in the transgenic mice compared with their negative siblings. It perhaps is surprising that TPA-induced expression of ODC was not inhibited by TAM67 expression. It has been shown that ODC expression is necessary for tumor promotion in mouse skin (33). Although the ODC promoter lacks AP-1 sites, whether ODC expression indirectly depends on AP-1 was previously unknown. These results establish ODC induction in vivo as occurring independently of AP-1. Finally, other growth-related genes excluded as TAM67 targets by this analysis include cyclin A and cyclin A1 (data not shown).
Figure 3.
TPA induces ODC, plasminogen activator inhibitor-2, and MMPs equally in transgenic and control mice. (A) Epidermal RNA from four control or four B6D2/N1/TAM67 transgenic mice, exposed to acetone or TPA for 6 hr, was used as a template to generate a 32P-labeled cDNA probe for expression array analysis (CLONTECH). RT probes were hybridized to individual cDNA blots overnight and washed, and gene expression was determined by scanning with a Molecular Dynamics PhosphorImager. Expression levels were determined densitometrically and were standardized to two or more of the housekeeping genes. Two representative sections from the arrays are shown. (B) Epidermal RNA from treated skin was reverse-transcribed, and 10%, 20%, or 40% of the cDNA product was amplified by 20 cycles of PCR in the presence of [33P]dCTP. The PCR products were separated by PAGE. Gene expression was determined by scanning with a Molecular Dynamics PhosphorImager. PCR amplification was determined to be linear when 2-fold additions of cDNA resulted in 2-fold amplification of product. Results are shown for four independent mice: two control, two transgenic. Col-1, collagenase-1; ST-3, stromelysin-3.
To measure the expression of individual genes that were either not present or not detectable on the array, RT-PCR analysis was used. Fig. 3B shows that TPA induced expression of the MMPs collagenase-1 and stromelysin-3 equally in the TAM67-expressing mice and in their negative siblings. These analyses indicate that the inhibitory effect of TAM67 on AP-1-luc is not generalized to all of the potentially AP-1-regulated endogenous genes. These results were somewhat surprising in that activation of MMP promoter-reporter constructs has been shown in some models to depend on AP-1, and expression of endogenous MMPs and other TPA-inducible genes has been interpreted as reflecting AP-1 activation. RNase protection assay using mouse cytokine mCK-2 or the jun/fos primers set from PharMingen showed equal induction of IL-1α, IL-1β, junB, and fra1 by TPA in both mice (data not shown). Taken together, these gene expression results appear to exclude multiple MMPs, cytokines, and cyclins, as well as ODC and members of the Jun and Fos family as K14-TAM67 target genes.
TPA-Induced Hyperproliferation Is Not Inhibited by TAM67.
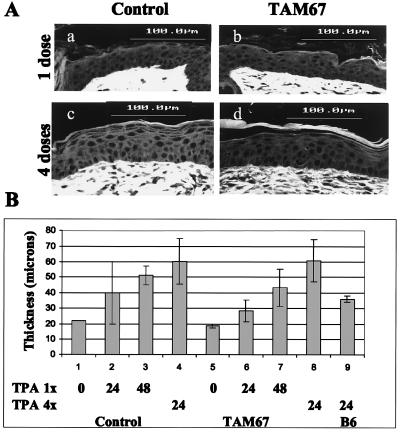
Acute treatment of mouse skin with the tumor promoter TPA has been shown to induce hyperproliferation of the epidermis (34), and prolonged treatment with TPA after tumor initiation can lead to papilloma formation (ref. 35 and reviewed in ref. 36). Furthermore, it has been shown that tumor promoter-induced hyperproliferation is necessary but not sufficient for the development of papillomas (which give rise to carcinomas) in mouse skin models (37–39). To determine the effects of TAM67 expression on hyperproliferation in mouse epidermis, transgene-positive and control mice were treated with TPA or acetone. Dorsal skin sections were analyzed by H&E staining at 24 hr and at 48 hr after treatment (Fig. 4A). TPA-induced hyperproliferation was measured as an increase in the thickness of the epidermis reflecting increased numbers of suprabasal dividing cells. TPA-induced hyperproliferation was apparent in both control and transgenic mice by 24 hr after a single treatment of TPA, and by 48 hr the degree of hyperproliferation in both groups was more than 2-fold that of the acetone control animals (Fig. 4B). Because a sustained hyperplasiagenic response after multiple exposures to TPA distinguishes promotion-sensitive from promotion-resistant mice (40, 41) we asked whether the hyperplasia in the TAM67 mice was sustained after four twice-weekly applications of TPA, conditions in which TPA induced AP-1-luc was shown to remain inhibited (data not shown). Measurements of the epidermal thickness showed that the magnitude of sustained hyperplasia in the tumor promotion-resistant C57BL/6 strain tested in parallel was diminished, as reported previously (40); however, it was undiminished in the TAM67-expressing B6D2/N1 mice compared with the TAM67-negative siblings. The mean numbers of basal to granular cell layers were 4.5 and 4.0 for the control and TAM67 mice, respectively, and only 2.4 for the C57BL/6 mice after TPA treatment. Furthermore, PCNA staining also showed no differential in the hyperproliferation response, indicating no differential entry into S phase (data not shown). Thus, expression of the dominant negative jun under conditions that inhibited AP-1 transactivation did not function to inhibit the TPA-induced hyperproliferation.
Figure 4.
TPA-induced hyperproliferation is not inhibited by TAM67. (A) Sections from dorsal skin of B6D2/N1/TAM67 (Right) and negative (Left) siblings treated with a single dose (Upper) or four twice-weekly doses (Lower) of TPA and stained with H&E 24 hr (Lower) or 48 hr (Upper) later. (B) Sections from dorsal skin of control mice (lanes 1–4) and TAM67 mice (lanes 5–8) were treated with a single dose of acetone (lanes 1 and 5) or TPA and 24 hr (lanes 2 and 6) or 48 hr (lanes 3 and 7) later stained with H&E, or treated with four twice-weekly doses of TPA (lanes 4 and 8) and 24 hr later stained with H&E. C57BL/6 (lane 9) was treated with four twice-weekly doses of TPA and stained 24 hr later. Sections were visualized under laser-induced confocal microscopy. The thickness of the epidermis was measured as described in Materials and Methods. An average of four measurements from two images from each section from two different animals (n = 16) for each time point was made.
Papillomagenesis Is Dramatically Inhibited by the Expression of TAM67.
In the mouse skin model, initiation can occur as a result of a single dose of a chemical carcinogen, such as DMBA, which mutationally activates H-ras (42) and promotion is induced by repeated treatments with a tumor promoter such as TPA (3). Naito et al. (43) showed that the B6D2/F1 offspring of a C57BL/6 crossed with DBA/2 were sensitive to DMBA initiation and TPA promotion of tumorigenesis, with tumor multiplicity intermediate between the resistant C57BL/6 and the sensitive DBA/2 parents. To test the activity of TAM67 in genetically sensitive mice, the B6D2F1/TAM67 founders were backcrossed with DBA/2 to generate (B6D2F1xDBA/2)N1 and (B6D2F1xDBA/2)N2.5 TAM67-positive and TAM67-negative siblings.
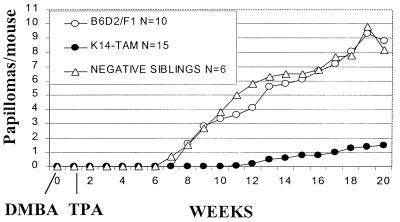
TAM67 mice, their negative siblings, and B6D2/F1 mice were subjected to DMBA initiation-TPA promotion; the results are shown in Fig. 5. Papillomas were first palpable by week 7 in the N2.5 TAM67-negative siblings and by week 8 in the B6D2/F1 mice. The incidence of papillomas (percent mice with papillomas) was 100% by week 9 in the TAM67-negative siblings and by week 11 in the B6D2/F1. On the other hand, papillomas were not detected in the (B6D2/F1xDBA/2)N2.5/TAM67 mice until week 11, and many of those mice remained tumor free. Furthermore, the maximal papilloma yield in the transgenic mice was 1.5 compared with 8.2–8.8 papillomas per mouse in the controls, indicating a greater than 80% inhibition of papillomagenesis. Similar results were seen in an independent experiment with the B6D2/F1xDBA/2)N1/TAM67 mice (data not shown). These results indicate that expression of K14-TAM67 in the epidermis appears to inhibit tumor promotion in the mouse skin model. Possible inhibition of tumor initiation by DMBA is not excluded, but is unlikely as TAM67 expression produced little or no inhibition of basal AP-1 activity. It is noteworthy that TAM67 expression is not accompanied by hypoproliferation, a response expected to inhibit initiation (35). The fact that the TPA-induced activation of AP-1 and papillomagenesis were both markedly inhibited in vivo is consistent with the hypothesis that AP-1 activation mediates tumor promotion and that inhibition of AP-1 transactivation can prevent carcinogenesis.
Figure 5.
Papillomagenesis is markedly inhibited by TAM67. (B6D2F1xDBA/2)N2.5 TAM67 (generated by crossing N3 males with N2 females), their negative siblings, and B6D2/F1 control mice were treated with a single dose of DMBA (400 nmol) and followed by twice-weekly doses of TPA (10 nmol) as described in Materials and Methods. Development of papillomas was determined by palpation. N in Inset indicates the number of animals. A similar result was obtained with B6D2/N1/TAM67 transgenics.
DISCUSSION
Transgenic mice expressing transactivation mutant c-jun are resistant to DMBA-TPA-induced skin papillomagenesis. Expression of dominant negative c-jun under the control of the human K14 promoter in the basal epidermis where tumor promotion occurs is sufficient to block tumor induction. Expression of TAM67 dramatically blocks TPA-induced activation of an AP-1 reporter. The possibility that the TAM67 mice are resistant not because they express TAM67 but because they express a segregating B6 resistance allele is unlikely because these resistance alleles are recessive (43). Furthermore, the lack of sustained hyperplasia that characterizes the resistant genotype of the C57BL/6 mice was absent in the K14-TAM67 mice. These results establish a requirement for AP-1-dependent gene expression in tumor promotion in vivo. They further demonstrate a genetic reagent effective in preventing tumor promotion and consequently tumor progression.
AP-1 Transactivation Is Required for Tumor Promotion.
Considering that a c-fos null status did not inhibit tumor promotion (19), it is perhaps surprising that blocking AP-1 transactivation by expressing TAM67 prevents tumor promotion. Whether AP-1 activation is inhibited in c-fos null mice is unknown. One possibility, compatible with the phenotypes of both the c-fos null and the K14-TAM67 transgenic mice is that cFos may not participate in the activated epidermal AP-1 transcription factor complex shown here to be necessary for tumor promotion. This finding suggests that tumor promotion is AP-1 dependent but c-fos independent. The tumor promotion inducing AP-1 complexes may contain Fos family members other than cFos. Elevated Fra-1 and Fos B have been found to be associated with progressive elevation of AP-1 and transformation in other models (16, 44, 45). Whether the cFos requirement for papilloma to carcinoma progression shown by Saez et al. (19) indicates a requirement for AP-1 activation currently is unknown. A more precise control of the expression of TAM67, for example with a tetracycline-regulated promoter (46), will allow determination of the role of AP-1 activation in papilloma to carcinoma progression under conditions in which papilloma induction is qualitatively and quantitatively unaffected. The use of inducible TAM67 also will permit separate examination of initiation and postinitiation events.
Hyperplasiagenesis May Occur Independently of AP-1 Activation.
Interestingly, TAM67 expression had no effect on basal epidermal thickness or on TPA-induced hyperproliferation. These phenotypes distinguish the TAM67 mice from the K14-HPVE7 (47, 48) and K14-IκBαM mice (49), both of which showed hyperproliferation in response to transgene expression. Tumor promoter-induced hyperproliferation appears to be necessary but not sufficient for tumor promotion (37–39). Although skin tumor promoters consistently induce hyperplasia, there are a few hyperplasiogenic agents such as ethyl phenyl propiolate that lack tumor-promoting activity (50). Our observations suggest that tumor promoter-induced AP-1 activation regulates expression of genes required for a promotion relevant event other than hyperproliferation. It is interesting that ODC induction was not inhibited by TAM67. Induction of ODC, which lacks AP-1 sites in its promoter, is necessary for tumor promotion as well as for hyperproliferation (33, 51).
Genes Excluded as Targets of Transactivation Mutant Jun.
Two putative AP-1-regulated candidate target genes, namely MMPs collagenase-1 and stromelysin-3, appear not to be TAM67 targets in vivo, at least in the epidermis, despite the implication of AP-1 activation. A number of candidate MMPs, including collagenase-I and stromelysin-1, also failed to be implicated as TAM67 targets in the mouse keratinocyte TPA-induced invasion model (17). The recalcitrance of endogenous epidermal collagenase-I and stromelysin-3 to TAM67 inhibition may reflect regulation by non-AP-1 factors. Alternatively, the expression of these genes may be AP-1 regulated, but by AP-1 complexes whose composition differs from that of the complexes regulating the 2× AP-1-luc reporter present in the mice. Thus, although AP-1 activation may be required for transformation, only a subset of potential AP-1-dependent genes are inhibited by TAM67 and therefore possibly responsible for inhibition of tumor promotion. Other TPA-induced epidermal genes excluded as targets of dominant negative Jun are ODC, plasminogen activator inhibitor-2, IL-1α, IL-β, and IL-1 Ra. Larger gene arrays from samples taken at multiple time points after TPA treatment appear to be needed to identify TAM67 target genes.
Potential for Prevention or Therapy.
Observations using retinoids to prevent tumor promotion in vivo (52) have suggested the utility for targeting AP-1 (16), but the question of the nonspecific toxicity of retinoids in vivo remains unresolved. The phenotype of the K14-TAM67 mice demonstrates the effectiveness of a genetic reagent for the prevention of tumor promotion, a rate-limiting stage of carcinogenesis. Expression of K14-TAM67 in mice had no obvious effects on the development or health of the transgenic animals. In fact, there was no detectable difference in any of the tissues tested when compared with control animals. Yet, the transgenic mice were dramatically protected from chemically induced carcinogenesis. The lack of toxicity combined with the efficiency at which K14-TAM67 inhibits a rate-limiting step of multistage carcinogenesis are valuable attributes befitting agents having potential utility for cancer prevention or therapy.
Acknowledgments
We thank Elaine Fuchs and her staff for help with the K14 vector, Jeff Green for advice on transgenic DNA, Rob Payne and the National Institute of Allergy and Infectious Diseases Transgenic Mouse Facility for generating the mice, Draginja Djuriskovic’s team for tumor experiments, Jim Resau for confocal laser scanning microscopy, Miriam Anver for pathology, Bill Pennie, Michael Birrer, David Margulies, Herbert Morse, and Zigang Dong for early discussions, and Colin Stewart and Paul Lambert for critical reading. This work was sponsored by the National Cancer Institute, Department of Health and Human Services, under contract with Advanced BioScience Laboratories (B.K.S.). R.A.F. is an Investigator of the Howard Hughes Medical Institute.
ABBREVIATIONS
- AP-1
activator protein-1
- K14
keratin-14
- TPA
12-O-tetradecanoylphorbol 13-acetate
- MMP
matrix metalloproteinase
- RT
reverse transcription
- ODC
ornithine decarboxylase
- hGH
human growth hormone
- H&E
hematoxylin/eosin
- DMBA
7,12-dimethylbenz[a]anthracene
- K-1
keratin-1
- AP-1-luc
AP-1 luciferase
References
- 1.Bickers D R, Lowy D R. J Invest Dermatol. 1989;92:121S–131S. doi: 10.1111/1523-1747.ep13075095. [DOI] [PubMed] [Google Scholar]
- 2.Boutwell R K. Prog Clin Biol Res. 1989;298:3–15. [PubMed] [Google Scholar]
- 3.Brown K, Balmain A. Cancer Metastasis Rev. 1995;14:113–124. doi: 10.1007/BF00665795. [DOI] [PubMed] [Google Scholar]
- 4.Bernstein L, Colburn N H. Science. 1989;244:566–569. doi: 10.1126/science.2541502. [DOI] [PubMed] [Google Scholar]
- 5.Dong Z, Birrer M, Watts R, Matrisian L, Colburn N H. Proc Natl Acad Sci USA. 1994;91:609–614. doi: 10.1073/pnas.91.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Li J J, Rhim J S, Schlegel R, Vousden K H, Colburn N H. Oncogene. 1998;21:2711–2721. doi: 10.1038/sj.onc.1201798. [DOI] [PubMed] [Google Scholar]
- 7.Domann F E, Levy J P, Birrer M J, Bowden G T. Cell Growth Differ. 1994;5:9–16. [PubMed] [Google Scholar]
- 8.Hennigan R F, Hawker K L, Ozanne B W. Oncogene. 1994;9:3591–3600. [PubMed] [Google Scholar]
- 9.Lamb R F, Hennigan R F, Turnbull K, Katsanakis K D, MacKenzie E D, Birnie G D, Ozanne B W. Mol Cell Biol. 1997;17:963–976. doi: 10.1128/mcb.17.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Angel P, Karin M. Biochim Biophys Acta. 1991;1072:129–157. doi: 10.1016/0304-419x(91)90011-9. [DOI] [PubMed] [Google Scholar]
- 11.Angel P, Imagawa M, Chiu R, Stein B, Imbra R J, Rahmsdorf H J, Jonat C, Herrlich P, Karin M. Cell. 1987;49:729–739. doi: 10.1016/0092-8674(87)90611-8. [DOI] [PubMed] [Google Scholar]
- 12.Matrisian L M. Ann NY Acad Sci. 1994;732:42–50. doi: 10.1111/j.1749-6632.1994.tb24723.x. [DOI] [PubMed] [Google Scholar]
- 13.Colburn N H, Former B F, Nelson K A, Yuspa S H. Nature (London) 1979;281:589–591. doi: 10.1038/281589a0. [DOI] [PubMed] [Google Scholar]
- 14.Colburn N H, Wendel E, Abruzzo G. Proc Natl Acad Sci USA. 1981;78:6912–6916. doi: 10.1073/pnas.78.11.6912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Brown P H, Alani R, Preis L H, Szabo E, Birrer M J. Oncogene. 1993;8:877–886. [PubMed] [Google Scholar]
- 16.Li J J, Dong Z, Dawson M I, Colburn N H. Cancer Res. 1996;56:483–489. [PubMed] [Google Scholar]
- 17.Dong Z, Crawford H C, Lavrovsky V, Taub D, Watts R, Matrisian L M, Colburn N H. Mol Carcinog. 1997;19:204–212. doi: 10.1002/(sici)1098-2744(199707)19:3<204::aid-mc8>3.0.co;2-d. [DOI] [PubMed] [Google Scholar]
- 18.Greenhalgh D A, Wang X J, Eckhardt J N, Roop D R. Cell Growth Differ. 1995;6:579–586. [PubMed] [Google Scholar]
- 19.Saez E, Rutberg S E, Mueller E, Oppenheim H, Smoluk J, Yuspa S H, Spiegelman B M. Cell. 1995;82:721–732. doi: 10.1016/0092-8674(95)90469-7. [DOI] [PubMed] [Google Scholar]
- 20.Hilberg F, Aguzzi A, Howells N, Wagner E F. Nature (London) 1993;365:179–181. doi: 10.1038/365179a0. [DOI] [PubMed] [Google Scholar]
- 21.Jain J, McCaffrey P G, Miner Z, Kerppola T K, Lambert J N, Verdine G L, Curran T, Rao A. Nature (London) 1993;365:352–355. doi: 10.1038/365352a0. [DOI] [PubMed] [Google Scholar]
- 22.Vassar R, Fuchs E. Genes Dev. 1991;5:714–727. doi: 10.1101/gad.5.5.714. [DOI] [PubMed] [Google Scholar]
- 23.Hunziker R, Margulies D H. Biotechnology. 1991;16:175–185. [PubMed] [Google Scholar]
- 24.Rincon M, Flavell R A. EMBO J. 1994;13:4370–4381. doi: 10.1002/j.1460-2075.1994.tb06757.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rho O, Bol D K, You J, Beltran L, Rupp T, DiGiovanni J. Mol Carcinog. 1996;17:62–69. doi: 10.1002/(SICI)1098-2744(199610)17:2<62::AID-MC2>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 26.Wang X, Zinkel S, Polonsky K, Fuchs E. Proc Natl Acad Sci USA. 1997;94:219–226. doi: 10.1073/pnas.94.1.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Arbeit J M, Howley P M, Hanahan D. Proc Natl Acad Sci USA. 1996;93:2930–2935. doi: 10.1073/pnas.93.7.2930. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Takami S, Getchell M L, Yamagishi M, Albers K M, Getchell T V. Cell Tissue Res. 1995;282:481–491. doi: 10.1007/BF00318880. [DOI] [PubMed] [Google Scholar]
- 29.Fuchs E. J Cell Sci. 1993;17,Suppl.:197–208. doi: 10.1242/jcs.1993.supplement_17.28. [DOI] [PubMed] [Google Scholar]
- 30.Oberyszyn T M, Sabourin C L, Bijur G N, Oberyszyn A S, Boros L G, Robertson F M. Mol Carcinog. 1993;7:238–248. doi: 10.1002/mc.2940070406. [DOI] [PubMed] [Google Scholar]
- 31.Robertson F M, Gilmour S K, Conney A H, Huang M T, Beavis A J, Laskin J D, Hietala O A, O’Brien T G. Cancer Res. 1990;50:4741–4746. [PubMed] [Google Scholar]
- 32.Dear A E, Costa M, Medcalf R L. FEBS Lett. 1997;402:265–272. doi: 10.1016/s0014-5793(97)00002-1. [DOI] [PubMed] [Google Scholar]
- 33.Takigawa M, Verma A K, Simsiman R C, Boutwell R K. Cancer Res. 1983;43:3732–3738. [PubMed] [Google Scholar]
- 34.DiGiovanni J, Slaga T J, Boutwell R K. Carcinogenesis. 1980;1:381–389. doi: 10.1093/carcin/1.5.381. [DOI] [PubMed] [Google Scholar]
- 35.Hennings H, Bowden G T, Boutwell R K. Cancer Res. 1969;29:1773–1780. [PubMed] [Google Scholar]
- 36.DiGiovanni J. In: Genes and Signal Transduction in Multistage Carcinogenesis. Colburn N H, editor. New York: Dekker; 1989. pp. 39–67. [Google Scholar]
- 37.Boutwell R K. CRC Crit Rev Toxicol. 1974;2:419–443. doi: 10.3109/10408447309025704. [DOI] [PubMed] [Google Scholar]
- 38.Schwarz J A, Viaje A, Slaga T J. Chem Biol Interact. 1977;17:331–347. doi: 10.1016/0009-2797(77)90096-5. [DOI] [PubMed] [Google Scholar]
- 39.Scribner J D, Slaga T J. J Natl Cancer Inst. 1974;52:1865–1868. doi: 10.1093/jnci/52.6.1865. [DOI] [PubMed] [Google Scholar]
- 40.Naito M, Naito Y, DiGiovanni J. Carcinogenesis. 1987;8:1807–1815. doi: 10.1093/carcin/8.12.1807. [DOI] [PubMed] [Google Scholar]
- 41.Sisskin E E, Gray T, Barrett J C. Carcinogenesis. 1982;3:403–407. doi: 10.1093/carcin/3.4.403. [DOI] [PubMed] [Google Scholar]
- 42.Balmain A, Pragnell I B. Nature (London) 1983;303:72–74. doi: 10.1038/303072a0. [DOI] [PubMed] [Google Scholar]
- 43.Naito M, Chenicek K J, Naito Y, DiGiovanni J. Carcinogenesis. 1988;9:639–645. doi: 10.1093/carcin/9.4.639. [DOI] [PubMed] [Google Scholar]
- 44.Bergers G, Graninger P, Braselmann S, Wrighton C, Busslinger M. Mol Cell Biol. 1995;15:3748–3758. doi: 10.1128/mcb.15.7.3748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Rosenberger S F, Bowden G T. Oncogene. 1996;12:2301–2308. [PubMed] [Google Scholar]
- 46.Gossen M, Bujard H. Proc Natl Acad Sci USA. 1992;89:5547–5551. doi: 10.1073/pnas.89.12.5547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gulliver G A, Herber R L, Liem A, Lambert P F. J Virol. 1997;71:5905–5914. doi: 10.1128/jvi.71.8.5905-5914.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Herber R, Liem A, Pitot H, Lambert P F. J Virol. 1996;70:1873–1881. doi: 10.1128/jvi.70.3.1873-1881.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Seitz C S, Lin Q, Deng H, Khavari P A. Proc Natl Acad Sci USA. 1998;95:2307–2312. doi: 10.1073/pnas.95.5.2307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Rose-John S, Furstenberger G, Krieg P, Besemfelder E, Rincke G, Marks F. Carcinogenesis. 1988;9:831–835. doi: 10.1093/carcin/9.5.831. [DOI] [PubMed] [Google Scholar]
- 51.Mitsunaga S, Clapper M, Litwin S, Watts P, Bauer B, Klein-Szanto A J. J Cell Biochem. 1997;28–29,Suppl.:81–89. [PubMed] [Google Scholar]
- 52.Huang C, Ma W Y, Dawson M I, Rincon M, Flavell R A, Dong Z. Proc Natl Acad Sci USA. 1997;94:5826–5830. doi: 10.1073/pnas.94.11.5826. [DOI] [PMC free article] [PubMed] [Google Scholar]