Abstract
It was reported recently that action potentials actively invade the sensory nerve terminals of corneal polymodal receptors, whereas corneal cold receptor nerve terminals are passively invaded (Brock, J.A., S. Pianova, and C. Belmonte. 2001. J. Physiol. 533:493–501). The present study investigated whether this functional difference between these two types of receptor was due to an absence of voltage-activated Na+ conductances in cold receptor nerve terminals. To address this question, the study examined the effects of polarizing current on the configuration of nerve terminal impulses recorded extracellularly from single polymodal and cold receptors in guinea-pig cornea isolated in vitro. Polarizing currents were applied through the recording electrode. In both receptor types, hyperpolarizing current (+ve) increased the negative amplitude of nerve terminal impulses. In contrast, depolarizing current (−ve) was without effect on polymodal receptor nerve terminal impulses but increased the positive amplitude of cold receptor nerve terminal impulses. The hyperpolarization-induced increase in the negative amplitude of nerve terminal impulses represents a net increase in inward current. In both types of receptor, this increase in inward current was reduced by local application of low Na+ solution and blocked by lidocaine (10 mM). In addition, tetrodotoxin (1 μM) slowed but did not reduce the hyperpolarization-induced increase in the negative amplitude of polymodal and cold nerve terminal impulses. The depolarization-induced increase in the positive amplitude of cold receptor nerve terminal impulses represents a net increase in outward current. This change was reduced both by lidocaine (10 mM) and the combined application of tetraethylammomium (20 mM) and 4-aminopyridine (1 mM). The interpretation is that both polymodal and cold receptor nerve terminals possess high densities of tetrodotoxin-resistant Na+ channels. This finding suggests that in cold receptors, under normal conditions, the Na+ conductances are rendered inactive because the nerve terminal region is relatively depolarized.
Keywords: action potential, tetrodotoxin, sodium channel, sensory receptor, extracellular recording
INTRODUCTION
A general assumption regarding the process of transduction in sensory receptors is that the transducer and action potential initiation site are functionally and spatially segregated (Shepherd, 1994). The nerve terminal transduces the sensory stimulus into a receptor potential, but does not support action potentials, and a site proximal to the nerve terminal is where the action potential is initiated. This functional segregation of transduction and action potential initiation has been demonstrated for low-threshold mechanoreceptors (Lowenstein, 1959) and olfactory sensory neurons (Firestein et al., 1990). However, almost nothing is known about the ability of free sensory nerve endings, with C or A delta axons, to support action potentials. Recently, it was suggested that the sensory nerve terminals of corneal cold receptors are invaded passively by action potentials generated at a site proximal to the nerve ending (Brock et al., 2001). This conclusion was based on the observation that the time course of nerve terminal impulses (NTIs)* was unaffected by local Na+ channel blockade. In contrast, local Na+ channel blockade slowed the time course of NTIs in polymodal receptors, indicating that action potentials propagate actively into their nerve endings. A question that arises from these observations is whether this functional difference between these two types of free nerve endings is due to an absence of voltage-activated Na+ conductances in cold receptor nerve terminals.
To address this question, NTIs have been recorded extracellularly from the sensory nerve terminals of corneal cold and polymodal receptors while polarizing current was passed through the recording electrode. Extracellularly applied current is expected to alter the membrane potential of the nerve terminal, immediately beneath the recording electrode, according to its direction and magnitude. In both cold-sensitive and polymodal receptors, hyperpolarization of the nerve terminal produced a large increase in inward ionic current that was blocked by the local anesthetic, lidocaine, but not the Na+ channel blocker, TTX (tetrodotoxin). The current is therefore likely to be mediated by a TTX-resistant Na+ conductance. The interpretation is that regenerative Na+ conductances are present in the nerve terminals of cold receptors and, that under normal conditions, they are rendered inactive because the nerve terminal region is relatively depolarized.
MATERIALS AND METHODS
The experiments were performed in accordance with the animal experimentation guidelines of the National Health and Medical Research Council of Australia and had prior approval of the University of New South Wales Animal Care and Ethics Committee. Guinea pigs of both sexes and in the weight range 200–400 g were used. Animals were anesthetized with an intraperitoneal injection of sodium pentobarbitone (100 mg/kg; Nembutal; Boehringer) and killed by decapitation. Both eyes were dissected free from their orbits and isolated along with a short length of optic nerve. After removal, eyes were superfused continuously with a physiological solution of the following composition (mM) NaCl, 133.4; KCl, 4.7; CaCl2, 2; MgCl2, 1.2; NaHCO, 16.3; NaH2PO4, 1.3 and glucose, 7.8, gassed with carbogen (95% O2, 5% CO2) to pH 7.4.
Eyes were pinned to the SYLGARD (Dow-Corning) coated base of a perspex chamber. The optic nerve and associated ciliary nerves were drawn into a suction-stimulating electrode. The ciliary nerves were electrically stimulated with a constant voltage stimulator (pulse width 0.1–0.5 ms, amplitude 10–60 V). The temperature of the solution entering the bath was controlled by a feedback regulated heater. During the experiments, the bath temperature was maintained in the range 30–32°C.
Nerve terminal activity was recorded using glass micropipette electrodes applied to the surface of the cornea and secured with slight suction. The pipettes had a tip diameter of ∼50 μm and were filled with physiological solution. Signals were recorded with respect to an Ag/AgCl pellet in the bath. Electrical activity was amplified (1,000×, Geneclamp 500; Axon Instruments), filtered (high pass 1 Hz, low pass 10 kHz; 432 Wavetek), digitized (VR-10B, Instrutech), and stored on video tape. Data was subsequently collected with a Maclab data acquisition system (ADInstruments) and analyzed with custom written software in Igor (WaveMetrics).
In all experiments, a polyethylene tube was positioned inside the recording electrode, close to the tip, to deliver agents locally at the site of recording (see Brock and Cunnane, 1995). These agents were made up in HEPES-buffered saline of the following composition (mM) HEPES, 10; NaCl, 151; KCl, 4.7; CaCl2, 2; MgCl2, 1.2; glucose, 7.8. The pH of this solution was adjusted to 7.4 using NaOH. In the experiments investigating the effects of perfusing the electrode with zero Ca2+ solution, CaCl2 was replaced with an equimolar concentration of MgCl2 and the solution also contained 1 mM EDTA. Low Na+ solutions were prepared by replacing all of the NaCl with an equimolar concentration of either choline chloride or TrisHCl (tris[hydroxymethyl]aminomethane hydrochloride). Perfusion of the recording electrode with HEPES-buffered saline alone has previously been shown to be without effect on polymodal and cold receptor NTI amplitude and time course (see Brock et al., 2001).
Chemicals
Lidocaine hydrochloride, 4-aminopyridine (4-AP), tetraethylammonium (TEA), and TTX were obtained from Sigma-Aldrich. Solutions containing lidocaine, 4-AP, and TEA were made up on the day of the experiment. TTX solutions were prepared from a 1 mM stock solution in distilled water.
Receptor Identification
In accord with the criteria of Brock et al. (1998), polymodal and cold receptors were distinguished on the basis of the ongoing frequency of spontaneous NTIs and responses to temperature and capsaicin. At bath temperatures in the range 30–32°C, cold receptors had a periodic, ongoing NTI discharge (in the range 5–20 Hz) that increased with cooling and decreased with heating. Polymodal receptors were identified by ciliary nerve stimulation and their sensitivity to capsaicin (0.2–0.5 μM). Ongoing NTI activity in polymodal receptors was either absent or occurred irregularly at a low frequency (<1 Hz). For cold receptors the spontaneously occurring NTIs were studied, whereas for polymodal receptors the electrically evoked antidromic NTIs were studied. In polymodal receptors, it has been demonstrated previously that both spontaneous and electrically evoked NTIs actively invade the sensory nerve endings (Brock et al., 2001).
Polarizing Current
Polarizing currents (in the range ±200 nA) were applied across the seal resistance for periods of 30–60 s using the constant current facility of the recording amplifier. In the guinea pig cornea, the majority of sensory axons terminate abruptly on arriving at the most superficial layer of the corneal epithelium and are orientated perpendicular to the corneal surface (Brock et al., 1998). Given the dimensions of the recording electrode relative to the axon, the application of constant current across the sealing resistance can be considered to place the axon in a graded field where the current density will be highest at the terminal and will become progressively weaker proximal to this site (i.e., deeper in to the corneal epithelium). Since the axonal membrane has a higher resistance than the surrounding extracellular space, most of the potential change will be across the membrane. Consequently, the distribution of potential along the axon should be primarily determined by the length constant of the axon. The maximum current applied to the tissue varied between recording sites on the cornea and was limited by the recording circuitry. Current direction is specified with respect to the recording electrode, i.e., positive current indicates that positive charge carriers move away from the recording electrode. Therefore, by virtue of the predicted effect on the nerve terminal membrane potential, positive and negative currents are designated as hyperpolarizing and depolarizing respectively.
NTI Analysis
The recorded extracellular signal at a nerve terminal is proportional to the net current across the membrane (Smith, 1988; Brock et al., 2001). For the recording configuration used in these experiments, positive deflections represent net outward current and negative deflections represent net inward current. Analyzed NTIs were averages of 30 or 50 individual NTIs. Prior to averaging, individual NTIs were aligned at their positive peak.
In the experiments investigating the effects of TTX, lidocaine, 4-AP, and TEA, the nerve terminals were hyperpolarized with a positive current strength that, under control conditions, produced at least a 100% increase in the negative amplitude of the NTI. A negative current of the same strength was used to analyze the effects of depolarizing the nerve terminal. At some recording sites, the largest positive current that could be passed without saturating the amplifier was not sufficient to produce a 100% increase in the negative amplitude of the NTI and these sites were not further investigated.
To assess the effects of polarizing current on NTIs, their peak positive and negative amplitude was measured. To compare the time course of the initial upstroke and the downstroke of the NTI recorded from different units, the maximum and minimum rates of change of voltage (dV/dt) were normalized to the peak positive amplitude.
Data are presented as mean ± SEM. All statistical comparisons were made with Statview (Abacus Concepts). In the absence of polarizing currents, comparisons were made with Student's t tests for paired data. The effects of polarizing current were assessed nonparametrically with Friedman tests followed by paired sign tests. Unless otherwise stated, cited P values are for paired sign tests. P < 0.05 was considered significant.
RESULTS
NTIs were recorded from 39 cold and 22 polymodal receptors innervating the guinea-pig cornea. In each case, NTIs were diphasic (positive-negative), with a larger positive-going component. In all experiments, the recorded NTIs were considered to originate from single axons. In support of this assumption, electrical stimulation of the ciliary nerves evoked a single, all-or-none NTI at the site of recording. This was an identifying feature of polymodal receptors and was observed in 30 of the 39 cold receptors. For the 9 cold receptors in which an NTI could not be electrically evoked, the presence of a rhythmic, ongoing NTI discharge and a consistent NTI shape were considered strong evidence that the NTI was from a single axon. In a separate group of 17 cold and 7 polymodal receptors, impulse collision was used to confirm axon singularity. In this group of receptors, the electrically-evoked NTI could be abolished, by collision, if the electrical stimulus was triggered at the occurrence of a spontaneous NTI.
Polarizing Current
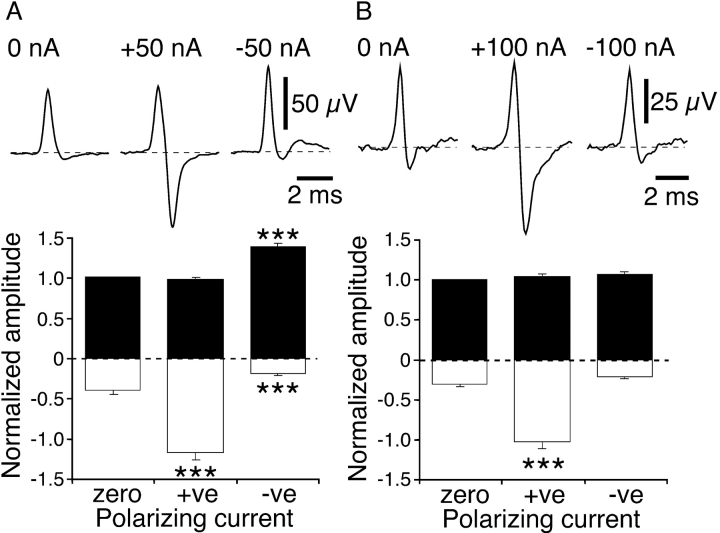
Hyperpolarizing and depolarizing currents produced consistent changes in cold (n = 28) and polymodal (n = 17) receptor NTI shape. Hyperpolarizing current increased the negative amplitude of the NTI in both types of receptor (Fig. 1, A and B) . Additionally, for cold receptor NTIs, depolarizing current increased the positive amplitude and reduced the negative amplitude (Fig. 1 A). In contrast, depolarizing current had no effect on the positive or negative amplitude of polymodal receptor NTIs (Fig. 1 B).
Figure 1.
Effects of polarization on NTIs recorded from cold receptors (A) and polymodal receptors (B). (A and B) The top traces are averaged NTIs recorded in single experiments and the bottom graphs show the mean effects of polarizing current on the positive (fill bars) and negative (open bars) amplitude of NTIs (cold receptors, n = 28; polymodal receptors, n = 17). In the graphs, the positive and negative amplitudes of NTIs were normalized to the positive amplitude measured in the absence of polarizing current (zero current). Statistical comparisons were made with paired sign tests. ***, P < 0.001.
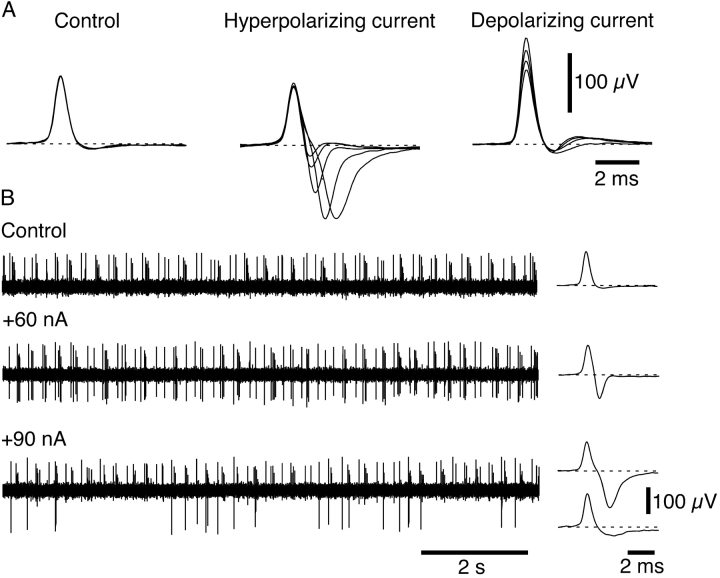
In both receptor types, the magnitude of the changes in NTI amplitude increased with polarizing current strength. An example of this behavior is shown for a cold receptor in Fig. 2 A. An increase in hyperpolarizing current strength produced a corresponding increase in the negative amplitude of the NTI. Similarly, increasing the strength of the depolarizing current increased the positive amplitude of the NTI. In some cold and polymodal receptors, increasing the strength of the hyperpolarizing current delayed the negative component of the NTI (Fig. 2 A). For both cold and polymodal receptors displaying this behavior, the hyperpolarization-induced increase in the negative amplitude of the NTI occurred in an all-or-none manner at current strengths above a particular value (Fig. 2 B).
Figure 2.
Effects of varying polarizing current strength on NTI shape in a cold receptor. (A) Overlaid averaged NTIs recorded under control conditions (three traces overlaid) and during application of hyperpolarizing current (30, 40, 60, 80, and 90 nA overlaid) and depolarizing current (−30, −50, −70, −90 nA overlaid). (B) The traces on the left show the pattern of NTI occurrence in the absence of polarizing current and during application of 60 and 90 nA. On the right, averaged NTIs recorded under each condition are shown. During application of 60 nA, the negative amplitude of all NTIs was increased. In contrast, during application of 90 nA, the increase in the negative amplitude of NTIs occurred in an all-or-none manner. At 90 nA, NTIs that had the large increase in negative amplitude were averaged separately from those which did not show this effect.
The initial upstroke of the NTI is produced by an outward capacitive current generated by electrotonic spread of potential from the arriving nerve impulse (see Brock et al., 2001). Assuming similarly shaped invading action potentials, the passive electrical properties of the nerve terminal membrane would be expected to be the primary determinant of the initial rate of rise of the NTI. In cold receptors, depolarizing and hyperpolarizing currents did not change the normalized maximum dV/dt of the NTIs (relative change; depolarizing current −0.02 ± 0.03, paired t test P = 0.54; hyperpolarizing current −0.02 ± 0.04, paired t test P = 0.64), suggesting that current application did not detectably change the passive electrical properties of their nerve terminals. Depolarizing current was also without effect on the normalized maximum dV/dt of polymodal receptor NTIs (relative change, −0.02 ± 0.02, paired t test P = 0.19), but hyperpolarizing current produced a small reduction in this value (relative decrease, −0.06 ± 0.02, paired t test P = 0.02). This slowing may reflect an increase in Na+ current early in the NTI.
In both cold and polymodal receptors, hyperpolarizing current increased the normalized minimum dV/dt; i.e., the speed of downstroke increased (relative increase, cold receptor 0.71 ± 0.09, paired t test P < 0.001; polymodal receptor 0.31 ± 0.06, paired t test P < 0.001). Conversely, depolarizing current decreased the normalized minimum dV/dt (relative decrease, cold receptor −0.26 ± 0.04, paired t test P < 0.001; polymodal receptor −0.22 ± 0.04, P < 0.001). As activation of Na+ channels would be expected to speed the downstroke of the NTI (see Brock et al., 2001), these effects on the normalized minimum dV/dt may be explained by changes in the number of inactivated Na+ channels produced by the influence of polarizing current on the resting membrane potential.
Effects of Low Na+ and Ca2+ Solutions
The hyperpolarization-induced increase in the negative amplitude of the NTI reflects a net increase in inward current that may be carried by either or both Na+ and Ca2+. Consequently, the effects of perfusing the recording electrode with either low Na+ solution or zero Ca2+ solution were investigated.
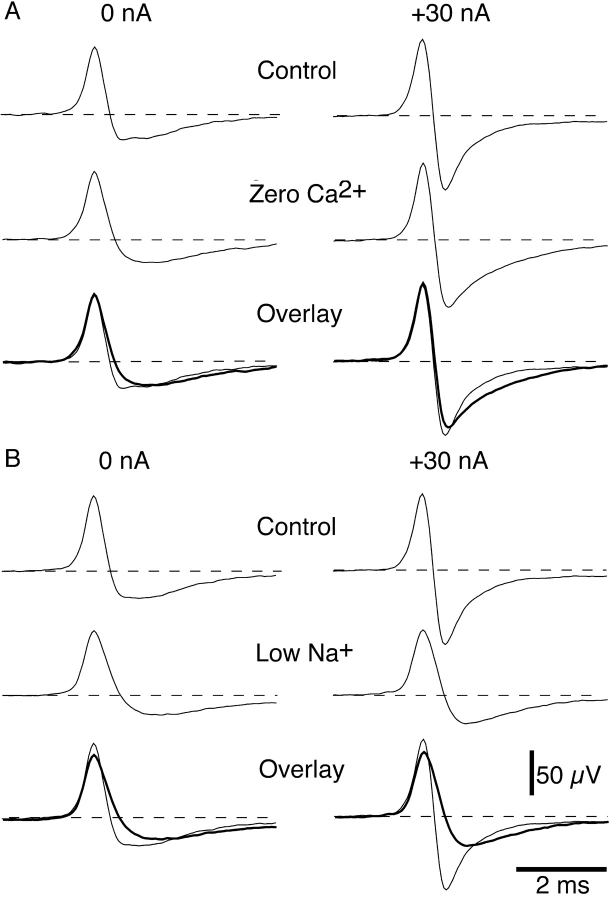
Fig. 3 shows the effects of perfusing the recording electrode with either zero Ca2+ solution or low Na+ solution on NTIs recorded from the same cold receptor. In the absence of polarizing current, perfusing the electrode with either zero Ca2+ or low Na+ solution slowed the downstroke of the NTI in this receptor. In addition, the low Na+ solution reduced the positive amplitude of the NTI. In the presence of zero Ca2+ solution, hyperpolarizing current still produced a large increase in the negative amplitude of the NTI but the time course of decay of the negative component was slowed. In contrast, perfusing the electrode with low Na+ solution markedly decreased the hyperpolarization-induced increase in the negative amplitude of the NTI.
Figure 3.
Effects of perfusing the recording electrode with zero Ca2+ solution (A) or low Na+ solution (B) on the hyperpolarization-induced changes in cold receptor NTI shape. (A and B) Averaged NTIs, from the same receptor, recorded before and during application of zero Ca2+ solution or low Na+ solution. In each case, NTIs were recorded in the absence of polarizing current and during application of +30 nA. The lower records in each panel show the control and treated (thick lines) NTIs overlaid. Between application of zero Ca2+ and low Na+ solution, the electrode was washed with control solution for 15 min. In this experiment, the low Na+ solution was prepared by replacing the NaCl with choline chloride.
For all cold and polymodal receptors tested, the effects of zero Ca2+ solution (cold receptors, n = 7; polymodal receptors, n = 4) and low Na+ solution (cold receptors, n = 9; polymodal receptors, n = 5) on the hyperpolarization-induced increase in the negative amplitude were similar to those shown in Fig. 3. Furthermore, the inhibitory action of low Na+ solution on the effects of hyperpolarizing current was observed when NaCl was replaced by choline chloride (cold receptors, n = 5; polymodal receptors, n = 3) or TrisHCl (cold receptors, n = 4; polymodal receptors, n = 2). These findings suggest that the hyperpolarization-induced increase in the negative amplitude of the NTI is due primarily to an increase in Na+ current. In further support of this conclusion, the hyperpolarization-induced increase in the negative amplitude of the NTI was unchanged by perfusion of the recording electrode with solution containing the calcium channel blocker, Cd2+ (0.1 mM), for both cold (n = 3) and polymodal receptors (n = 3).
Effects of TTX
In the absence of polarizing currents, local application of TTX (1 μM, n = 9) produced no significant change in the shape of cold receptor NTIs (Table I , Fig. 4 A). In contrast, for polymodal receptors (n = 9), TTX reduced the positive amplitude of NTIs (Table I, Fig. 4 C) and decreased their normalized minimum dV/dt (Table I).
TABLE I.
Effects of Locally Applied Agents on NTIs Recorded from Cold and Polymodal Receptors in the Absence of Polarizing Currents
| Receptor type | Positive amplitude |
Negative amplitude |
Maximum dV/dt (normalized) |
Minimum dV/dt (normalized) |
Ratio | n | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| μV | μV | |||||||||||
| Cold | 55 ± 5 | −19 ± 2 | 2,853 ± 122 | −3,270 ± 376 | 1.02 ± 0.06 | 28 | ||||||
| Polymodal | 50 ± 4 | −14 ± 1 | 2,780 ± 227 | −3,504 ± 398 | 0.85 ± 0.04 | 17 | ||||||
| Relative change | ||||||||||||
| Receptor type and treatment | Positive amplitude |
Negative amplitude |
Maximum dV/dt |
Minimum dV/dt |
Ratio | n | ||||||
| Cold | ||||||||||||
| 10 mM lidocaine | −0.14 ± 0.05a | −0.35 ± 0.10b | −0.09 ± 0.03a | −0.13 ± 0.04a | 0.05 ± 0.05 | 10 | ||||||
| 1 μM TTX | −0.11 ± 0.07 | −0.10 ± 0.07 | −0.06 ± 0.03 | −0.12 ± 0.10 | 0.13 ± 0.11 | 9 | ||||||
| 20 mM TEA + 1 mM 4-AP | −0.07 ± 0.03 | −0.40 ± 0.07c | −0.01 ± 0.02 | −0.02 ± 0.07 | 0.04 ± 0.06 | 9 | ||||||
| Polymodal | ||||||||||||
| 10 mM lidocaine | −0.27 ± 0.05b | −0.26 ± 0.17 | −0.15 ± 0.03b | −0.38 ± 0.05b | 0.45 ± 0.15a | 8 | ||||||
| 1 μM TTX | −0.18 ± 0.06a | 0.21 ± 0.19 | −0.07 ± 0.05 | −0.15 ± 0.06a | 0.14 ± 0.09 | 9 | ||||||
All values are the means ± SEM. The top section shows measurements for NTIs recorded under control conditions. The bottom section shows the relative change in each of the parameters produced by local application of lidocaine, TTX, and the combination of TEA and 4-AP. TEA and 4-AP were only tested in cold receptors. The maximum and minimum dV/dt (V s−1) were normalized by dividing them by the positive NTI amplitude. The ratio was calculated by dividing the maximum dV/dt by the minimum dV/dt. Statistical comparisons were made with two sided, paired t tests.
P < 0.05.
P < 0.01.
P < 0.001.
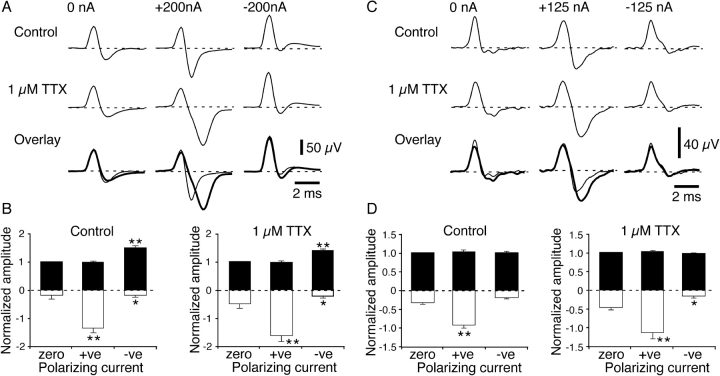
Figure 4.
Effects of locally applied TTX (1 μM) on the polarization-induced changes in NTI shape in cold (A and B) and polymodal receptors (C and D). (A and C) Averaged NTIs record in single experiments before and during TTX application. In each case, NTIs were recorded in the absence of polarizing current and during application of hyperpolarizing (+ve) and depolarizing (−ve) current. The lower records in (A) and (C) show control and TTX (thick line) traces overlaid. (B and D) Graphs showing the mean effects of polarizing current on the positive (fill bars) and negative (open bars) amplitude of NTIs before and during application of TTX (cold receptors n = 9; polymodal receptors n = 9). In the graphs, the positive and negative amplitudes of NTIs were normalized to the positive amplitude measured in the absence of polarizing current (zero current). Statistical comparisons were made with paired sign tests. *, P < 0.05; **, P < 0.01.
The effects of polarizing current on cold receptor NTI shape in the presence of TTX (1 μM, Fig. 4, A and B) were similar to those observed in the absence of this agent. Hyperpolarizing current still increased the negative amplitude of the NTI, but the magnitude of this change was significantly increased in the presence of TTX (relative increase, control 3.57 ± 0.83, TTX 4.97 ± 1.58, P = 0.04). Depolarizing current produced an increase in the positive amplitude of the NTI similar in magnitude to that observed in the absence of TTX (relative increase, control 0.49 ± 0.09, TTX 0.38 ± 0.07, P = 0.51). The reduction in the negative amplitude of the NTI produced by depolarizing current was also unaffected by TTX (relative decrease, control −0.51 ± 0.10, TTX −0.45 ± 0.14, P = 0.99).
For polymodal receptors, although the shape of the control NTIs was changed by TTX, the effects of polarizing current remained similar to those observed before its application (Fig. 4, C and D). In TTX, the negative amplitude of the NTI increased with hyperpolarizing current by an amount similar to that observed in the absence of TTX (relative increase, control 2.04 ± 0.32, TTX 1.71 ± 0.38, P = 0.18). Depolarizing current had no significant effect on the positive or negative amplitude of polymodal receptor NTIs before or in the presence of TTX.
The effects of hyperpolarizing and depolarizing current on the normalized maximum dV/dt of polymodal and cold receptor NTIs before and in the presence of TTX were similar. In cold receptors, hyperpolarizing and depolarizing current did not change the normalized maximum dV/dt (relative change, hyperpolarizing current 0.07 ± 0.12, P > 0.99; depolarizing current 0.08 ± 0.11, P > 0.99). In polymodal receptors, the normalized maximum dV/dt was decreased by hyperpolarizing current (relative decrease, −0.14 ± 0.04, P < 0.01) but unaffected by depolarizing current (relative change, −0.06 ± 0.05, P = 0.51).
In TTX, the downstroke of both cold and polymodal receptor NTIs, recorded during hyperpolarizing current, often had an inflection close to the point where the polarity of the signal reversed (Fig. 4, A and C). However, the normalized minimum dV/dt of cold receptor NTIs was still increased by hyperpolarizing current (relative increase, 0.65 ± 0.21, P < 0.01) and decreased by depolarizing current (relative decrease, −0.19 ± 0.06, P < 0.01). In contrast, in polymodal receptors, hyperpolarizing and depolarizing current failed to significantly change the normalized minimum dV/dt (hyperpolarizing current, relative change, 0.13 ± 0.12, P = 0.51; depolarizing current, relative change, −0.13 ± 0.06, P = 0.18). In both receptor types, during hyperpolarizing current, the negative-going phase of the NTI was slowed in time course (Fig. 4, A and C). This effect produced an increase in the interval between the positive and negative peaks of the NTI during hyperpolarizing current (cold receptor, control 0.77 ± 0.11 ms, TTX 1.03 ± 0.14 ms, P < 0.01; polymodal receptor, control 1.09 ± 0.20 ms, TTX 1.55 ± 0.22 ms, P = 0.04).
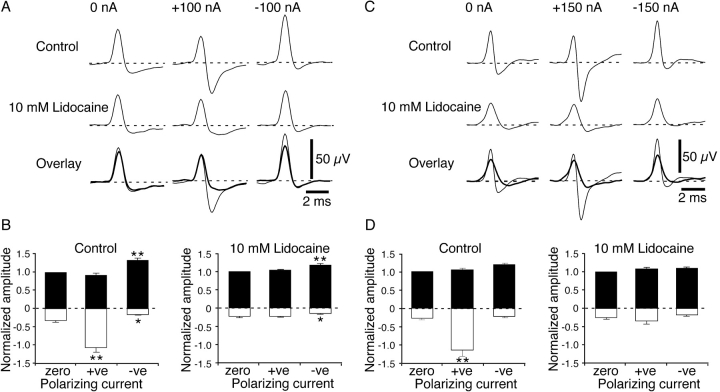
Effects of Lidocaine
In the absence of polarizing current, the effects of perfusing the electrode with the local anesthetic, lidocaine (10 mM), on cold and polymodal receptor NTIs were similar to those previously reported (Brock et al., 2001). In cold receptors (n = 10), perfusion of the recording electrode with lidocaine (10 mM) reduced the positive and negative amplitude of NTIs (Table I, Fig. 5 A). Additionally, in the present study, lidocaine produced a small reduction in the normalized maximum and minimum dV/dt, but the ratio between these values was unchanged (Table I). For polymodal receptor NTIs (n = 9), lidocaine reduced the positive amplitude and the normalized maximum and minimum dV/dt (Table I, Fig. 5 C). In addition, lidocaine markedly increased the ratio between the maximum and minimum dV/dt (Table I). In contrast to the previous study (Brock et al., 2001), locally applied lidocaine did not significantly reduce the negative amplitude of the NTI in polymodal receptors.
Figure 5.
Effects of locally applied lidocaine (10 mM) on the polarization-induced changes in NTI shape in cold (A and B) and polymodal receptors (C and D). (A and C) Averaged NTIs recorded in single experiments before and during lidocaine application. In each case, NTIs were recorded in the absence of polarizing current and during application of hyperpolarizing (+ve) and depolarizing (−ve) current. The lower records in (A) and (C) show control and lidocaine (thick line) traces overlaid. (B and D) Graphs showing the mean effects of polarizing current on the positive (fill bars) and negative (open bars) amplitudes of NTIs before and during application of lidocaine (cold receptors n = 10; polymodal receptors n = 9). In the graphs, the positive and negative amplitudes of NTIs were normalized to the positive amplitude measured in the absence of polarizing current (zero current). Statistical comparisons were made with paired sign tests. *, P < 0.05; **, P < 0.01.
Locally applied lidocaine significantly altered the effects of polarizing currents on NTI shape. For both cold and polymodal receptors, lidocaine abolished the increase in the negative amplitude of the NTI produced by hyperpolarizing current (Fig. 5). The effects of depolarizing current on cold receptor NTIs were similar to those observed before lidocaine application (Fig. 5, A and B), but the magnitude of the depolarization-induced increase in positive amplitude was reduced (relative increase, control 0.33 ± 0.06, lidocaine 0.17 ± 0.04, P = 0.02). For polymodal receptors, depolarizing current had no significant effect on NTI amplitude before or during lidocaine application.
In polymodal receptors, hyperpolarizing and depolarizing current in the presence of lidocaine was without effect on the normalized maximum dV/dt (relative change, hyperpolarizing current 0.06 ± 0.05, P = 0.73; depolarizing current 0.03 ± 0.05, P = 0.73). However, for cold receptors in the presence of lidocaine, hyperpolarizing current did reduce the maximum dV/dt (relative decrease, −0.10 ± 0.02, P < 0.01) but this parameter was unaffected by depolarizing current (relative change, −0.01 ± 0.02, P = 0.75).
Hyperpolarizing current did not change the normalized minimum dV/dt of cold and polymodal receptor NTIs in the presence of lidocaine (relative change, cold receptor 0.06 ± 0.06, P = 0.75; polymodal receptor 0.13 ± 0.05, P = 0.29). The normalized minimum dV/dt was also unaffected by depolarizing current in both types of receptor (relative change, cold receptor −0.05 ± 0.04, P = 0.75; polymodal receptor −0.01 ± 0.06, P > 0.99). These findings support the view that polarization-induced changes in the normalized minimum dV/dt, in the absence of lidocaine, may reflect changes in the resting level of Na+ channel inactivation.
Effects of K+ Channel Blockers
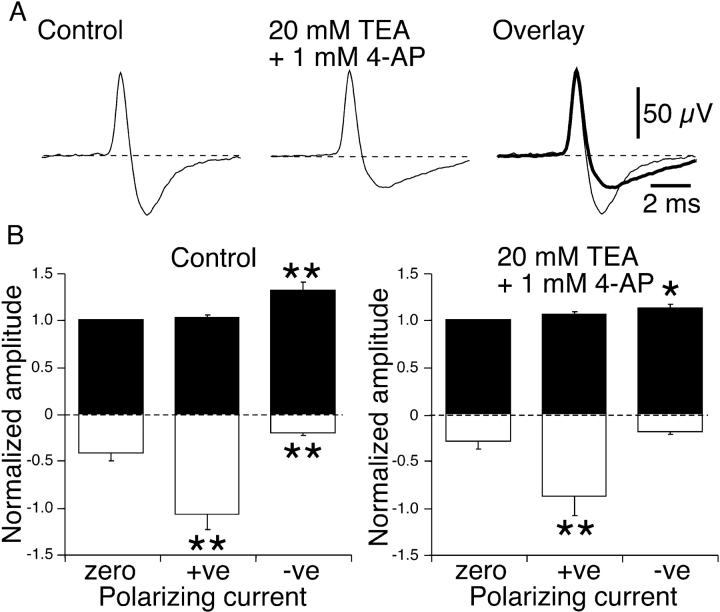
For cold receptor NTIs, depolarizing current produced an increase in the positive amplitude (Figs. 1 A, 2 A, 4 A, and 5 A). This change represents a net increase in outward current across the nerve terminal membrane. Local application of lidocaine reduced the depolarization-induced increase in net outward current (see above) and this effect may be due to its known blocking action on some voltage-dependent K+ channels (Olschewski et al., 1998; Komai and McDowell, 2001). To investigate this possibility, the effects of the combined application of the K+ channel blockers, TEA and 4-AP, on the polarization-induced changes in NTI amplitude were examined in cold receptors.
In the absence of polarizing current, perfusion of the recording electrode with a solution containing TEA (20 mM) and 4-AP (1 mM) produced a decrease in the negative amplitude of the NTI but did not significantly change the positive amplitude or the normalized maximum and minimum dV/dt (n = 9, Table I, Fig. 6 A). The observed reduction in the negative amplitude of the NTI is expected for blockade of K+ channels that contribute to repolarization during the action potential and this change reflects a reduction in the magnitude of the inward capacitive current. In all experiments, the combination of K+ channel blockers slowed the decay of the negative component of the NTI (Fig. 6 A).
Figure 6.
Effects of locally applied TEA (20 mM) and 4-AP (1 mM) on polarization-induced changes in NTI shape in cold receptors. (A) Averaged NTIs recorded in the absence of polarizing current in a single experiments before and during application of TEA and 4-AP. The right hand records show the control and treated (thick line) traces overlaid. (B) Graphs showing the mean effects of polarizing current on the positive (fill bars) and negative (open bars) amplitude of NTIs before and during application of TEA and 4-AP (n = 9). In the graphs, the positive and negative amplitudes of NTIs were normalized with respect to the positive amplitude measured in the absence of polarizing current (zero current). Statistical comparisons were made with paired sign tests. *, P < 0.05; **, P < 0.01.
In the presence of TEA and 4-AP, the effects of polarizing currents on cold receptor NTI shape were similar to those observed before drug application (Fig. 6 B). Hyperpolarizing current increased the negative amplitude and depolarizing current increased the positive amplitude. The only difference was that depolarizing current did not significantly reduce the negative amplitude of the NTI. In the presence of TEA and 4-AP, the magnitude of the hyperpolarization-induced increase in the negative amplitude of the NTI was not different from pretreatment control values (relative increase, control 1.84 ± 0.40; TEA and 4-AP 3.20 ± 1.4, P = 0.51). In contrast, the combination of TEA and 4-AP reduced the increase in the positive amplitude of the NTI produced by depolarizing current (relative increase, control 0.32 ± 0.10, TEA and 4-AP 0.13 ± 0.03, P = 0.04).
DISCUSSION
The signals recorded extracellularly from the surface of the cornea are proportional to net membrane current and this is comprised of capacitive and ionic components. Under control conditions, where the biphasic NTIs (positive-negative) recorded from both cold and polymodal receptors are largely positive going, the net membrane current is predominantly outward and is due primarily to the capacitive current produced when the nerve impulse invades the nerve terminal (see Smith, 1988). Consistent with this interpretation, NTIs remained largely positive going after local application of lidocaine and when K+ channel blockers were applied. In polymodal receptors, local application of lidocaine markedly slowed the downstroke of the NTI and this slowing reflects the blockade of the inward current normally produced by the rapid activation of Na+ channels (see also Brock et al., 2001).
In this study, it was assumed that application of constant current through the recording electrode would change nerve terminal membrane potential according to the applied current's magnitude and direction. Since polarizing current would not be expected to change the specific capacitance of the nerve terminal membrane, the observed changes in NTI shape are likely to reflect changes in the contribution of ionic currents to the net membrane current. In accord with this suggestion, in cold receptors, both hyperpolarizing and depolarizing currents had no detectable affect on the time course of the initial rising phase of the NTI (i.e., normalized maximum dV/dt), a component of the NTI that is determined primarily by the passive membrane properties. In polymodal receptors, depolarizing current also had no effect on the time course of the initial rising phase of the NTI but hyperpolarizing current produced a slight slowing that may be explained by an increase in Na+ current early in the NTI.
Nerve Terminal Hyperpolarization
In both cold and polymodal receptors, hyperpolarizing current increased the negative amplitude of the NTI, indicating a net increase in inward current across the nerve terminal membrane. This hyperpolarization-induced increase in inward current was reduced by local application of low Na+ solution and largely unaffected by local application of zero Ca2+ solution. This finding is consistent with hyperpolarization increasing the inward Na+ current across the nerve terminal membrane. Furthermore, the differential effects of TTX and lidocaine on the hyperpolarization-induced inward current suggest that it is due primarily to activation of TTX-resistant Na+ channels. This conclusion indicates that sensory nerve terminals of both polymodal and cold receptors have high densities of TTX-resistant Na+ channels. The slowing in time course of the hyperpolarization-induced inward current produced by TTX may reflect the slower activation kinetics of TTX-resistant Na+ channels (Roy and Narahashi, 1992) and also indicates that the nerve terminals possess TTX-sensitive Na+ channels (see also Brock et al., 1998, 2001). Previous studies have shown that both spontaneous and sensory stimulus–evoked NTIs in polymodal and cold receptors persist in the presence of bath applied TTX (Brock et al., 1998, 2001; see also Strassman and Raymond, 1999). This finding indicates that the density of TTX-resistant Na+ channels at the site of impulse initiation is sufficient to support action potentials.
In some experiments, increasing the hyperpolarizing current strength beyond a certain level caused the increase in inward current to be evoked in an all-or-none manner (Fig. 2 B). These failures to evoke an increase in net inward current under conditions of nerve terminal hyperpolarization are most readily explained if the membrane potential was hyperpolarized to a point where the invading impulse was occasionally subthreshold for evoking a regenerative Na+ current in the nerve terminal. Such all-or-none behavior was never observed when depolarizing current was applied.
In the absence of polarizing current, it has been reported previously that voltage-activated Na+ channels do not appear to contribute significantly to the generation of cold receptor NTIs (Brock et al., 2001). In the present study, where a larger number of receptors were studied, lidocaine produced a small but significant slowing in the time course of both the initial upstroke and the downstroke of the NTI. However, in contrast to polymodal receptor NTIs, the ratio between the normalized maximum and minimum dV/dt for cold receptor NTIs was not changed by lidocaine (see also Brock et al., 2001). This finding suggests that lidocaine-sensitive Na+ channels make a negligible contribution to cold receptor NTIs, as blockade of Na+ channels would be expected to produce a more marked slowing of the downstroke of the NTI (Brock et al., 2001). Hyperpolarization of cold receptor nerve terminals produced a large increase in net inward current that was sensitive to Na+ channel blockade, indicating that Na+ channels are indeed present in the nerve terminal. A simple explanation for their lack of contribution to the NTI, under control conditions, is that the nerve terminals have a relatively low membrane potential and, as a result, most of the Na+ channels present are inactivated. Hyperpolarization of the nerve terminals would relieve Na+ channel inactivation and, as a result, allow their activation during impulse invasion. We assume that the relatively high levels of ongoing NTI activity recorded in cold receptors are determined by the spread of depolarization from the sensory nerve endings to a more proximal site in the axon where there are sufficient Na+ channels available for action potential initiation.
An alternative explanation for why Na+ channel activation may not contribute to cold receptor NTIs is that the current spreading electrotonically from the invading action potential depolarizes the nerve terminal membrane to the Na+ equilibrium potential too rapidly for the activated Na+ channels to produce significant inward current. Such a process is thought to occur in nerve terminals of mouse intercostalis intimi motoneurons where focal application of TTX to the nerve terminal does not change the extracellularly recorded signal (Konishi and Sears, 1984). In this case, the unmyelinated nerve terminal is short (20–30 μm) and the current spreading from the penultimate node and heminode rapidly depolarizes the nerve terminal. Although it is likely that guinea-pig corneal cold receptor neurons have a thinly myelinated main axon (Aδ-fibers), all corneal sensory axons are unmyelinated from a point close to their site of entry into the cornea at the limbus (Whitear, 1960). As a result, the distance between the heminode and the site of recording at the nerve terminal is likely to be several hundred micrometers. In this case, it is unlikely that there is a focal source of current close to the nerve endings.
Two TTX-resistant Na+ channels, Nav1.8 and Nav1.9, have been identified in small diameter sensory neurons in dorsal root ganglia and trigeminal ganglia. In cell bodies of mouse sensory neurons, the currents generated by Nav1.8 channels are activated at relatively positive potentials (about −40 mV) and inactivate more slowly than the TTX-sensitive currents (Akopian et al., 1999). In contrast, the currents generated by Nav1.9 channels are activated at around −70 mV and their rates of activation and inactivation are much slower than those generated by Nav1.8 channels (Cummins et al., 1999). At present there are no pharmacological agents that selectively inhibit Nav1.8 or Nav1.9 channels, so their function remains a matter of speculation. TTX-resistant action potentials recorded in the cell bodies are postulated to be due to activation of Nav1.8 channels (Renganathan et al., 2001). The Nav1.9 channels are thought to produce a persistent Na+ current that contributes to setting the resting membrane potential (Dib-Hajj et al., 2002). Therefore, the regenerative TTX-resistant Na+ currents recorded from the nerve terminals are probably due to activation of Nav1.8 channels. In mouse sensory neurons, the steady-state inactivation curve for current generated by Nav1.8 channels is shifted to more positive potentials than the TTX-sensitive current, with a V1/2 of about −30 mV (Akopian et al., 1999). Assuming that the TTX-resistant Na+ channels in the cold receptor nerve endings have similar properties to the Nav1.8 channels in mouse sensory neurons, then the membrane potential of the nerve terminals is likely to be lower than −30 mV.
It could be questioned whether the relatively low membrane potential of cold receptor nerve endings is an artifact of the in vitro technique. The eyes were bathed in a conventional physiological saline used to study guinea-pig peripheral tissues in vitro. The ionic composition of this solution is similar to the plasma of guinea pigs (Mitruka and Rawnsley, 1981). In humans and rabbits, the ionic composition of tears is also similar to plasma for all ions except for K+, which ranges between 6 and 24 mM (Botelho and Martinez, 1973; Van Haeringen, 1981). The physiological saline used in these experiments contained 4.7 mM K+, which is close to the measured plasma concentration in guinea pigs (4.8–5.1 mM; Mitruka and Rawnsley, 1981). Therefore, if the concentration of K+ in tears were important in determining the membrane potential of the sensory nerve terminals, the extent of depolarization may actually have been underestimated. However, in cold receptors, changes in the frequency of NTI discharge and NTI shape are only observed when the K+ content of the solution superfusing the cornea is raised above 20 mM (unpublished data). Perhaps the tight surface layer of the corneal epithelium (Klyce and Beuerman, 1988) limits changes in the extracellular concentration of this ion within the cornea.
Nerve Terminal Depolarization
Depolarizing current changed the amplitude of cold receptor NTIs but was without effect on the amplitude of polymodal receptor NTIs. In cold receptors, depolarizing current increased the positive amplitude and reduced the negative amplitude of the NTI. The increase in the positive amplitude of the NTI represents a net increase in outward current and this effect of depolarizing current was reduced both by lidocaine and combined application of the K+ channel blockers, TEA, and 4-AP. As lidocaine has been reported to block both transient and sustained voltage-activated K+ currents in rat dorsal root ganglion cells (Komai and McDowell, 2001), the depolarization-induced increase in the positive amplitude of NTIs may be produced by an increase in the voltage-activated K+ current. The reason why depolarizing current has no detectable effect on the positive amplitude of polymodal receptor NTIs is unknown. However, it is possible that this difference reflects the differential expression of K+ channels types in polymodal and cold receptors.
In the absence of polarizing current, the combined blockade of TEA and 4-AP–sensitive K+ channels was without effect on the positive amplitude of cold receptor NTIs, indicating that outward current through these channels does not normally contribute significantly to this component of the signal. However, lidocaine did reduce the positive amplitude of cold receptor NTIs in the absence of polarizing current. As this reduction in the positive amplitude of the NTI was not associated with a detectable change in NTI time course, we suggested previously that this effect of lidocaine might be due to a decrease in the leak conductance of the nerve terminal (Brock et al., 2001). In accord with this suggestion, local anesthetics, such as lidocaine, are known to block some two-pore domain K+ channels (Kindler et al., 1999), which are believed to be leak channels involved in regulating resting membrane potential (Patel and Honore, 2001). If lidocaine does reduce the resting conductance of the nerve terminal membrane, the increase in membrane time constant produced by this change could explain the slight slowing in the time course of both the initial upstroke and the downstroke of cold receptor NTIs observed in the present study. Interestingly, TREK-1, or a similar two-pore domain K+ conductance, has recently been suggested to have an important role in cold transduction in sensory neurons (Reid and Flonta, 2001; see also Viana et al., 2002)
In conclusion, the present study provides further evidence of differences in the electrophysiological properties of polymodal and cold receptor nerve endings in the guinea-pig cornea. Under control conditions, action potentials propagate actively into the sensory nerve endings of polymodal receptors. In the cornea, capsaicin-sensitive sensory receptors are known to contain and release calcitonin gene related peptide and substance P (see Belmonte et al., 1997). The ability of action potentials to propagate actively into their nerve endings may therefore be important for their efferent function (see Brock et al., 2001). In cold receptors, although Na+ channels are present in the nerve terminal, their activation during impulse invasion of the nerve terminal makes only a minor contribution to the shape of cold receptor NTIs. This finding is most readily explained if the nerve terminals have a relatively low membrane potential and, as a consequence, most of the Na+ channels are inactivated. As cold receptors have continuous ongoing activity that is modulated by temperature, a low membrane potential in their receptive endings may be integral to their sensory function.
Acknowledgments
This work was supported by the Australian Research Council. J. Brock is a Senior Research Fellow of the Australian National Health and Medical Research Council.
Footnotes
Abbreviations used in this paper: 4-AP, 4-aminopyridine; NTI, nerve terminal impulse; TEA, tetraethylammonium; TTX, tetrodotoxin.
References
- Akopian, A.N., V. Souslova, S. England, K. Okuse, N. Ogata, J. Ure, A. Smith, B.J. Kerr, S.B. McMahon, S. Boyce, et al. 1999. The tetrodotoxin-resistant sodium channel SNS has a specialized function in pain pathways. Nat. Neurosci. 2:541–548. [DOI] [PubMed] [Google Scholar]
- Botelho, S.Y., and E.V. Martinez. 1973. Electrolytes in lacrimal gland fluid and in tears at various flow rates in the rabbit. Am. J. Physiol. 225:606–609. [DOI] [PubMed] [Google Scholar]
- Belmonte, C., J. Garcia-Hirschfeld, and J. Gallar. 1997. Neurobiology of ocular pain. Prog. Retin. Eye Res. 16:117–156. [Google Scholar]
- Brock, J.A., and T.C. Cunnane. 1995. Effects of Ca2+ and K+ channel blockers on nerve impulses recorded from guinea-pig postganglionic sympathetic nerve terminals. J. Physiol. 489:389–402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock, J.A., E. McLachlan, and C. Belmonte. 1998. Tetrodotoxin-resistant impulses in single nociceptor nerve terminals in guinea-pig cornea. J. Physiol. 512:211–217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brock, J.A., S. Pianova, and C. Belmonte. 2001. Differences between nerve terminal impulses of polymodal nociceptors and cold sensory receptors of the guinea-pig cornea. J. Physiol. 533:493–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummins, T.R., S.D. Dib-Hajj, J.A. Black, A.N. Akopian, J.N. Wood, and S.G. Waxman. 1999. A novel persistent tetrodotoxin-resistant sodium current in SNS-null and wild-type small primary sensory neurons. J. Neurosci. 19:RC43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dib-Hajj, S., J.A. Black, T.R. Cummins, and S.G. Waxman. 2002. NaN/Nav1.9: a sodium channel with unique properties. Trends Neurosci. 25:253–259. [DOI] [PubMed] [Google Scholar]
- Firestein, S., G.M. Shepherd, and F.S. Werblin. 1990. Time course of the membrane current underlying sensory transduction in salamander olfactory receptor neurones. J. Physiol. 430:135–158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler, C.H., C.S. Yost, and A.T. Gray. 1999. Local anesthetic inhibition of baseline potassium channels with two pore domains in tandem. Anesthesiology. 90:1092–1102. [DOI] [PubMed] [Google Scholar]
- Klyce, S.D., and R.W. Beuerman. 1988. Structure and function of the cornea. The Cornea. H.E. Kaufman, B.A Barron, M.B. McDonald, and S.R. Waltman, editors. Churchill Livingstone, Inc., New York. 3–54.
- Komai, H., and T.S. McDowell. 2001. Local anesthetic inhibition of voltage-activated potassium currents in rat dorsal root ganglion neurons. Anesthesiology. 94:1089–1095. [DOI] [PubMed] [Google Scholar]
- Konishi, T., and T.A. Sears. 1984. Electrical activity of mouse motor nerve terminals. Proc. R. Soc. Lond. B Biol. Sci. 222:115–120. [DOI] [PubMed] [Google Scholar]
- Lowenstein, W.R. 1959. Generation of electrical activity in a nerve ending. Ann. NY Acad. Sci. 81:367–387. [DOI] [PubMed] [Google Scholar]
- Mitruka, B.M., and H.M. Rawnsley. 1981. Clinical biochemical and hematological values in normal experimental animals and normal humans. Madison Publishing USA, New York. 172 pp.
- Olschewski, A., G. Hempelmann, W. Vogel, and B.V. Safronov. 1998. Blockade of Na+ and K+ currents by local anesthetics in the dorsal horn neurones of the spinal cord. Anesthesiology. 88:172–179. [DOI] [PubMed] [Google Scholar]
- Patel, A.J., and E. Honore. 2001. Properties and modulation of mammalian 2P domain K+ channels. Trends Neurosci. 24:339–346. [DOI] [PubMed] [Google Scholar]
- Renganathan, M., T.R. Cummins, and S.G. Waxman. 2001. Contribution of Na(v)1.8 sodium channels to action potential electrogenesis in DRG neurons. J. Neurophysiol. 86:629–640. [DOI] [PubMed] [Google Scholar]
- Reid, G., and M. Flonta. 2001. Cold transduction by inhibition of a background potassium conductance in rat primary sensory neurones. Neurosci. Lett. 297:171–174. [DOI] [PubMed] [Google Scholar]
- Shepherd, G.M. 1994. Neurobiology. 2nd ed. Oxford University Press, New York. 208 pp.
- Smith, D.O. 1988. Determinants of nerve terminal excitability. Neurology and Neurobiology. Vol 35. Long-term potentiation. P.W. Lanfield and S.A. Deadwyler, editors. Alan Liss Inc., New York. 411–438.
- Strassman, A.M., and S.A. Raymond. 1999. Electrophysiological evidence for tetrodotoxin-resistant sodium channels in slowly conducting dural sensory fibers. J. Neurophysiol. 81:413–424. [DOI] [PubMed] [Google Scholar]
- Roy, M.L., and T. Narahashi. 1992. Differential properties of tetrodotoxin-sensitive and tetrodotoxin-resistant sodium channels in rat dorsal root ganglion neurons. J. Neurosci. 12:2104–2111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Haeringen, N.J. 1981. Clinical biochemistry of tears. Surv. Ophthalmol. 26:84–96. [DOI] [PubMed] [Google Scholar]
- Viana, F., E. de la Pena, and C. Belmonte. 2002. Specificity of cold thermotransduction is determined by differential ionic channel expression. Nat. Neurosci. 5:254–260. [DOI] [PubMed] [Google Scholar]
- Whitear, M. 1960. An electron microscope study of the cornea in mice, with special reference to the innervation. J. Anat. 94:387–409. [PMC free article] [PubMed] [Google Scholar]