Hodgkin and Huxley (1952) demonstrated the presence of voltage-activated sodium (Na+) and potassium (K+) permeabilities in the squid giant axon. Ever since then, biophysicists have tried to understand how voltage activates the ionic pathways responsible for the action potential. Hodgkin and Huxley (1952) concluded that there had to be charges or charge dipoles in the membrane that move in response to changes in the voltage across the membrane, turning the Na+ and K+ permeabilities on and off. We now know that Na+ and K+ ions go through voltage-activated Na+ and K+ channels. These voltage-activated ion channels are composed of: (a) a pore-forming domain that contains the ion permeation pathway and the gates that control the flow of ions, (b) a voltage-sensing domain that contains the voltage sensor, and (c) a coupling mechanism that links the voltage sensor to the gates in the pore (Fig. 1) . We have come a long way in our understanding of how ion channels work since Hodgkin and Huxley's first voltage-clamp experiments. However, we still do not fully understand the molecular mechanism of how changes in voltage open and close these channels, and, we know even less about the coupling mechanism between the voltage sensors and the gates. In this Perspective, I give my view of the molecular mechanism of gating in voltage-activated K+ (Kv) channels and closely related ion channels (both voltage-gated and voltage-independent ion channels).
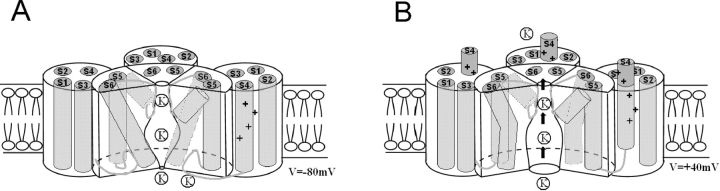
Figure 1.
Model of a voltage-activated ion channel. Cartoon of a Kv channel (only three out of the four subunits are shown) in the closed state (A) and the open state (B). S5, S6, and the S5-S6 loop (the P loop) from all four subunits contribute to the pore-forming domain. S1-S4 from each subunit form a voltage-sensing domain. S4 charges move outwards in response to a depolarization, triggering the opening of the activation gate (located at the bundle crossing at the COOH-terminal end of S6). The S4-S5 loop is here suggested as the coupling mechanism between S4 movement and the opening of the activation gate.
Molecular Mechanism of Voltage-activated K+ Channels
Most of what we know about voltage activation of ion channels comes from studies of Kv channels. These ion channels have four subunits, each of which has six transmembrane segments, S1-S6. S1-S4 form the voltage-sensing domain, and S5-S6 form the pore domain (Fig. 1).
The Pore and the Activation Gate(s)
The pore is the best understood part of voltage-activated ion channels. This is, in part, because of the extensive array of electrophysiology and molecular biology experiments that have been conducted since Hodgkin and Huxley, but mostly because we have two crystal structures of bacterial ion channels that are homologous to the pore domain of Kv channels: the KcsA K+ channel, presumably in the closed state, and the MthK K+ channel, presumably in the open state (Doyle et al., 1998; Jiang et al., 2002a; Fig. 2) .
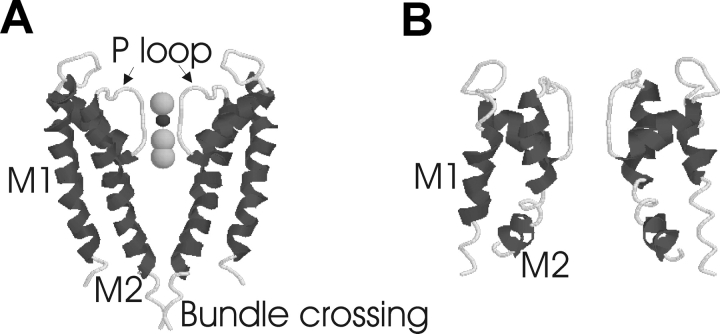
Figure 2.
Opening and closing of the activation gate in bacterial K+ channels. Molecular models of the KcsA K+ channel (A) and the MthK K+ channel (B) (Doyle et al., 1998; Jiang et al., 2002a). Only two of four subunits are shown for simplicity. The larger circles are K+ ions, and the smaller, darker circle is a water molecule. The KcsA channel is presumably in a closed state, and the MthK channel is in an open state. The difference between the two structures suggests that bending of the M2 transmembrane segment opens K+ channels (Jiang et al., 2002b).
Like Kv channels, these bacterial channels are tetramers, but each subunit has only two transmembrane segments, M1 and M2, which are homologous transmembrane segments to S5 and S6 in Kv channels. S5 and S6, together with the P loop connecting the two, are thought to form the pore domain of Kv channels (Yellen, 1998). The structure of KcsA shows how four subunits come together to form the pore, with four P-loops forming the selectivity filter and the outer part of the pore, whereas the COOH-terminal part of four M2s forms the intracellular half of the pore (Fig. 2).
Most likely, the KcsA structure is that of a closed channel, since, at the bundle crossing of the four M2s (Fig. 2), the pore has a small radius and is lined with hydrophobic residues (Jiang et al., 2002b). The state-dependent accessibility of S6 residues suggests that the pore of Kv channels is also closed at the bundle crossing (Yellen, 1998). Cysteines introduced above the bundle crossing are only accessible in the open state of Kv channels, whereas cysteines introduced below the bundle crossing are equally accessible in both the open and the closed states of Kv channels (Yellen, 1998). The closing of the gate excludes even small ions, such as Ag and Cd, from entering the pore, suggesting that the flow of K+ ions is controlled by this gate (del Camino and Yellen, 2001).
A comparison of the closed structure of the KcsA channel and the open structure of the MthK channel gives us an idea of how the gate at the cytosolic end of M2 opens and closes the pore (Jiang et al., 2002b). The main difference between the open-state structure of the MthK channel and the closed-state structure of the KcsA channel is a pronounced bend of the inner helix M2 in the MthK channel, so that the cytosolic half of the pore is much wider (Fig. 2). This bend in M2 literally reduces the pore to the selectivity filter (Jiang et al., 2002b). Additional support for the gate being located at the cytosolic end of the pore in the KcsA channel comes from experiments measuring the spin–spin coupling between spin labels attached to M2 residues, which suggest that the four M2s rotate relative to each other and move apart when the KcsA channel opens (Perozo et al., 1999). The wide opening of the cytosolic end of the pore is consistent with data that show that large cysteine reagents, organic blockers, and the inactivation ball peptide can bind deep within the open pore of Kv channels (Armstrong, 1992; del Camino and Yellen, 2001; Zhou et al., 2001a).
In addition to the gate at the cytosolic end of S6, the selectivity filter has been proposed to form a second gate. This hypothesis is based on the appearance of subconductance states during the activation or deactivation of K+ channels, and on the finding that the subconductance states have a different ion selectivity from the ion selectivity in the fully opened state (for review see Yellen, 1998). In addition, crystals of the KcsA channel incubated in low versus high concentration of potassium ions have been found to display different structures of the selectivity filter, where the “low concentration” structure seems to be that of a closed selectivity filter (Zhou et al., 2001b). The selectivity gate could work independently or in concert with the cytosolic gate. Alternatively, the large changes in the S6 gate during opening and closing could indirectly lead to changes both in conductance and selectivity for the pore. So, the question remains: are there one or two gates, and how does the voltage sensor control the opening and closing of the gate(s)?
The Voltage Sensor(s)
When the first voltage-activated Na+ and K+ channels were cloned in the 1980s, the fourth transmembrane domain of these channels, S4, was proposed as the molecular identity of the moving charges (gating charges) that Hodgkin and Huxley had originally envisioned. This hypothesis was based on the finding that S4 in all voltage-activated ion channels has a unique pattern—a positively charged residue at every third position. For these charges to be the gating charges, they have to move outwards relative to the electric field in response to a depolarization of the membrane. The movement of these charges relative to the electric field provides the energy to open the ion channels.
S4 is now widely accepted as the major voltage sensor in both Na+ and K+ channels, based on the voltage-dependent accessibility of residues in S4, voltage-dependent changes in fluorescence of S4-linked probes, and the effect of neutralizing the charged residues in S4 (for review see Yellen, 1998; Horn, 2000).
Accessibility studies of cysteines introduced into S4 of Na+ and K+ channels showed that S4 moves outwards in response to a depolarization. In particular, some S4 residues moved the entire way from the intracellular solution to the extracellular solution when channels went from closed to open, providing direct evidence that S4 charges move relative to the electric field and, thus, act as gating charges. In addition, histidines introduced in S4 were found to transport protons across the membrane in a voltage-dependent fashion, supporting the hypothesis that, during gating, S4 residues move from one side of the membrane to the other. Furthermore, fluorescence changes from fluorescent probes attached to S4 residues correlated in time and voltage dependence to the movement of the gating charge, providing further evidence that S4 is the voltage sensor.
Charge-neutralization studies showed that the amount of gating charge that moves in response to a depolarization depends on the number of charges in S4. Neutralizing an S4 charge reduces the total amount of gating charge per channel in such a way that most, if not all, gating charges could be explained by the outward, transmembrane movement of the four most external charges in S4. One caveat with all mutagenesis is that the mutation itself (charge neutralization, or introduction of the cysteine or histidine) may have secondary effects, such as altering the movement of S4 or the movement of other charged residues. At least, it is reassuring that all studies indicate S4 charges as the major gating charges. However, even if S4 is the major voltage sensor, we still do not know how S4 movement causes the gates of the pore to open and close.
The Voltage Sensor-to-gate Coupling
So, how is the movement of the voltage sensor coupled to the opening of the activation gate? The search is on, but so far, there is no direct evidence for what constitutes the physical coupling mechanism between the voltage sensor and the gates. The coupling mechanism is not necessarily a direct physical linker between the voltage sensor and the activation gate. The coupling mechanism could be indirect since it depends on the total energy of the interactions of the two domains (the pore domain and the voltage-sensing domain), thus making it hard to identify a specific region of the protein that is responsible for the coupling mechanism. However, the interaction surface between the pore domain and the voltage-sensing machinery likely plays an important role in the coupling mechanism, and it should be possible to identify this interaction surface.
Different hypotheses about the coupling mechanism have been proposed. One hypothesis is that linkers between the transmembrane segments, such as the S4-S5 loop, provide the coupling between the movement of the voltage sensor (transmembrane domain S4) to the opening of the activation gate (transmembrane domain S6) (Yellen, 1998; Horn, 2000). Chimeric channels between KcsA and Shaker K+ channels that transfer the whole pore domain of KcsA into Shaker K+ channels give rise to nonfunctional channels, whereas chimeric channels that include part of the linkers between S4-S5 and the S6-C terminus from Shaker K+ channels result in voltage-gated channels (Lu et al., 2001), supporting the hypothesis that the linker regions are important for functional voltage-gated ion channels. Another hypothesis is that the coupling is at the interaction surfaces between transmembrane domains. In other words, the movement of one transmembrane domain directly pushes, pulls, or rotates another transmembrane domain. Mutations in transmembrane domains S5 and S6 suggest that interactions between transmembrane domains are important for the opening and closing of channels (Kanevsky and Aldrich, 1999; Horn, 2000; Espinosa et al., 2001).
Gating Mechanisms of Other Members in the Super Family of Voltage-gated Ion Channels
In the super family of voltage-activated ion channels, all depolarization-activated channels, including Kv, Na+, and Ca2+ channels, probably use a similar activation mechanism, in which an outward movement of S4 opens an activation gate located at the intracellular end of S6. The molecular nature of the coupling mechanism between sensors and gates of these channels is not known, but there is no reason to believe that it would be different among Kv, Na+, and Ca2+ channels. However, not all members of the superfamily of voltage-activated ion channels use the same sensors, gates, and coupling mechanisms to control the flow of ions.
CNG and Kv Channels: Different Sensors and Different Gates
Even though the opening of cyclic nucleotide–gated (CNG) channels is not voltage dependent, CNG channels are considered members of the superfamily of voltage-activated ion channels because of amino acid sequence homology with Kv channels, including an S4 with a positive charge at every third position. Because CNG channels are voltage independent, however, the role of the S4 domain in CNG channels is still a mystery. If the S4 charges in CNG channels do not serve as voltage sensors, perhaps they stabilize the channel structure by interacting with negative charges in other transmembrane domains. Instead of being activated by voltage, the CNG channels are activated by the binding of cyclic nucleotides to the COOH-terminal of each of the four subunits. In addition to being activated by a different stimulus than Kv channels, CNG channels also use a different gate to control the flow of ions. Cysteine accessibility studies of S6 in CNG channels suggest that the gate is not located at the intracellular end of the pore, but at the narrow selectivity filter toward the extracellular end of the pore (for review see Flynn et al., 2001). The gate may also be involved in activation gating in Kv channels (although not as the primary gate, see “the pore and the activation gates”) because selectivity changes have been seen during the opening and closing processes in Kv channels.
HERG and Kv Channels: Similar Sensors and Gates, Different Use of the Gates
HERG is an ion channel in the superfamily of voltage-activated ion channels that conducts much more current at hyperpolarized potentials than at depolarized potentials, that is, it displays inward rectification. This rectification is opposite to the outward rectification that most voltage-activated ion channels display.
The inward rectification of the HERG channel is due to a fast inactivation gate in combination with a slow activation gate. In response to a depolarization, the HERG channel opens slowly but inactivates quickly, which results in no outward macroscopic current. Upon hyperpolarization, the HERG channel recovers quickly from inactivation but closes slowly, which results in large K+ currents that decay slowly (Yellen, 1998). The fast inactivation gate in the HERG channel is similar to the slow inactivation (C-type inactivation) gate in Kv channels. Most likely, the activation gate in the HERG channel is similar to the activation gate in the Kv channels, because the HERG activation gate can trap intracellular blockers in a similar manner to the activation gate in Kv channels (Mitcheson et al., 2000). Fluorescence studies show that S4 movement accompanies the opening and the closing of both the inactivation gate and the activation gate, suggesting that S4 is the voltage sensor for both of these gates in the HERG channel (Smith and Yellen, 2002).
HCN and Kv Channels: Similar Voltage Sensor and Gate, Probably Different Coupling Mechanisms
There are other members in the superfamily of voltage-activated ion channels, such as KAT and HCN channels (for review see Santoro and Tibbs, 1999), that also are inward rectifying. These channels appear to use a different mechanism to display inward rectification from that used by the HERG channel. Instead of using an additional gate, as does the HERG channel, the HCN channels appear to use a similar gate and a similar voltage sensor to those used in the Kv channels, but a different coupling mechanism between the gate and the sensor.
Similar Activation Gate
In classic experiments, Armstrong showed that intracellular blockers could only access the pore of squid Kv channels when the activation gate is open, and once these blockers are inside the pore, closure of the activation gate traps the blockers (for review see Armstrong, 1992; Yellen, 1998). These results suggested that the activation gate of Kv channels is located at the intracellular end of the pore. Shin et al. (2001) used blockers to show that, most likely, the activation gate in HCN channels is also located at the intracellular end of the pore. The specific HCN channel blocker ZD7288 was found to access the pore only when the HCN channels were open, and ZD7288 could be trapped in the closed state (Shin et al., 2001). In addition, as for Kv channels, a cysteine introduced in S6 of HCN channels was only accessible to intracellular Cd2+ when the channels were open (Rothberg et al., 2002). This finding suggests that Kv and HCN channels have a similar activation gate. However, in HCN channels, the gate opens upon hyperpolarization, but in Kv channels, it opens upon depolarization.
Similar Voltage Sensor
If HCN and Kv channels use a similar gate, perhaps HCN channels use a different voltage sensor from the one Kv channels use, which makes the gate open in response to different voltage steps in HCN from those in Kv channels. Männikkö et al. (2002) tested whether S4 in HCN channels moves as a voltage sensor, as it does in Kv channels. Indeed, the cysteine accessibility of cysteine introduced in S4 showed that S4 moves outward in response to a depolarization, just as it does in Kv channels. This S4 movement is conserved between Kv and HCN channels, suggesting that S4 is the voltage sensor in HCN channels as well as in Kv channels (Männikkö et al., 2002).
The KAT channel, an inwardly rectifying channel cloned from plants, probably uses a gating mechanism similar to the one used in HCN channels. Single-channel analysis, in combination with mutations in the S4 domain, suggests that inward S4 movement opens the KAT channel (Zei and Aldrich, 1998). Furthermore, preliminary cysteine accessibility studies suggest that S4 moves inward when the membrane is hyperpolarized (Latorre et al., 2001). These results suggest that S4 is the voltage sensor in the KAT channel and that the KAT channel opens when S4 is in a retracted position.
So, the inwardly rectifying channels in the superfamily of voltage-activated ion channels appear to use different gating strategies. In HERG channels, outward S4 movement appears to open the activation gate and inward S4 movement appears to close the activation gate. In contrast, KAT and HCN channels appear to be gated in a different manner: inward S4 movement appears to open the activation gate and outward S4 movement appears to close the activation gate. HCN and KAT channels appear to use only the activation gate to control the flow of ions, whereas HERG channels use an additional inactivation gate, probably also controlled by S4, to prevent outward currents.
Different Coupling Mechanisms
The movement of both the voltage sensor and the activation gate in HCN and Kv channels are likely to be similar. What about the coupling mechanism? As argued by Männikkö et al. (2002), the coupling mechanisms are likely to be different in HCN and Kv channels. This causes HCN channels to open upon hyperpolarization and Kv channels to open upon depolarization, although S4 moves outwards in response to depolarizations in both channels (Männikkö et al., 2002). Unfortunately, the coupling mechanism is not known for any voltage-activated ion channel, so we still do not know what determines the opposite responses to a voltage pulse in these two channels.
Areas Where Additional Information Is Needed
What information do we need to unravel the coupling mystery between the voltage sensor and the gates? And what methods can we use to identify the molecular nature of the coupling mechanism? While we await a crystal structure of a Kv channel in both the closed and open states, we can still learn a lot about the coupling mechanism from experiments using other biophysical techniques. Using electrophysiology, biochemistry, and fluorescence methods, we should be able to refine our knowledge of the following: (a) the location of S4 relative to the pore, (b) the relative motion of S4 and the pore, and (c) the physical interaction (e.g., electrostatic, hydrogen bonding, hydrophobic, and steric) between S4 and the pore. Our expanding knowledge in these three areas will most likely advance our understanding of the coupling mechanism.
S4 Location Relative to The Pore
In the absence of a crystal structure for a complete voltage-activated ion channel, we need to identify the spatial relationship of S4 to the pore to better understand how S4 influences the pore and its gates.
Li-Smerin et al. (2000) made a tryptophan scan of the pore domain to identify residues that interact with the voltage-sensing domain by studying how these residues influence the voltage dependence of Kv channels. The idea is that mutations of residues important for coupling between S4 and the pore will affect the open state differently from the closed state. This is because residues involved in coupling most likely undergo some kind of conformational rearrangement during gating, making a mutation better tolerated in one state than in another state. For example, a bulky side chain can be sterically constrained in the closed conformation, but not in the open conformation. Therefore, these mutations alter the stability of the open state relative to the closed state, inducing a voltage shift in the voltage dependence of the channel. A number of residues were found that drastically shift the voltage dependence of opening when mutated (Li-Smerin et al., 2000). However, they cannot be unambiguously identified as being located at the interaction surface with the voltage sensor because this type of mutagenesis scan cannot distinguish between pore residues that interact with the voltage sensor from pore residues that interact with other moving parts of the channel. For example, mutations of a pore residue that moves relative to another pore residue during the opening of the gate could also give rise to voltage shifts.
To estimate the location of S4, Elinder et al. (2001a) introduced charged residues at the extracellular surface around the pore region in the Shaker K+ channel and measured the effect that these charges have on S4 motion. The underlying hypothesis was that an introduced, positively charged residue electrostatically interacts with the emerging S4 charges and, thus, destabilizes the open state and causes a voltage shift of the voltage dependence to more positive potentials. The closer the location of the introduced charge to S4, the bigger the shift. The introduction of a negatively charged residue was expected to have the opposite effect, shifting the voltage dependence an equal amount to more negative potentials. Elinder et al. (2001a) introduced the charges in situ by modifying cysteines with either the positively charged MTSET or the negatively charged MTSES. A number of residues were identified that shifted the voltage dependence to more negative potentials when modified by MTSES and to more positive potentials when modified by MTSET. Modification of A419C at the extracellular end of S5 gave the largest voltage shift, indicating that S4 is located close to S5, outside the interface between S5 and S6 from the same subunit. The model presented by Elinder et al. (2001a) places S4 not in the middle of the subunit but on one side of the subunit, so that one face (the hydrophobic face) of S4 is in contact with the lipid bilayer. This placement would make it easier for S4 to undergo large translocation movements, such as a helical screw movement (see S4 motion relative the pore). Forming disulfide bonds between S4 residues and pore residues would confirm the proposed location of S4 (preliminary results have been reported; Gandhi and Isacoff, 2002b; Laine et al., 2002).
Other methods that have been used to measure distances in Kv channels include FRET (see next section) and a toxin with variable-length linkers. In the latter method, pore-blocking toxins are tethered to a specific cysteine (for example, in S4) with different length linkers. An upper estimate of the distance between the cysteine and the pore would be the length of the linker for the toxin with the shortest linker that still allows for pore blockage (Blaustein et al., 2000).
Because there are intrinsic uncertainties of these methods and the fact that these methods have not been used in a larger scale mapping of different residues of Kv channels, we still do not know the 3D arrangement of the six transmembrane domains (S1–S6) in the open or the closed state.
S4 Motion Relative to the Pore
The cysteine accessibility studies, the fluorescent labeling studies, and the charge neutralization studies of S4 were all designed to identify the important gating charges in S4. These studies showed that these gating charges move across the membrane through the plane of the membrane. However, these studies did not provide information about how the gating charges move relative to the pore and how the movement of these gating charges influences the gates in the pore.
Recent studies have suggested two different models for S4 motion: a twist motion without significant translational motion (Bezanilla, 2000) and a helical screw (or sliding helix) motion (for review see Keynes and Elinder, 1999; see also in this issue, Gandhi and Isacoff, 2002a; Bezanilla, 2002). Support for a twist motion was obtained using fluorescent resonance energy transfer (FRET) between fluorophores on S4s in different subunits (Cha et al., 1999; Glauner et al., 1999). The energy transfer is strongly dependent on the distance between fluorophores (1/r6), so that changes in the distance between fluorophores are readily seen as changes in the fluorescence signals. The FRET studies were consistent with the notion that S4 rotates up to 180 degrees during activation (Cha et al., 1999; Glauner et al., 1999). In another fluorescent study, the kinetics of the fluorescence changes was compared between neighboring residues in S4. Residues that had similar fluorescent changes were located in a helical pattern along S4 (Gandhi et al., 2000), which suggests that S4 moves in a helical motion.
The helical screw model of S4 motion implies that S4 residues move substantially during gating (>10Å). This model has been met with skepticism because of the large size of the proposed movement. However, such large transmembrane movements in proteins are not without precedent, as a >50 amino acid portion of colicin undergoes a voltage-driven transmembrane movement across the membrane (Qui et al., 1996). In addition, S4 may move in several steps across the membrane, making each step a smaller conformational change (Baker et al., 1998). Furthermore, negative charges in S2 and S3, which are thought to act as counter ions to the positive S4 charges, appear to interact with different S4 charges in different states, in a manner compatible with a helical screw motion of S4 (Tiwari-Woodruff et al., 2000).
Elinder et al. (2001b) showed that S4 charges undergo large conformational motions during activation. They studied the electrostatic effect that S4 charges have on pore residues by measuring the reaction rate of both positively and negatively charged cysteine reagents with cysteines located in S5. They found that the top charge of S4, R362, speeds up the reaction rate of the negatively charged cysteine reagents only in the open state, as if R362 were located very close to S5 in the open state. However, in the closed state, R362 was found not to influence the reaction rates, which suggests that R362 and the cysteine are far apart in the closed state. Elinder et al. (2001b) conclude that R362 moves more than 12 Å relative to the pore, between the closed and open states. The nature of the conformational change that gives rise to this movement remains to be determined. But, the large movement is compatible with either the helical screw model for S4 motion (for review see Keynes and Elinder, 1999) or a large, purely rotational motion for S4 (Bezanilla, 2000), because even a purely rotational motion, such as a 180° rotation of a 10 Å diameter S4 helix, would translocate individual S4 residues a large distance (>10 Å).
The formation of disulfides between S4 and pore residues in the two different states would provide even more direct evidence about the nature of the S4 movement (see S4 location relative the pore).
S4 Effects on the Pore
So far, no one has provided any direct evidence of how S4 interacts with the pore and causes the gates to open and to close. A direct interaction between S4 charges and pore residues has been suggested for the coupling between S4 and the slow inactivation gate in the Shaker K+ channel (Gandhi et al., 2000; Larsson and Elinder, 2000; Loots and Isacoff, 2000; Ortega-Saenz et al., 2000). Elinder et al. (2001b) showed that the most extracellular charges on S4 indeed interact electrostatically with residues in S5—residues that are important for slow inactivation. However, Elinder et al. (2001b) also showed that removing this interaction, by neutralizing the relevant S4 charges, did not affect the inactivation time course, indicating that this electrostatic interaction does not contribute substantially to the coupling mechanism between the voltage sensor and the slow inactivation gate.
Further studies of the direct effects of S4 residues on nearby transmembrane domains should give valuable information about the coupling mechanism between the voltage sensor and the gates.
Conclusions
Although we now know a great deal about the pore and the gates of voltage-activated ion channels and about the voltage sensor in these channels, we still have very little understanding of how movement of the voltage sensor causes the gates to open or to close the pores in these channels. Identification of the molecular mechanism underlying this coupling mechanism will (hopefully) be one of the next great discoveries in ion channel research.
Acknowledgments
I thank Drs. F. Elinder, R.L. Brown, and J. Maylie for comments and suggestions, and S. Oster for editing the manuscript.
References
- Armstrong, C.M. 1992. Voltage-dependent ion channels and their gating. Physiol. Rev. 72:S5–S13. [DOI] [PubMed] [Google Scholar]
- Baker, O.S., H.P. Larsson, L.M. Mannuzzu, and E.Y. Isacoff. 1998. Three transmembrane conformations and sequence-dependent displacement of the S4 domain in Shaker K+ channel gating. Neuron. 20:1283–1294. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F. 2000. The voltage sensor in voltage-dependent ion channels. Physiol. Rev. 80:555–592. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F. 2002. Voltage sensor movements. J. Gen. Physiol. 120:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blaustein, R.O., P.A. Cole, C. Williams, and C. Miller. 2000. Tethered blockers as molecular ‘tape measures’ for a voltage-gated K+ channel. Nat. Struct. Biol. 7:309–311. [DOI] [PubMed] [Google Scholar]
- Cha, A., G.E. Snyder, P.R. Selvin, and F. Bezanilla. 1999. Atomic scale movement of the voltage-sensing region in a potassium channel measured via spectroscopy. Nature. 402:809–813. [DOI] [PubMed] [Google Scholar]
- del Camino, D., and G. Yellen. 2001. Tight steric closure at the intracellular activation gate of a voltage- gated K+ channel. Neuron. 32:649–656. [DOI] [PubMed] [Google Scholar]
- Doyle, D.A., J. Morais-Cabral, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Elinder, F., P. Arhem, and H.P. Larsson. 2001. a. Localization of the extracellular end of the voltage sensor S4 in a potassium channel. Biophys. J. 80:1802–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elinder, F., R. Männikkö, and H.P. Larsson. 2001. b. S4 charges move close to residues in the pore domain during activation in a K+ channel. J. Gen. Physiol. 118:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espinosa, F., R. Fleischhauer, A. McMahon, and R.H. Joho. 2001. Dynamic interaction of S5 and S6 during voltage-controlled gating in a potassium channel. J. Gen. Physiol. 118:157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flynn, G.E., J.P. Johnson, and W.N. Zagotta. 2001. Cyclic nucleotide-gated channels: shedding light on the opening of a channel pore. Nat. Rev. Neurosci. 2:643–651. [DOI] [PubMed] [Google Scholar]
- Gandhi, C.S., and E.Y. Isacoff. 2002. a. Molecular models of voltage sensing. J. Gen. Physiol. 120:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, C.S., and E.Y. Isacoff. 2002. b. Optical measurements from a voltage-gated ion channel reveal interactions between voltage-sensing and opening. Biophys. J. 82:232a. [Google Scholar]
- Gandhi, C.S., E. Loots, and E.Y. Isacoff. 2000. Reconstructing voltage sensor-pore interaction from a fluorescence scan of a voltage-gated K+ channel. Neuron. 27:585–595. [DOI] [PubMed] [Google Scholar]
- Glauner, K.S., L.M. Mannuzzu, C.S. Gandhi, and E.Y. Isacoff. 1999. Spectroscopic mapping of voltage sensor movement in the Shaker potassium channel. Nature. 402:813–817. [DOI] [PubMed] [Google Scholar]
- Hodgkin, A.L., and A.F. Huxley. 1952. A quantatitive description of membrane currents and its application to conduction and excitation in nerve. J. Physiol. 117:500–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, R. 2000. Conversation between voltage sensors and gates of ion channels. Biochemistry. 39:15653–15658. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. a. Crystal structure and mechanism of a calcium-gated potassium channel. Nature. 417:515–522. [DOI] [PubMed] [Google Scholar]
- Jiang, Y., A. Lee, J. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. b. The open pore conformation of potassium channels. Nature. 417:523–526. [DOI] [PubMed] [Google Scholar]
- Kanevsky, M., and R.W. Aldrich. 1999. Determinants of voltage-dependent gating and open-state stability in the S5 segment of Shaker potassium channels. J. Gen. Physiol. 114:215–242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keynes, R.D., and F. Elinder. 1999. The screw-helical voltage gating of ion channels. Proc. R. Soc. Lond. B Biol. Sci. 266:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laine, M., J.P.A. Bannister, W.R. Silverman, M.A. Lin, A.F. Mock, D.M. Papazian. 2002. Structural interactions between voltage sensor and pore in Shaker K+ channels. Biophys. J. 82:231a. [Google Scholar]
- Larsson, H.P., and F. Elinder. 2000. A conserved glutamate is important for slow inactivation in K+ channels. Neuron. 27:573–583. [DOI] [PubMed] [Google Scholar]
- Latorre, R., B. Claudia, C. Gonzalez, O. Alvarez, and D. Cosmelli. 2001. KAT1, a K+ channel from Arabidopsis thaliana, possesses an intrinsic voltage sensor. Biophys. J. 80:436a. [Google Scholar]
- Li-Smerin, Y., D.H. Hackos, and K.J. Swartz. 2000. A localized interaction surface for voltage-sensing domains on the pore domain of a K+ channel. Neuron. 25:411–423. [DOI] [PubMed] [Google Scholar]
- Loots, E., and E.Y. Isacoff. 2000. Molecular coupling of S4 to a K+ channel's slow inactivation gate. J. Gen. Physiol. 116:623–636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu, Z., A.M. Klem, and Y. Ramu. 2001. Ion conduction pore is conserved among potassium channels. Nature. 413:809–813. [DOI] [PubMed] [Google Scholar]
- Männikkö, R., F. Elinder, and H.P. Larsson. 2002. Voltage-sensing mechanism is conserved among ion channels gated by opposite voltages. Nature. In press. [DOI] [PubMed] [Google Scholar]
- Mitcheson, J.S., J. Chen, and M.C. Sanguinetti. 2000. Trapping of a methanesulfonalide by closure of the HERG potassium channels activation gate. J. Gen. Physiol. 115:229–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ortega-Saenz, P., R. Pardal, A. Castellano, and J. Lopez-Barneo. 2000. Collapse of conductance is prevented by a glutamate residue conserved in voltage-dependent K+ channels. J. Gen. Physiol. 116:181–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perozo, E., D.M. Cortes, and L.G. Cuello. 1999. Structural rearrangements underlying K+-channel activation gating. Science. 285:73–78. [DOI] [PubMed] [Google Scholar]
- Qui, X.-Q., K.S. Jakes, P.K. Kienker, A. Finkelstein, and S.L. Slatin. 1996. Major transmembrane movement associated with colicin Ia channel gating. J. Gen. Physiol. 107:313–328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rothberg, B.S., K.S. Shin, P.S. Phale, and G. Yellen. 2002. Voltage-controlled gating at the intracellular entrance to a hyperpolarization-activated cation channel. J. Gen. Physiol. 119:83–91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santoro, B., and G.R. Tibbs. 1999. The HCN gene family: molecular basis of the hyperpolarization-activated pacemaker channels. Ann. NY Acad. Sci. 868:741–764. [DOI] [PubMed] [Google Scholar]
- Shin, K., B. Rothberg, and G. Yellen. 2001. Blocker state dependence and trapping in hyperpolarization-activated cation channels. Evidence for an intracellular activation gate. J. Gen. Physiol. 117:91–102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith, P.L., and G. Yellen. 2002. Fast and slow voltage sensor movements in HERG potassium channels. J. Gen. Physiol. 119:275–293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiwari-Woodruff, S.K., M.A. Lin, C.T. Schulteis, and D.M. Papazian. 2000. Voltage-dependent structural interactions in the Shaker K+ channel. J. Gen. Physiol. 115:123–138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen, G. 1998. The moving parts of voltage-gated ion channels. Q. Rev. Biophys. 31:239–295. [DOI] [PubMed] [Google Scholar]
- Zei, P.C., and R.W. Aldrich. 1998. Voltage-dependent gating of single wild-type and S4 mutant KAT1 inward rectifier potassium channels. J. Gen. Physiol. 112:679–713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, M., J.H. Morais-Cabral, S. Mann, and R. MacKinnon. 2001. a. Potassium channel receptor site for the inactivation gate and quaternary amine inhibitors. Nature. 411:657–661. [DOI] [PubMed] [Google Scholar]
- Zhou, Y., J.H. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. b. Chemistry of ion coordination and hydration revealed by a K+ channel-Fab complex at 2.0 A resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]