Ion channels, like most other proteins, are designed to move. To preserve ionic gradients across cell membranes, they are usually closed and open their gates only when called upon to do electric work. Ionic fluxes through open channels are exploited by cells for a variety of tasks, including the generation of explosive action potentials in excitable cells. A singular feature of the ion channels underlying action potentials is that their gates are regulated by the voltage across the plasma membrane.
Membrane potential has two roles in voltage-gated ion channels. It controls the open probability of their gates, especially the so-called activation gate, and it drives ions through open channels. The activation gate in this class of ion channel tends to be highly sensitive to small changes in membrane potential, so much so that a depolarization of only 5 mV can produce a 10-fold increase of open probability (Schoppa et al., 1992; Hirschberg et al., 1995).
What underlies this exquisite sensitivity? Three functional components of the channel are required: a permeation pathway (pore), gates that permit or deny the movement of ions through the pore, and a voltage sensor that changes its conformation in response to changes of membrane potential. Movement of the voltage sensor must be coupled energetically to the gates. The steep voltage dependence of activation in many types of potassium channels requires that at least 12 elementary charges (e0) cross the membrane electric field between completely closed and open conformations (Sigworth, 1994; Sigg and Bezanilla, 1997). The energetic coupling between membrane potential and the activation gate is the defining characteristic that makes these channels voltage dependent.
Nature chose to make the voltage sensor and the activation gate separate structures. It didn't have to be that way. If the activation gate were sufficiently charged and moved through the electric field as it opened and closed, it would also serve as a voltage sensor. However, the activation gate is formed by the cytoplasmic convergence of four uncharged transmembrane segments (S6 segments) that line the pore (Liu et al., 1997; Del Camino et al., 2000; Del Camino and Yellen, 2001). These S6 segments are contributed either from four separate α subunits in potassium channels, or from each of four homologous domains on a single α subunit in sodium and calcium channels. Even an uncharged gate can itself cause charge movement across the electric field when it opens or closes an aqueous pathway, because gating transitions will change the shape of the electric field across the pore. For example, when the intracellular activation gate is open, most of the electric field will fall across the narrowest part of the pore, the short selectivity filter at its extracellular end (Jiang et al., 2002). When the activation gate is closed, however, some of the electric field will fall across this hydrophobic obstruction. This will tend to produce a charge transfer when the transmembrane potential has a finite magnitude, partly because less of the electric field will fall across the ions in the selectivity filter. If there is an excess of cations within the selectivity filter, for example in a cation-selective ion channel, hyperpolarization will tend to favor the open state for simple energetic reasons (see Fig. 5 in Sigworth [1994] for the explanation behind this inference). To make a precise prediction, however, it is necessary to know the positions of all charged, or partially charged, species within the electric field in both open and closed states (Sigworth, 1994; Sigg and Bezanilla, 1997), something we can only dream about for the moment.
In spite of our ignorance, it is generally accepted that the main voltage sensor is the positively charged S4 transmembrane segment and not the gate. This fact is supported by five classes of information. First, neutralizing S4 mutations reduce the number of charges coupled to gating (Aggarwal and MacKinnon, 1996; Seoh et al., 1996). Second, the voltage-dependent transfer of S4's charged residues across the membrane, measured by cysteine and histidine scanning, can largely account for the number of charges expected from the steepness of activation (3–4 e0 per S4 segment; for review see Bezanilla, 2000). Third, the movement of the S4 segment, tracked by fluorescence, approximately corresponds in kinetics and steady-state voltage dependence to the gating current of voltage sensors (Bezanilla, 2000). Fourth, immobilizing S4 segments reduces both gating current and opening of the activation gate, as expected quantitatively if S4 is the principle voltage sensor underlying activation (Horn et al., 2000; Ding and Horn, 2001). Finally, some nonvoltage-dependent ion channels (e.g., KcsA potassium channels) have an activation gate and pore homologous to those in voltage-gated channels, but lack a voltage-sensing domain with S4 segments.
Although S4 segments are believed to be the main voltage sensors, they may have cohorts that share the load. Of the six transmembrane segments in each potassium channel subunit or sodium channel domain, the majority of charges are found on S4 segments, each of which typically has 4–8 basic residues. The S5 and S6 segments, closest to the central axis surrounding the pore, are uncharged in many types of voltage-dependent channels. The S1-S3 segments do, however, contain conserved charged residues (Keynes and Elinder, 1999). In sodium and calcium channels S1 segments typically have an acidic residue, either Asp or Glu, near the cytoplasmic end. S2 segments have two highly conserved charged residues, one basic and one acidic, in the cytoplasmic half of the transmembrane segment. S3 segments also have a highly conserved Asp near their cytoplasmic ends. S2 and S3 segments also have more variable acidic residues near their extracellular ends.
Two roles in gating have been proposed for the acidic residues in S2 and S3. They might interact electrostatically with the basic residues of S4, and they might themselves be involved in voltage sensing, i.e., by moving through the electric field in response to changes of membrane potential. There is good evidence for an electrostatic role (e.g., Papazian et al., 1995). However, even though neutralizing these negatively charged residues can produce large effects on the voltage sensitivity of gating, the jury is still out on whether S2 or S3 segments should be considered bona fide voltage sensors (Seoh et al., 1996; Nguyen and Horn, 2002).
In this perspective I will consider two topics. First, what kind of movement does the S4 segment make in response to a depolarization? Second, in what ways could S4 movement cause activation gates to open?
S4 Movement
A variety of movements of the S4 segment might account for the translocation of its positive charges across the membrane electric field when a channel is depolarized, the most obvious being an outward translational movement. Such a movement would simply explain the translocation of S4 residues, substituted by cysteine, from a cytoplasmic to an extracellular vestibule during a depolarization (Larsson et al., 1996; Yang et al., 1996). Other types of movements could achieve the same result, including an axial rotation around S4's helical axis, lateral motion among a bundle of transmembrane helices, changes of tilt angle of the S4 segment or of other transmembrane segments, and a “helical screw” movement that combines translation and rotation (see discussion in Yellen, 1998; Bezanilla, 2000; Horn, 2000). It is even possible for S4 to transfer its charged residues across the electric field without itself moving with respect to the lipid bilayer, if instead the electric field moves. This could occur if the hydrophobic transmembrane segments surrounding the S4 segment move inward, for example, in response to a depolarization (Yang et al., 1996). Because S4 segments are almost completely surrounded by hydrophilic crevices (Larsson et al., 1996; Yang et al., 1996; Starace and Bezanilla, 2001), only a small movement is needed to transfer the necessary 3–4 e0 during a depolarization. The extensive hydrophilic crevices furthermore focus the membrane electric field over a distance considerably shorter than the thickness of the bilayer, facilitating the movement of the voltage sensor, much as the short selectivity filter facilitates permeant ion movement (Miller, 1982; Jiang et al., 2002).
Other Perspectives in this issue (Bezanilla, 2002; Gandhi and Isacoff, 2002; Larsson, 2002) will consider the intricacies of S4 movement in more detail. Although S4 rotation is generally believed to accompany charge movement, axial translation of S4 with respect to the lipid bilayer is more controversial. I only intend to fuel the fire by referring to one of our experimental results that argues against pure rotation as the mechanism of charge transfer. Fig. 1 shows such a rotational model for the S4 segment of the fourth domain of a sodium channel. The four consecutive residues shown, two arginines (designated R2 and R3) separated by a leucine and an alanine, were individually substituted by a cysteine, and accessibility to hydrophilic cysteine reagents was examined from both sides of the membrane. Assuming that the S4 segment is an α helix, R2 and R3 will be situated on opposite sides of the helix from these two neutral residues, as shown. Depolarization moves R2C and R3C from an intracellular to an extracellular hydrophilic vestibule (Yang et al., 1996). Fig. 1 shows how this could be accomplished with a pure rotational movement. Interestingly, the membrane electric field in this case now lies perpendicular rather than parallel to the membrane and falls across the S4 segment itself. Although this simplistic model can account for charge transfer, it makes a prediction that is not supported by our data, namely that the two neutral residues between R2 and R3 translocate in the opposite direction, from extracellular to intracellular, upon depolarization (Fig. 1). Our cysteine-scanning measurements, however, show that all four residues are internally accessible at hyperpolarized voltages, whereas depolarization renders L1452C and A1453C inaccessible on either side of the membrane (Yang et al., 1997). This result is inconsistent with the model shown, but does not eliminate the possibility that S4 undergoes a rotation when depolarized. However, something is missing from the picture. Either S4's movement is more complicated, e.g., a helical screw motion, or else the surrounding transmembrane segments also move, thereby altering the structure of the hydrophilic crevices.
Figure 1.
Model of the fourth homologous domain (D4) of the human skeletal muscle sodium channel (hNaV1.4) with the S4 segment depicted as a rotating cylinder. Four S4 residues are shown: Arg1451 (R2), Leu1452, Ala1453, and Arg1454 (R3). The positions of the residues around the S4 segment roughly correspond to those of an α helical model. A depolarizing rotation transfers R2 and R3 from an intracellularly to an extracellularly accessible crevice, whereas Leu1452 and Ala1453 are translocated in the opposite direction.
Coupling
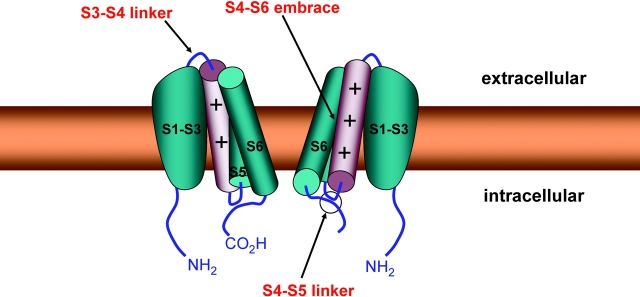
Two experimental results indicate that the opening of the activation gate involves a significant separation of the tail ends of the four S6 segments. First, large diameter (up to ∼12 Å) blockers and hydrophilic reagents can enter the open pore from its cytoplasmic face (Yellen, 1998). Second, the crystal structure of an open potassium channel reveals an ∼12 Å intracellular entrance to the pore, in striking contrast to the tight bundle crossing of a closed KcsA channel (Jiang et al., 2002). How does the conformation of the S4 segment influence the bottom of the S6 segment? Clearly, the coupling between voltage sensor movement and opening of the activation gate is robust (Sigworth, 1994; Sigg and Bezanilla, 1997). Fig. 2 shows three structural possibilities for coupling. S4 movement could affect: (a) the S3-S4 linker, (b) the S4-S5 linker, or (c) adjacent transmembrane segments, including the S6 segment itself. Pulling or twisting linkers would have to be communicated allosterically to the activation gate.
Figure 2.
Hypothetical model of two potassium channel subunits oriented opposite one another across the permeation pathway. The other two subunits, in front and behind, are removed for clarity. The activation gate is the convergence at the cytoplasmic ends of the S6 segments. The S4 segment, the positively charged cylinder, lies next to the “pore domain” (S5-S6). Three potential coupling sites are highlighted: (a) the S3-S4 linker that might interact with the top of the pore domain; (b) the S4-S5 linker that might interact with the tail end of the S6 segment; and (c) an interaction between the S4 segment and the pore domain within the transmembrane region.
The least critical participant in coupling is likely to be the extracellular S3-S4 linker. Although voltage-dependent S4 movement causes concomitant movement in this linker (Bezanilla, 2000), deletion of the entire linker leaves a crippled but functional channel (Gonzalez et al., 2001). Furthermore, the length of this linker is highly variable among voltage-gated ion channels. By contrast, the S4-S5 linker is much more sensitive to even conservative point mutations, which sometimes produce drastic changes in channel gating (e.g., McCormack et al., 1991). Moreover, our laboratory has systematically inserted single alanines all along the S4-S5 linker of Shaker potassium channels, and virtually all of these constructs are nonfunctional (unpublished data). These results suggest an important role for the S4-S5 linker in coupling. The coupling mechanism is speculative, but it has been proposed that the S4-S5 linker is in direct contact with the tail end of the S6 segment (Fig. 2), so that S4 movement would have a rather intimate link to a region near the activation gate (Lu et al., 2001; Ding and Horn, 2002; Tristani-Firouzi et al., 2002).
The final alternative is that S4 movement causes a repacking of the transmembrane segments that surround it, including the S6 segment. Evidence for this possibility is based primarily on tryptophan scanning of the S6 segment of Shaker potassium channels (Li-Smerin et al., 2000). This study revealed stripes of “high-impact” residues on the backside of S6 (facing away from the central axis of the channel). As interpreted in other tryptophan scanning studies, high-impact residues are especially sensitive to mutation and are therefore likely to be involved in protein–protein interactions. High-impact mutants typically show strong shifts in the closed-open equilibrium, suggesting a disruption of either closed or open states by the introduced tryptophan.
The above speculations fail to address how S4 movement produces movement of the activation gate. Although the coupled movements will certainly involve changes in tertiary structure, for example as the relative positions of transmembrane segments are transmuted, the energetics of interaction remain to be elucidated. A voltage-dependent change in the relative positions between two transmembrane segments, for example, or between any two side chain residues, does not reveal why these relationships were altered.
These coupling puzzles will be addressed in future studies, but it is worthwhile considering some possible scenarios, assuming for the moment that charged S4 segments are driven to move through the electric field in response to a change of membrane potential. (a) Either hydrophobic or electrostatic forces may adhere S4 segments to other transmembrane segments so that they move relatively in concert. One example from our laboratory involves voltage-dependent changes in reactivity of cysteines substituted into the outer third of an S3 segment of sodium channels. The direction of voltage dependence could be explained by the S4 segment dragging its next door neighbor outward upon depolarization (Nguyen and Horn, 2002). (b) Rigid-body movement of an S4 segment may cause movement of an adjacent transmembrane segment because of steric interaction between the side chains of the two transmembrane segments. For example, rotation of one helix could cause its neighbor to rotate, the way a key engages the tumblers of a lock. Such a mechanism has been proposed to explain coupled rotation between S5 and S6 segments of a potassium channel (Espinosa et al., 2001). (c) The path of S4 movement could pry apart tertiary or quaternary interactions at its leading edge and leave vacancies in its wake. Entropic and enthalpic forces would favor subsequent rearrangements that could produce movement of the activation gate. (d) Finally, as suggested above, S4 movement might pull or twist a linker that could allosterically open or close the activation gate.
These and other coupling mechanisms are amenable to experimental exploration; however it is unlikely that one of them is solely responsible for all of the coupling energy between S4 and gate movements. Helpful clues about important coupling factors will likely come from studies of the gating mechanisms of other ion channels, as well as allosteric movements in other proteins (Perutz, 1989).
References
- Aggarwal, S.K., and R. MacKinnon. 1996. Contribution of the S4 segment to gating charge in the Shaker K+ channel. Neuron. 16:1169–1177. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F. 2000. The voltage sensor in voltage dependent ion channels. Physiol. Rev. 80:555–592. [DOI] [PubMed] [Google Scholar]
- Bezanilla, F. 2002. Voltage sensor movements. J. Gen. Physiol. 120:465–473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Del Camino, D., M. Holmgren, Y. Liu, and G. Yellen. 2000. Blocker protection in the pore of a voltage-gated K+ channel and its structural implications. Nature. 403:321–325. [DOI] [PubMed] [Google Scholar]
- Del Camino, D., and G. Yellen. 2001. Tight steric closure at the intracellular activation gate of a voltage-gated K+ channel. Neuron. 32:649–656. [DOI] [PubMed] [Google Scholar]
- Ding, S., and R. Horn. 2001. Slow photo-cross-linking kinetics of benzophenone-labeled voltage sensors of ion channels. Biochemistry. 40:10707–10716. [DOI] [PubMed] [Google Scholar]
- Ding, S., and R. Horn. 2002. Pivotal role of the tail end of the S6 segment in coupling the voltage sensor and activation gate of Shaker K channels. Biophys. J. 82:174a. [Google Scholar]
- Espinosa, F., R. Fleischhauer, A. McMahon, and R.H. Joho. 2001. Dynamic interaction of S5 and S6 during voltage-controlled gating in a potassium channel. J. Gen. Physiol. 118:157–170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gandhi, C.S., and E.Y. Isacoff. 2002. Molecular models of voltage sensing. J. Gen. Physiol. 120:455–463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gonzalez, C., E. Rosenman, F. Bezanilla, O. Alvarez, and R. Latorre. 2001. Periodic perturbations in Shaker K+ channel gating kinetics by deletions in the S3-S4 linker. Proc. Natl. Acad. Sci. USA. 98:9617–9623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirschberg, B., A. Rovner, M. Lieberman, and J. Patlak. 1995. Transfer of twelve charges is needed to open skeletal muscle Na+ channels. J. Gen. Physiol. 106:1053–1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horn, R. 2000. A new twist in the saga of charge movement in voltage-dependent ion channels. Neuron. 25:511–514. [DOI] [PubMed] [Google Scholar]
- Horn, R., S. Ding, and H.J. Gruber. 2000. Immobilizing the moving parts of voltage-gated ion channels. J. Gen. Physiol. 116:461–475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang, Y.X., A. Lee, J.Y. Chen, M. Cadene, B.T. Chait, and R. MacKinnon. 2002. The open pore conformation of potassium channels. Nature. 417:523–526. [DOI] [PubMed] [Google Scholar]
- Keynes, R.D., and F. Elinder. 1999. The screw-helical voltage gating of ion channels. Proc. R. Soc. Lond. B. Biol. Sci. 266:843–852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, H.P. 2002. The search is on for the voltage sensor-to-gate coupling. J. Gen. Physiol. 120:475–481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson, H.P., O.S. Baker, D.S. Dhillon, and E.Y. Isacoff. 1996. Transmembrane movement of the Shaker K+ channel S4. Neuron. 16:387–397. [DOI] [PubMed] [Google Scholar]
- Li-Smerin, Y.Y., D.H. Hackos, and K.J. Swartz. 2000. A localized interaction surface for voltage-sensing domains on the pore domain of a K+ channel. Neuron. 25:411–423. [DOI] [PubMed] [Google Scholar]
- Liu, Y., M. Holmgren, M.E. Jurman, and G. Yellen. 1997. Gated access to the pore of a voltage-dependent K+ channel. Neuron. 19:175–184. [DOI] [PubMed] [Google Scholar]
- Lu, Z., A.M. Klem, and Y. Ramu. 2001. Ion conduction pore is conserved among potassium channels. Nature. 413:809–813. [DOI] [PubMed] [Google Scholar]
- McCormack, K., M.A. Tanouye, L.E. Iverson, J.-W. Lin, M. Ramaswami, T. McCormack, J.T. Campanelli, M.K. Mathew, and B. Rudy. 1991. A role for hydrophobic residues in the voltage-dependent gating of Shaker K+ channels. Proc. Natl. Acad. Sci. USA. 88:2931–2935. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller, C. 1982. Feeling around inside a channel in the dark. Transport in Biological Membranes. R. Antolini, editor. Raven, New York. 99–108.
- Nguyen, T.P., and R. Horn. 2002. Movement and crevices around a sodium channel S3 segment. J. Gen. Physiol. 120:419–436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Papazian, D.M., X.M. Shao, S.-A. Seoh, A.F. Mock, Y. Huang, and D.H. Wainstock. 1995. Electrostatic interactions of S4 voltage sensor in Shaker K+ channel. Neuron. 14:1293–1301. [DOI] [PubMed] [Google Scholar]
- Perutz, M.F. 1989. Mechanisms of cooperativity and allosteric regulation in proteins. Q. Rev. Biophys. 22:139–236. [DOI] [PubMed] [Google Scholar]
- Schoppa, N.E., K. McCormack, M.A. Tanouye, and F.J. Sigworth. 1992. The size of gating charge in wild-type and mutant Shaker potassium channels. Science. 255:1712–1715. [DOI] [PubMed] [Google Scholar]
- Seoh, S.A., D. Sigg, D.M. Papazian, and F. Bezanilla. 1996. Voltage-sensing residues in the S2 and S4 segments of the Shaker K+ channel. Neuron. 16:1159–1167. [DOI] [PubMed] [Google Scholar]
- Sigg, D., and F. Bezanilla. 1997. Total charge movement per channel - The relation between gating charge displacement and the voltage sensitivity of activation. J. Gen. Physiol. 109:27–39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sigworth, F.J. 1994. Voltage gating of ion channels. Q. Rev. Biophys. 27:1–40. [DOI] [PubMed] [Google Scholar]
- Starace, D.M., and F. Bezanilla. 2001. Histidine scanning mutagenesis of basic residues of the S4 segment of the Shaker K+ channel. J. Gen. Physiol. 117:469–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tristani-Firouzi, M., J. Chen, and M.C. Sanguinetti. 2002. Interactions between S4-S5 linker and S6 transmembrane domain modulate gating of HERG K+ channels. J. Biol. Chem. 277:18994–19000. [DOI] [PubMed] [Google Scholar]
- Yang, N., A.L. George, and R. Horn. 1997. Probing the outer vestibule of a sodium channel voltage sensor. Biophys. J. 73:2260–2268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, N., A.L. George, Jr., and R. Horn. 1996. Molecular basis of charge movement in voltage-gated sodium channels. Neuron. 16:113–122. [DOI] [PubMed] [Google Scholar]
- Yellen, G. 1998. The moving parts of voltage-gated ion channels. Q. Rev. Biophys. 31:239–295. [DOI] [PubMed] [Google Scholar]