Abstract
Recent molecular dynamic simulations and electrostatic calculations suggested that the external TEA binding site in K+ channels is outside the membrane electric field. However, it has been known for some time that external TEA block of Shaker K+ channels is voltage dependent. To reconcile these two results, we reexamined the voltage dependence of block of Shaker K+ channels by external TEA. We found that the voltage dependence of TEA block all but disappeared in solutions in which K+ ions were replaced by Rb+. These and other results with various concentrations of internal K+ and Rb+ ions suggest that the external TEA binding site is not within the membrane electric field and that the voltage dependence of TEA block in K+ solutions arises through a coupling with the movement of K+ ions through part of the membrane electric field. Our results suggest that external TEA block is coupled to two opposing voltage-dependent movements of K+ ions in the pore: (a) an inward shift of the average position of ions in the selectivity filter equivalent to a single ion moving ∼37% into the pore from the external surface; and (b) a movement of internal K+ ions into a vestibule binding site located ∼13% into the membrane electric field measured from the internal surface. The minimal voltage dependence of external TEA block in Rb+ solutions results from a minimal occupancy of the vestibule site by Rb+ ions and because the energy profile of the selectivity filter favors a more inward distribution of Rb+ occupancy.
Keywords: K+ channels, ion permeation, tetraethylammonium, rubidium
INTRODUCTION
TEA ions have, for many years, been useful probes of the structure and function of K+ channels (Armstrong, 1966; Hille, 1967; Armstrong and Hille, 1972; Kirsch et al., 1991), perhaps because TEA is positively charged, like K+ ions, and about the same size as a hydrated K+ ion. External TEA blocks many types of K+ channels but with >1,000-fold range of effective concentrations (Kavanaugh et al., 1991). Much of this difference can be attributed to the amino acid at a single position in the outer entrance to the pore (MacKinnon and Yellen, 1990; Gomez-Hernandez et al., 1997). Amino acids with an aromatic side chain at this location (residue 449 in Shaker K+ channels) confer high sensitivity to block by external TEA ions.
The nature of this external TEA binding site has been the subject of several studies designed to probe the structure of the outer entrance to the pore. The high-affinity form of the site is very specific for TEA: tetramethylammonium is essentially ineffective and tetrapropylammonium blocks with at least a 10-fold lower affinity (Heginbotham and MacKinnon, 1992). The binding free energy change includes contributions from all four subunits of the channel (Heginbotham and MacKinnon, 1992; Kavanaugh et al., 1992), perhaps via cation-π orbital interactions (Heginbotham and MacKinnon, 1992).
The change from the low-affinity to the high-affinity form of the external TEA receptor in Shaker channels was unexpectedly accompanied by a change in the voltage dependence of TEA block. TEA blocks the low-affinity form (with a threonine at position 449) as if the TEA ion moves ∼19% into the membrane electric field to reach its binding site (Heginbotham and MacKinnon, 1992). The high-affinity form (with a tyrosine at 449), however, is blocked as if TEA ions only need to traverse 4% of the electric field. These results led Heginbotham and MacKinnon (1992) to conclude that replacing the tyrosines with threonines moved the TEA binding site further into the pore.
The solution of the bacterial K+ channel, KcsA, crystal structure (Doyle et al., 1998) has raised doubts about this picture of the external TEA binding site. The position and orientation of the aromatic ring of the relevant tyrosines in this channel is not consistent with favorable cation-π orbital interactions (Crouzy et al., 2001; Luzhkov and Aqvist, 2001). Molecular dynamics calculations revealed that replacing the tyrosine with a threonine changed the position of the TEA binding site, as suggested by the changes in voltage dependence described above (Crouzy et al., 2001). However, while the voltage dependence of TEA block suggested that the tyrosine to threonine replacement shifted the TEA binding site further away from the pore entrance, these molecular dynamics simulations predict that the site would move closer to the pore entrance. Moreover, electrostatic calculation showed that both locations should be well outside the membrane electric field (Roux et al., 2000) and so TEA block should be independent of voltage for both the high and low affinity forms of the receptor.
In an attempt to resolve these apparent contradictions we have reexamined the voltage dependence of external TEA block in Shaker K+ channels with the native, low-affinity TEA receptor that contains a threonine at position 449. In agreement with previous results we found that, with K+ as the permeant ion, external TEA blocked these channels in a voltage-dependent manner as if it moved through ∼21% of the membrane electric field. However, this voltage dependence all but disappeared with solutions containing Rb+ as the permeant ion. Ion replacement studies showed that the identity of the permeant ion in the internal solution determined the voltage dependence of external TEA block. This result suggests that the external TEA binding site is not within the membrane electric field but that the voltage dependence of TEA block in K+ solutions arises through a coupling with the movement of K+ ions through some part of the membrane electric field. Thus, it might be expected that the voltage dependence of external TEA block would be reduced as the concentration of K+ ions in the intracellular solution is reduced. However, we found just the opposite: with 20 mM internal K+, TEA appeared to move through ∼27% of the membrane electric field. The resolution to this apparent contradiction lies in two types of coupling of external TEA block with K+ ions in the pore: (a) K+ ions in the selectivity filter must move through ∼37% of the membrane electric field in order to allow TEA to bind to its receptor; and (b) the block of the channel by TEA promotes internal K+ occupancy of a binding site located ∼13% into the membrane electric field measured from the internal surface. The minimal voltage dependence of TEA block in Rb+ solutions results, in this view, from a different pattern of occupancy of specific sites in the pore by Rb+ and K+ ions.
MATERIALS AND METHODS
K Channel Constructs and Expression
The experiments reported here were done on the inactivation-deletion version of Shaker B, ShB Δ6–46 (Hoshi et al., 1990). Frog (Xenopus laevis) maintenance, oocyte isolation, and RNA injection used standard methods (Goldin, 1992) and have been described previously in detail (Thompson and Begenisich, 2000, 2001). The procedures for animal handling, maintenance, and surgery were approved by the University of Rochester Committee on Animal Resources. Frogs were anesthetized with 0.2% tricaine (Sigma-Aldrich). Isolated ovarian lobes were defolliculated by incubation for 60–90 min with 2 mg/ml of collagenase Type IA (Sigma-Aldrich).
Electrophysiological Recordings
Potassium channel currents were recorded 1 to 5 d after RNA injection. Electrophysiological recordings were done at room temperature (20–22°C) with excised inside/out or outside/out macropatches using Axopatch 1-D or 200B amplifiers (Axon Instruments, Inc.). Patch pipettes had tip diameters of ∼3-6 μm constructed from Corning 7052 (Garner Glass Co.) or GC-150 glass (Warner Instruments, Inc.). The measured junction potentials for the solutions used were all within 4 mV of one another and so no correction for these was applied. The holding potential was −70 mV in most cases; however, we used a holding potential of −90 mV with some experiments, especially those with 20 mM internal K+, in order to minimize the amount of slow inactivation (Baukrowitz and Yellen, 1996a,b). Data acquisition was performed using a 12-bit analogue/digital converter controlled by a personal computer. Current records were filtered at 5 kHz.
The standard external solution contained (in mM): 5 KCl, 135 N-methyl-D-glucamine (NMDG)-Cl, 2 CaCl2, 2 MgCl2, 10 mM HEPES, pH of 7.2 (with NMDG). External TEA was added to this solution by equimolar replacement of NMDG. The standard internal solution consisted of (in mM): 110 KCl, 25 KOH, 10 EGTA, 10 HEPES, pH 7.2 (with HCl). In some experiments Rb+ completely replaced the K+ in either the internal or external solution or in both. The K+ or Rb+ content in the external or internal solutions was altered by equimolar replacement with NMDG.
Data Analysis
The apparent Kd values for Shaker channel inhibition by external TEA were obtained from measurements of current block by different TEA concentrations. Several (4–6) concentrations of external TEA, bracketing the block mid-point, were used. The K app values were obtained by fitting these dose–response relations with the standard single-component Langmuir equation:
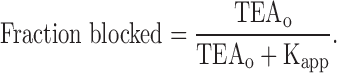 |
(1) |
Except for experiments with the lowest internal K+ concentration (20 mM), this equation provided an accurate fit to the data. The shape of the dose–response relation in 20 mM internal K+ was less steep than predicted by Eq. 1; for this concentration of internal K+, the interpolated concentration at 50% block was used to estimate the K app value.
The voltage dependence of the apparent affinity constant was assessed through a simple form of the Woodhull (1973) equation:
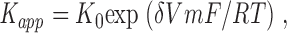 |
(2) |
where K 0 is the K app value at 0 mV; δ is the effective electrical distance, and R, T, and F have their usual thermodynamic meanings. Shaker channel activation gating occurs between about −60 and −10 mV (Yellen et al., 1991). Thus, in order to minimize any apparent voltage dependence coupled to channel gating, we only used membrane potentials between −10 and 70 mV.
Fits of equations to the data were done using the Levenberg-Marquardt algorithm as implemented in Origin 6.1 (OriginLab Corp.). Error limits for the fitted parameters are the estimated errors from the fitting routine.
RESULTS
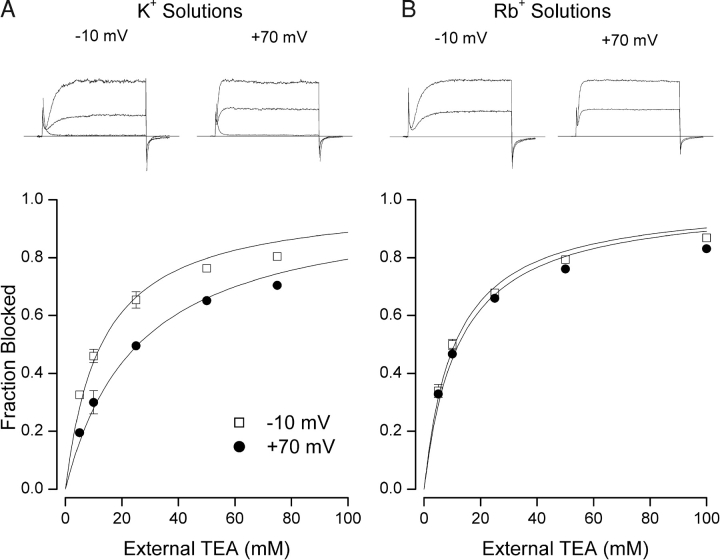
The top part of Fig. 1 A depicts Shaker K+ channel currents recorded in solutions that contained 5 mM external and 135 mM internal K+. Shown are records at −10 and 70 mV in the absence and presence (smaller currents) of 20 mM external TEA. This concentration of TEA produced less block at the more positive potential. The main part of Fig. 1 A shows the dose-dependent block of Shaker channels by external TEA at −10 (□) and 70 mV (•). These data were fit by Eq. 1 with K app values for TEA block of 12.3 ± 0.81 mM and 25.2 ± 1.4 mM at −10 and 70 mV, respectively. That is, external TEA was more effective at −10 than 70 mV with K+ as the permeant ion.
Figure 1.
External TEA block of Shaker K+ channels. (A) TEA block in K+ solutions (5 mM external K+//135 mM internal K+). (Top) Raw current records obtained with step changes in membrane potential from −70 mV to the indicated voltages. Lower record of each pair recorded in the presence of 20 mM TEA. Superimposed on each set of records is the current at the indicated voltage recorded from an uninjected oocyte illustrating the lack of any significant endogenous currents. The current records have not been leak corrected- the small current from the uninjected oocyte is likely through the ∼7 GΩ seal resistance. (Main) Fraction of channel current block by external TEA at −10 (□) and 70 mV (•), respectively. Lines are fits of Eq. 1 to the data with K app values of 12.3 ± 0.81 and 25.2 ± 1.4 mM at −10 and 70 mV, respectively. (B) TEA block in Rb+ solutions (5 mM external Rb+//135 mM internal Rb+). (Top) Raw current records obtained with step changes in membrane potential from −70 mV to the indicated voltages. Lower record of each pair recorded in the presence of 20 mM TEA. (Main) Fraction of channel current block by external TEA at −10 (□) and 70 mV (•), respectively. Lines are fits of Eq. 1 to the data with K app values of 10.9 ± 0.74 and 12.5 ± 1.2 mM at −10 and 70 mV, respectively.
The voltage dependence seen in Fig. 1 A may not be an intrinsic property of external TEA because it was absent in solutions in which K+ ions were replaced by Rb+. Fig. 1 B illustrates TEA block of Shaker K+ channels in such Rb+ solutions. The apparent affinity for TEA block at −10 mV (□) was 10.9 ± 0.74 mM only slightly less than the value of 12.5 ± 1.2 mM for the block at 70 mV (•).
In a manner similar to that illustrated in Fig. 1, we determined the K app values for external TEA block at several values of membrane potential between −10 and 70 mV and with solutions of different K+ and Rb+ composition. The results of this analysis are illustrated in Fig. 2 .
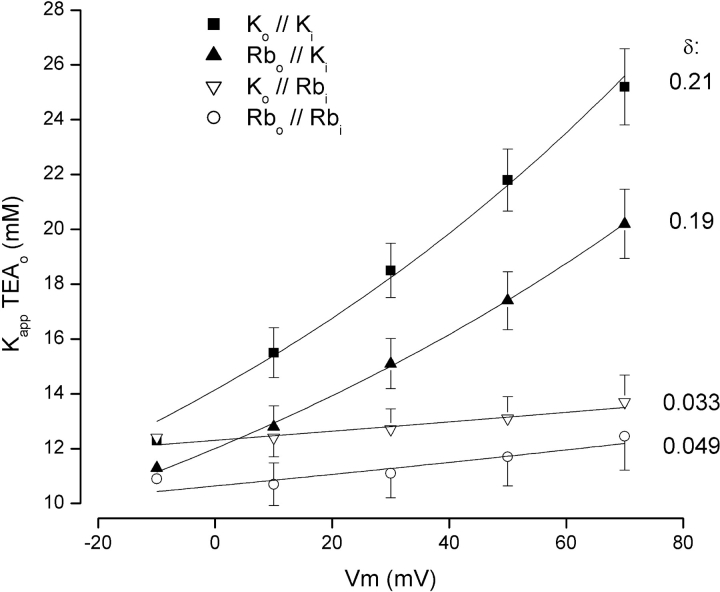
Figure 2.
Voltage dependence of TEA block in K+ and Rb+ solutions. The apparent Kd for external TEA block, K app, is shown as a function of membrane potential for solutions containing K+ on both sides of the channel (▪), solutions with external and internal Rb+ (○), external Rb+ and internal K+ (▴), and external K+ with internal Rb+ (▿). Lines are fits of Eq. 2 to the data with the indicated value of δ.
One set of K app values (▪) was obtained (as in Fig. 1 A) with K+ solutions consisting of 5 mM external and 135 mM internal K+. These data were fit (solid line) by the appropriate form of the Woodhull (1973) equation (Eq. 2, see materials and methods) to obtain an estimate for the effective electrical distance, δ associated with external TEA block. In these K+ solutions, the electrical distance was ∼0.21, quite similar to the value of 0.19 obtained by Heginbotham and MacKinnon (1992) with similar K+ solutions.
We also determined the apparent Kd for channel block in solutions in which all the K+ was replaced by equimolar substitution with Rb+ (○). The voltage dependence all but disappeared with Rb+ ions replacing K+ as the charge carriers. The electrical distance parameter, δ, was estimated to be 0.049 ± 0.045 in these Rb+ solutions.
Subsequent experiments revealed that the determinant factor for a voltage sensitive apparent Kd for external TEA block was the presence of internal K+. As shown in Fig. 2 (▴), the voltage dependence was preserved in solutions with external Rb+ but with internal K+ (δ = 0.19). TEA block was essentially voltage insensitive with external K+ and internal Rb+ (▿, δ = 0.033).
Contrary to previous suggestions (Heginbotham and MacKinnon, 1992), the lack of voltage dependence of external TEA block with internal Rb+ ions presented in Fig. 2 suggests that TEA may not penetrate far enough into the pore selectivity filter to be directly influenced by the membrane electric field. Rather, the voltage dependence of block seen with K+ solutions may arise because K+ but not Rb+ ions must move through some part of the membrane electric field before TEA can block the pore. Thus, in the context of Eq. 2, the electrical distance parameter, δ, or the apparent affinity at zero-voltage, K 0, or both might depend on the concentration of external or internal K+ ions (or both). In an earlier study (Thompson and Begenisich, 2001) we found that K 0 is insensitive to changes in external K+ over the 200-fold range of 0.2 to 40 mM. To determine if the electrical distance parameter was sensitive to external K+, we determined the K app for TEA block in solutions with 0.2 and 40 mM external K+ with the same type of analysis as illustrated in Fig. 2. We found values of the electrical distance parameter, δ, of 0.18 ± 0.008 and 0.17 ± 0.002 for solutions with 0.2 and 40 mM external K+, respectively, quite similar to the value of 0.21 for the 5 mM external K+ data of Fig. 2 (▪). Thus, external TEA block appears to be entirely insensitive to external K+ concentrations over the 200-fold range of 0.2 to 40 mM.
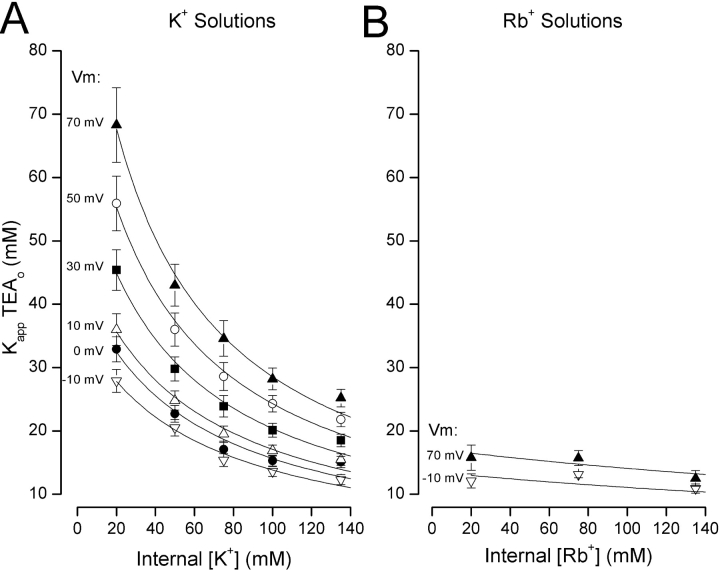
In contrast to the total lack of effect of changes in external K+ on external TEA block, the K app for external TEA is reciprocally related to the internal K+ concentration as illustrated in Fig. 3 A. We previously demonstrated (Thompson and Begenisich, 2001) this relationship at 0 mV, which is reproduced here in Fig. 3 A (•). The other data in Fig. 3 A show that the reciprocal relationship between the K app for external TEA block and internal K+ seen at 0 mV occurred for other voltages as well. Indeed, the steepness of this reciprocal relationship increased at more depolarized potentials.
Figure 3.
Sensitivity of apparent affinity for external TEA block to internal K+ and Rb+. (A) Internal K+ dependence of K app for TEA block at the indicated membrane potentials. Lines are fits of Eq. 4 to the data with parameters as described in text. Data at 0 mV (•) from Thompson and Begenisich (2001). (B) Internal Rb+ dependence of K app for TEA block at the indicated membrane potentials. Lines are simulations from Eq. 4 with parameters as described in text.
Thus, with increased internal K+, block by external TEA increases—exactly the opposite result expected from classical models of multiion pores (e.g., Hille and Schwarz, 1978) in which internal K+ ions would pass through the pore, compete with external TEA, and so reduce block. We have shown previously that this behavior is expected if TEA block of K+ efflux promotes internal K+ occupancy of a site near the inner end of the pore (Thompson and Begenisich, 2001). Internal K+ ions have essentially no effect on internal TEA block in the absence of external TEA, but act like mutually exclusive competitors in the presence of external TEA (Thompson and Begenisich, 2001). Thus, internal K+ significantly occupies a site in the inner end of the pore (the internal TEA site) ONLY when external TEA inhibits K+ efflux. This concept can be quantitatively described by the following scheme:
That is, external TEA binds to the channel with an affinity constant, K T, and the TEA-blocked channel by inhibiting K+ efflux through the pore, placing the inner end of the pore in true equilibrium with the internal aqueous solution. Internal K+ ions can then occupy the internal site with an affinity, K K. Since, as noted above, K+ ions rarely occupy the internal site in the absence of external TEA, we can exclude this state from Scheme I .
SCHEME I.
In this scheme, at any concentration of TEA and internal K+, the channels will be distributed among three states: unblocked, blocked-without-internal-K+, and blocked-with-internal K+. As the internal K+ concentration increases at a particular concentration of TEA, a larger fraction of the channels will occupy the blocked-with-internal K+ state, reducing the fraction of channels in the unblocked state. Thus, this mass action effect makes it appear that external TEA becomes more effective at increased concentrations of internal K+. In mathematical terms, the apparent affinity for external TEA block has the following form (Thompson and Begenisich, 2001):
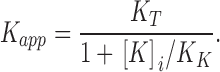 |
(3) |
This equation predicts the reciprocal relationship between the apparent affinity for external TEA and the internal K+ concentration seen at all potentials between −10 and 70 mV (Fig. 3 A). We did a similar investigation with Rb+ solutions and found (Fig. 3 B) that the apparent Kd for external TEA block was essentially insensitive to internal Rb+ concentration and membrane potential. Therefore, as previously suggested (Thompson and Begenisich, 2001), the internal site that can be readily occupied by K+ ions when external TEA blocks pore efflux appears to have a negligible affinity for Rb+ ions.
The almost total lack of Rb+ or voltage sensitivity of TEA block in Rb+ solutions (Fig. 3 B) suggests that the intrinsic affinity of external TEA for the Shaker pore has little or no voltage dependence and may have a value near 12 mM. It is, of course, possible that TEA binding in Rb+ solutions is voltage dependent but is coupled to some other process (e.g., Rb+ ion movement) with an exactly opposite voltage dependence, such that TEA block just appears to be entirely voltage independent. While possible, it doesn't seem likely that two such processes would be “equal but opposite” over the 80 mV voltage and almost sevenfold Rb+ concentration ranges.
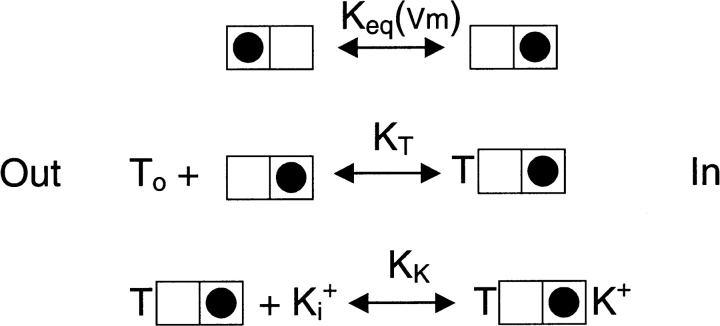
Thus, if the intrinsic binding of external TEA to the pore is voltage independent, then the voltage sensitivity of TEA block in K+ solutions must arise from a coupling of block with some voltage dependent process (or processes). The data of Fig. 3 A clearly show that external TEA block is coupled to some process involving internal K+ ions and our analysis (Eq. 3) suggests that this is due to K+ occupancy of an internal site in TEAo-blocked channels. If this were the source of the voltage dependence of external TEA block, then this voltage sensitivity should disappear in low internal K+ solutions. Inspection of the data of Fig. 3 A reveals that the voltage dependence of external TEA block actually increased at low internal K+. Thus, external TEA block must be coupled to another voltage-dependent process in addition to its coupling to internal K+ ion occupancy of the internal site. We suggest that this other process is a voltage-dependent rearrangement of K+ ions in the pore selectivity filter that is required to allow block by external TEA. This process, combined with the scheme described above for the coupling of external TEA block to internal K+ ions, leads to the following diagram for block by external TEA:
where the first line of the diagram represents a K+ ion (filled circle) occupying two different positions within the selectivity filter which is within the membrane electric field. Therefore, the equilibrium constant controlling occupancy of the two states is, as indicated, a function of membrane potential. According to this scheme (second line), external TEA can only block the pore when the K+ ion is in the rightmost location in the filter. Finally, the last line illustrates the concept that block of K+ efflux by external TEA allows K+ ions from the internal solution to occupy a site toward the inner end of the pore.
External TEA blocks the pore in this scheme according to Eq. 1 with an apparent affinity, K app, given by:
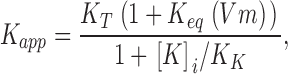 |
(4) |
where K T is the intrinsic affinity of TEA for its receptor in the outer part of the K+ channel pore, K eq(Vm) is the equilibrium constant controlling the distribution of K+ ions in the selectivity filter, and K K is the apparent affinity constant for binding of internal K+ ions to the pore. As discussed above, K T is likely to have a value near 12 mM, independent of membrane potential. While much of the voltage dependence of external TEA block is likely a result of the voltage-dependent distribution of K+ ions in the selectivity filter through the K eq(Vm) term, pore occupancy by internal K+ ions, represented by the K K term, may be voltage-dependent as well.
A more complex model could be considered in which TEA binds to the pore with two different affinities depending on the position of the K+ ion in the selectivity filter. However, we will show that the simpler model can account for the data without any additional complexities. It should, however, be noted that the selectivity filter almost certainly contains more than a single K+ ion occupying two positions (Morais-Cabral et al., 2001) and so the parameter, K eq, in this model represents the apparent equilibrium constant for the aggregate movement of K+ ions necessary to allow external TEA binding. This issue is considered in more detail in discussion.
As a test of the model, we fit Eq. 4 to the data in Fig. 3 A (lines) at each membrane voltage. As noted above, the apparent affinity for TEA block in Fig. 1 appears to asymptotically approach a value near 12 mM that provides an estimate for the intrinsic TEA affinity, K T. With this fixed value for K T, this simple equation is able to quantitatively account for the internal K+ sensitivity of external TEA block at all tested voltages and internal K+ concentrations. From these fits we obtained estimates for both the voltage dependence of the apparent equilibrium for K+ ions in the selectivity filter, K eq(Vm), and for internal K+ ion occupancy, K K. These values, determined at the indicated membrane potentials, are illustrated in Fig. 4 .
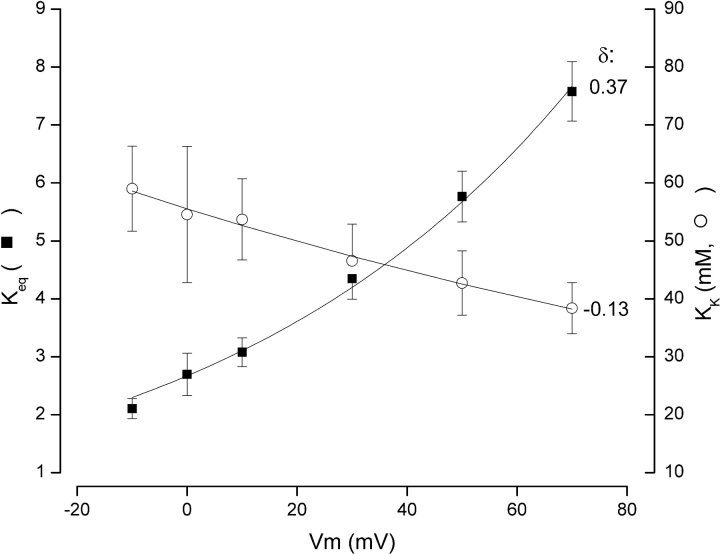
Figure 4.
Voltage dependence of the selectivity filter effective K+ equilibrium, K eq, and the inner vestibule K+ site affinity, K K. The K eq (▪) and K K (○) data were obtained as described in text. The lines are fits of Eq. 2 to the data. The electrical distance parameter, δ, for the selectivity filter equilibrium is 0.37, measured from the external side. The electrical distance for the vestibule site is 0.13 into the membrane electric field from the inner side, indicated by the minus sign. Other parameters from the fit are described in the text.
As expected, the equilibrium constant for K+ ion rearrangement in the selectivity filter, K eq, exhibits a strong voltage sensitivity (▪, Fig. 4). Eq. 2 provided a good fit to these data (line) with a K 0 value of 2.7 ± 0.07 and a δ value of 0.37 ± 0.01. That is, the selectivity filter equilibrium constant has a voltage dependence equivalent to a single K+ ion moving through ∼37% of the membrane electric field.
Also shown in Fig. 4 (○) is the voltage dependence of the binding affinity for internal K+ ions, K K. A fit of Eq. 2 to these data indicates that, at 0 mV, the internal K+ site has an affinity of 56 ± 0.5 mM for K+ ions—not far from the 65 mM value reported previously (Thompson and Begenisich, 2001). The value of δ from this fit suggests that this site is located ∼13% into the membrane electric field from the internal side.
Thus, the effective electrical distance of 0.21 for external TEA block in normal K+ solutions (▪, Fig. 2) appears to be the net result of two oppositely directed voltage-dependent processes: (a) a voltage-dependent rearrangement of K+ ions in the selectivity filter required before TEA can bind, and (b) a voltage-dependent occupancy of an internal K+ site after TEA binding. The fact that external TEA block was almost entirely insensitive to membrane potential in Rb+ solutions or to internal Rb+ concentration (Fig. 3 B) is, according to this analysis, the result of two differences between Shaker pore occupancy by Rb+ and K: (a) the equilibrium for Rb+ occupancy of the selectivity filter favors the inner site, and (b) the ion binding site very near the inner end of the pore has a very low affinity for Rb+ ions. Indeed, the lines in Fig. 3 B are simulations from Eq. 4 with the same electrical distance value of 0.37 as for K+ ions but with an 18-fold lower value for the K 0 part of K eq and with a value of 450 mM for the affinity of the internal site for Rb+ ions. That is, voltage controls the distribution of Rb+ in the selectivity filter just as it does K+ ions but some property of the selectivity filter biases this distribution to favor the more internal position. The very small value of K 0 (compared with unity in the numerator of Eq. 4) minimizes the voltage dependence of K app with Rb+ solutions even with the same value of δ as for K+ ions.
DISCUSSION
The results of this study showed that block of the Shaker pore by external TEA was voltage dependent only in solutions with internal K+ ions. The magnitude and voltage sensitivity of block was reciprocally related to the internal K+ concentration. External TEA block was essentially insensitive to membrane voltage in Rb+ solutions and insensitive to changes in internal Rb+ concentration. We showed that these properties are quantitatively consistent with a specific mechanism for external TEA block: (a) external TEA binding to the pore has no intrinsic voltage dependence; (b) external TEA cannot block unless ions in the selectivity filter are in a certain configuration disfavored at positive membrane voltages; and (c) when efflux is inhibited by external TEA block, internal ions occupy a site very near the inner end of the pore. TEA block in K+ and Rb+ solutions differ because, in this view, these two ions occupy the Shaker pore quite differently: Rb+ ions preferentially occupy the selectivity filter in a manner that allows external TEA block and the internal ion binding site exhibits a strong preference for K+ but not Rb+ ions.
How can ion occupancy of certain sites in the selectivity filter control external TEA block? One way, of course, is that occupancy of a certain configuration could induce a conformational change at the TEA receptor. Alternatively, ion occupancy of certain sites in the selectivity filter may, through steric and/or electrostatic constraints, effect external TEA block. This latter view is consistent with the physical picture illustrated in Scheme II .
SCHEME II.
We suggest that when the permeant ion in the selectivity filter is in the outermost position, external TEA cannot bind. The simplest mechanism for this is destabilization of TEA because of electrostatic repulsion from the permeant ion; however, other mechanisms are possible, including unfavorable steric constraints from associated water molecules (not depicted in the figure). Thus, in this simple model, TEA ions can block the channel only when the ion in the selectivity filter is away from the most external site. Since membrane potential controls the distribution of K+ ions in these two sites, TEA block is voltage dependent even though it binds outside the membrane electric field.
In Scheme II, K+ ions from the cytoplasmic solution can occupy some location in the inner vestibule of the pore. In a conducting channel, ions spend little time occupying this area. However, when TEA blocks the external end of the pore, the inner end comes into equilibrium with the internal solution. Under these conditions, K+ ions occupy the inner vestibule with an affinity of a few 10's of mM depending on membrane voltage (Fig. 4). This vestibule has a negligible affinity for Rb+ ions. These properties account (Fig. 3) for the decrease in K app for TEAo block with increased internal K+ and the lack of effect with Rb+.
According to this picture of the pore, the major source of voltage dependence of external TEA block is the movement of the permeant ion between the two positions in the selectivity filter which are within the membrane electric field. We found that, in K+ solutions, the voltage dependence of external TEA block is equivalent to a single charge crossing 37% of the electric field. The almost complete lack of voltage sensitivity of external TEA block in Rb+ solutions is, in this view, not because Rb+ ions don't occupy the same sites in the selectivity filter, but because the equilibrium position for these ions favors occupancy of the inner site.
According to recent analyses of KcsA crystallographic data (Morais-Cabral et al., 2001) and molecular dynamic simulations (Berneche and Roux, 2001), the selectivity filter of this channel may often be occupied by two permeant ions making concerted, extremely rapid transitions back and forth between inner and outer configurations. If this is also true for Shaker K+ channels in the conditions used in our current study, then the apparent voltage dependence of external TEA block would represent this aggregate movement with each of the two ions moving over a smaller fraction of the membrane potential. If the sites are equally distributed, then, from our measured voltage dependence, each site in the selectivity filter would be separated by 18–19% of the electric field. Roux et al. (2000) computed the membrane potential profile for KcsA and for a KcsA-like structure with open inner helices as might occur in a conducting channel. These calculations predict that, in a conducting channel, the movement of the two K+ ions from the outer to the inner sites should represent a net crossing of ∼35% of the membrane potential—unreasonably close to our estimate of 37% obtained from the voltage sensitivity of TEA block.
As described in introduction, molecular dynamics simulations (Crouzy et al., 2001) and continuum electrostatic calculations based on the KcsA structure (Roux et al., 2000) predict that external TEA binds outside the membrane electric field. According to these analyses, this is true both for channels with a tyrosine at the “TEA site” (as in KcsA) and for channels with a threonine (as in wild-type Shaker). Our results support this view that the TEA site in Shaker K+ channels with a threonine residue is not within the membrane electric field. The observed voltage dependence of TEA block appears to be coupled to movements of permeant ions through different parts of the pore. Differences in TEA block in K+ and Rb+ solutions results from differences in pore occupancy by these permeant ions.
Acknowledgments
We thank Dr. Robert Dirksen for discussions on several aspects of this study and he and Dr. Bertil Hille for comments on the manuscript.
This work was supported by a grant from the National Science Foundation (IBN-0090662).
Lawrence G. Palmer served as editor.
References
- Armstrong, C.M. 1966. Time course of TEA+-induced anomalous rectification in squid giant axons. J. Gen. Physiol. 50:491–503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Armstrong, C.M., and B. Hille. 1972. The inner quaternary ammonium ion receptor in potassium channels of the node of Ranvier. J. Gen. Physiol. 59:388–400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz, T., and G. Yellen. 1996. a. Two functionally distinct subsites for the binding of internal blockers to the pore of voltage-activated K+ channels. Proc. Natl. Acad. Sci. USA. 93:13357–13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baukrowitz, T., and G. Yellen. 1996. b. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science. 271:653–656. [DOI] [PubMed] [Google Scholar]
- Berneche, S., and B. Roux. 2001. Energetics of ion conduction through the K+ channel. Nature. 414:73–77. [DOI] [PubMed] [Google Scholar]
- Crouzy, S., S. Berneche, and B. Roux. 2001. Extracellular blockade of K+ channels by TEA: results from molecular dynamics simulations of the KcsA channel. J. Gen. Physiol. 118:207–218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D.A., J. Morais-Cabral, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Goldin, A.L. 1992. Maintenance of Xenopus laevis and oocyte injection. Methods Enzymol. 207:266–279. [DOI] [PubMed] [Google Scholar]
- Gomez-Hernandez, J.M., C. Lorra, L.A. Pardo, W. Stuhmer, O. Pongs, S.H. Heinemann, and A.A. Elliott. 1997. Molecular basis for different pore properties of potassium channels from the rat brain Kv1 gene family. Pflugers Arch. 434:661–668. [DOI] [PubMed] [Google Scholar]
- Heginbotham, L., and R. MacKinnon. 1992. The aromatic binding site for tetraethylammonium ion on potassium channels. Neuron. 8:483–491. [DOI] [PubMed] [Google Scholar]
- Hille, B. 1967. The selective inhibition of delayed potassium currents in nerve by tetraethylammonium ion. J. Gen. Physiol. 50:1287–1302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hille, B., and W. Schwarz. 1978. Potassium channels as multi-ion single-file pores. J. Gen. Physiol. 72:409–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi, T., W.N. Zagotta, and R.W. Aldrich. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:533–538. [DOI] [PubMed] [Google Scholar]
- Kavanaugh, M.P., R.S. Hurst, J. Yakel, M.D. Varnum, J.P. Adelman, and R.A. North. 1992. Multiple subunits of a voltage-dependent potassium channel contribute to the binding site for tetraethylammonium. Neuron. 8:493–497. [DOI] [PubMed] [Google Scholar]
- Kavanaugh, M.P., M.D. Varnum, P.B. Osborne, M.J. Christie, A.E. Busch, J.P. Adelman, and R.A. North. 1991. Interaction between tetraethylammonium and amino acid residues in the pore of cloned voltage-dependent potassium channels. J. Biol. Chem. 266:7583–7587. [PubMed] [Google Scholar]
- Kirsch, G.E., M. Taglialatela, and A.M. Brown. 1991. Internal and external TEA block in single cloned K+ channels. Am. J. Physiol. 261:C583–C590. [DOI] [PubMed] [Google Scholar]
- Luzhkov, V.B., and J. Aqvist. 2001. Mechanisms of tetraethylammonium ion block in the KcsA potassium channel. FEBS Lett. 495:191–196. [DOI] [PubMed] [Google Scholar]
- MacKinnon, R., and G. Yellen. 1990. Mutations affecting TEA blockade and ion permeation in voltage-activated K+ channels. Science. 250:276–279. [DOI] [PubMed] [Google Scholar]
- Morais-Cabral, J.H., Y. Zhou, and R. MacKinnon. 2001. Energetic optimization of ion conduction rate by the K+ selectivity filter. Nature. 414:37–42. [DOI] [PubMed] [Google Scholar]
- Roux, B., S. Berneche, and W. Im. 2000. Ion channels, permeation, and electrostatics: insight into the function of KcsA. Biochemistry. 39:13295–13306. [DOI] [PubMed] [Google Scholar]
- Thompson, J., and T. Begenisich. 2000. Interaction between quaternary ammonium ions in the pore of potassium channels. Evidence against an electrostatic repulsion mechanism. J. Gen. Physiol. 115:769–782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson, J., and T. Begenisich. 2001. Affinity and location of an internal K+ ion binding site in shaker K channels. J. Gen. Physiol. 117:373–384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woodhull, A.M. 1973. Ionic blockage of sodium channels in nerve. J. Gen. Physiol. 61:687–708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yellen, G., M.E. Jurman, T. Abramson, and R. MacKinnon. 1991. Mutations affecting internal TEA blockade identify the probable pore-forming region of a K+ channel. Science. 251:939–942. [DOI] [PubMed] [Google Scholar]