Abstract
The interlobular duct cells of the guinea-pig pancreas secrete HCO3 − across their luminal membrane into a HCO3 −-rich (125 mM) luminal fluid against a sixfold concentration gradient. Since HCO3 − transport cannot be achieved by luminal Cl−/HCO3 − exchange under these conditions, we have investigated the possibility that it is mediated by an anion conductance. To determine whether the electrochemical potential gradient across the luminal membrane would favor HCO3 − efflux, we have measured the intracellular potential (Vm) in microperfused, interlobular duct segments under various physiological conditions. When the lumen was perfused with a 124 mM Cl−-25 mM HCO3 − solution, a condition similar to the basal state, the resting potential was approximately −60 mV. Stimulation with dbcAMP or secretin caused a transient hyperpolarization (∼5 mV) due to activation of electrogenic Na+-HCO3 − cotransport at the basolateral membrane. This was followed by depolarization to a steady-state value of approximately −50 mV as a result of anion efflux across the luminal membrane. Raising the luminal HCO3 − concentration to 125 mM caused a hyperpolarization (∼10 mV) in both stimulated and unstimulated ducts. These results can be explained by a model in which the depolarizing effect of Cl− efflux across the luminal membrane is minimized by the depletion of intracellular Cl− and offset by the hyperpolarizing effects of Na+-HCO3 − cotransport at the basolateral membrane. The net effect is a luminally directed electrochemical potential gradient for HCO3 − that is sustained during maximal stimulation. Our calculations indicate that the electrodiffusive efflux of HCO3 − to the lumen via CFTR, driven by this gradient, would be sufficient to fully account for the observed secretory flux of HCO3 −.
Keywords: pancreatic ducts, membrane potentials, bicarbonate ion, cystic fibrosis transmembrane conductance regulator, sodium-bicarbonate cotransporter
INTRODUCTION
Pancreatic duct cells secrete a HCO3 −-rich, isotonic fluid in response to stimulation with secretin (Case and Argent, 1993). It is now generally accepted that HCO3 − accumulation across the basolateral membrane is achieved both by Na+-HCO3 − cotransport and by Na+- H+ exchange (Ishiguro et al., 2001). The mechanism of HCO3 − secretion across the luminal membrane is less clear. Electrophysiological studies of rat pancreatic ducts have led to the proposal that HCO3 − secretion is mediated by a Cl−-HCO3 − exchanger operating in parallel with the cystic fibrosis transmembrane conductance regulator (CFTR)* Cl− channel (Gray et al., 1988; Novak and Greger, 1988b). In this model, HCO3 − enters the lumen in exchange for Cl−, which is supplied to the lumen by CFTR (Novak and Pahl, 1993). In support of this model, patch-clamp electrophysiology (Gray et al., 1989) and immunolocalization studies (Crawford et al., 1991; Marino et al., 1991) have confirmed the presence of CFTR in the luminal membrane of human pancreatic ducts. In addition, measurements of intracellular pH have revealed evidence of cAMP-stimulated Cl−-HCO3 − exchange at the luminal membrane in several species (Lee et al., 1999; Ishiguro et al., 2000), although the molecular identity of the exchanger has not yet been determined (Lohi et al., 2000; Roussa et al., 2001). A recent study of CFTR mutants expressed in HEK293 cells suggests that CFTR may also have important regulatory effects on anion exchangers (Choi et al., 2001).
However, this model can only account for HCO3 − secretion in species like the rat, in which the maximal HCO3 − concentration in the juice is ∼70 mM (Sewell and Young, 1975; Sohma et al., 2000). It cannot explain the much higher concentrations of HCO3 −, often as high as 140 mM, observed in the pancreatic juice of humans (Domschke et al., 1976), dogs, and guinea-pigs (Case and Argent, 1993). Maximal stimulation of the guinea-pig pancreas with secretin in vivo evokes the production of a juice containing ∼140 mM HCO3 − and only 20 mM Cl− (Padfield et al., 1989). Furthermore, in interlobular duct segments isolated from guinea-pig pancreas, stimulated HCO3 − secretion appears not to be dependent on the presence of Cl− in the lumen (Ishiguro et al., 1996b). We have also shown that secretin can evoke a HCO3 −-rich secretion even when the lumen is first filled by microinjection with a solution containing 125 mM HCO3 − and 24 mM Cl− (Ishiguro et al., 1998). With the luminal membrane facing such a high concentration of HCO3 − and a relatively low concentration of Cl−, an active Cl−-HCO3 − exchanger at the luminal membrane would mediate HCO3 − absorption rather than secretion. To avoid this, the activity of the luminal Cl−-HCO3 − exchanger appears to be inhibited by the high luminal HCO3 − concentration (Ishiguro et al., 2000).
In searching for other mechanisms that might account for HCO3 − secretion against the steep concentration gradient at the luminal membrane, we have measured intracellular HCO3 − ([HCO3 −]i) and Cl− ([Cl−]i) concentrations in microperfused interlobular ducts isolated from guinea-pig pancreas (Ishiguro et al., 2000, 2002). When the duct lumen was perfused with a solution containing 125 mM HCO3 − and 24 mM Cl−, forskolin stimulation reduced [Cl−]i to as little as ∼7 mM. This seems to be a consequence of Cl− efflux via CFTR at the luminal membrane and the limited capacity of the basolateral Cl− uptake mechanisms (Ishiguro et al., 2002). In contrast, [HCO3 −]i is maintained at ∼20 mM by powerful acid/base transporters at the basolateral membrane. The depletion of intracellular Cl− during maximal stimulation would favor an HCO3 −-rich secretion if an anion conductance with sufficient permeability to HCO3 − were present at the luminal membrane. HCO3 − secretion would be further facilitated if, as has been suggested, the Cl− conductance of CFTR is suppressed by the high luminal HCO3 − concentration (O'Reilly et al., 2000; Sohma et al., 2000). Whether HCO3 − secretion via a luminal membrane conductance is feasible will also depend on the direction and magnitude of the electrochemical potential gradient for HCO3 −. To evaluate the gradient it is necessary to measure the membrane potential.
In the present study, we have measured the intracellular potential in microperfused interlobular ducts isolated from guinea-pig pancreas. Our experiments have focused primarily on the changes in membrane potential, both transient and steady-state, after stimulation with cAMP and following alterations in the anion composition of the luminal perfusate. We have also examined the contribution of the basolateral Na+-HCO3 − cotransporter to the driving force for luminal HCO3 − secretion.
MATERIALS AND METHODS
Isolation and Culture of Interlobular Ducts
Female Hartley guinea-pigs (350–450 g) were killed by cervical dislocation. All procedures were approved by the Ethical Committee of Nagoya University. The body and tail of the pancreas were removed, and interlobular ducts (diameter 100–150 μm, length 800–1200 μm) were isolated and cultured overnight as described previously (Ishiguro et al., 1996a).
Solutions
The standard Hepes-buffered solution contained (mM): 140 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose, and 10 Hepes, equilibrated with 100% O2. pH was adjusted at 37°C to 7.4 or 8.2 by addition of NaOH. The standard HCO3 −-buffered solution (high Cl−) contained (mM): 115 NaCl, 5 KCl, 1 CaCl2, 1 MgCl2, 10 D-glucose, and 25 NaHCO3, equilibrated with 95% O2 + 5% CO2. The low Cl−-low HCO3 − solution contained 25 mM HCO3 −, 24 mM Cl−, and 100 mM glucuronate, and was equilibrated with 95% O2 + 5% CO2. In each case, pH was adjusted to 7.4 at 37°C. The high-HCO3 − solution contained 125 mM HCO3 − and 24 mM Cl−, was equilibrated with 95% O2 + 5% CO2, and had a pH of ∼8.2. The concentrations of the other solutes in these modified solutions were unchanged.
The Na+-free, HCO3 −-buffered solution (high Cl−) contained 115 mM N-methyl-D-glucamine chloride (NMDG Cl) in place of NaCl, 25 mM choline bicarbonate in place of NaHCO3, and 10 μM atropine (to avoid any muscarinic effects resulting from the high concentration of choline), and was equilibrated with 95% O2 + 5% CO2. The pH was adjusted to 7.4. The Na+-free, high-HCO3 − solution contained 15 mM NMDG Cl, 125 mM choline bicarbonate, and 50 μM atropine, and was equilibrated with 95% O2 + 5% CO2. The pH was adjusted to 8.2.
Microperfusion of Isolated Ducts
The duct lumen was microperfused using a concentric pipette assembly attached to one end of the duct as described previously (Ishiguro et al., 1999). Effluent luminal perfusate emerging from the open end of the duct was washed away by the flow of solution through the bath which was continuously perfused at 3 ml min−1 and maintained at 37°C.
Measurement of Membrane Potential
Membrane potential was measured by impaling the basolateral membrane of microperfused ducts with glass microelectrodes filled with 0.5 M KCl. The microelectrodes were drawn on a micropipette puller (Sutter Instruments Co.) from borosilicate glass with an internal filament (o.d., 1.0 mm; i.d., 0.78 mm; Clark Electromedical Instruments). Microelectrodes, connected to an electrometer (World Precision Instruments, Inc.), were advanced toward the duct at an angle of ∼30° from the axis of the duct and impalement was obtained with the aid of a water pressure-operated micromanipulator (Narishige). An indifferent Ag-AgCl electrode (Radiometer Copenhagen) made contact with the bath solution through a 3 M KCl agar bridge. The intracellular potential (Vm) was measured with reference to the grounded bath. The criteria for successful impalements were: (a) a sudden deflection of Vm to a value more negative than −40 mV when the electrode entered the cell, (b) a stable resting potential lasting longer than 30 s, and (c) the return of the potential to the same baseline upon withdrawal of the microelectrode.
Since one end of the duct was always open to the bath, the apical and basolateral membrane potentials were equal under the conditions of the experiments. However, this is unlikely to have adversely affected our measurements of Vm. The pancreatic duct has a leaky epithelium (88 Ω cm2 in the rat; Novak and Greger, 1988a) and the apical and basolateral membrane potentials in vivo will not differ by more than a few millivolts. In support of this, the reported transepithelial potential differences of intra/interlobular ducts range from 1–5 mV (lumen negative) in isolated rat ducts (Novak and Greger, 1991) to 5–9 mV (lumen negative) recorded in situ in intact rabbit pancreas (Schulz, 1980).
Materials
Dihydro-4,4′-diisothiocyanatostilbene-2,2′-disulphonic acid (H2DIDS) was obtained from Molecular Probes, dibutyryl cyclic AMP (dbcAMP) was from Sigma-Aldrich, and secretin was from the Peptide Institute.
Statistics
Averaged data are presented as the mean ± SEM. Tests for statistically significant differences were made with Student's t test for paired or unpaired data as appropriate.
RESULTS
For measurements of Vm in guinea-pig pancreatic duct cells under resting conditions, the duct lumen and bath were perfused initially with a HCO3 −-buffered solution containing 124 mM Cl− and 25 mM HCO3 −. A representative experiment is shown in Fig. 1 A. After impalement of the basolateral membrane, the intracellular potential generally reached a steady value within 30 s. Under these conditions, the mean steady-state value of Vm in eight experiments was −60.6 ± 2.6 mV.
Figure 1.
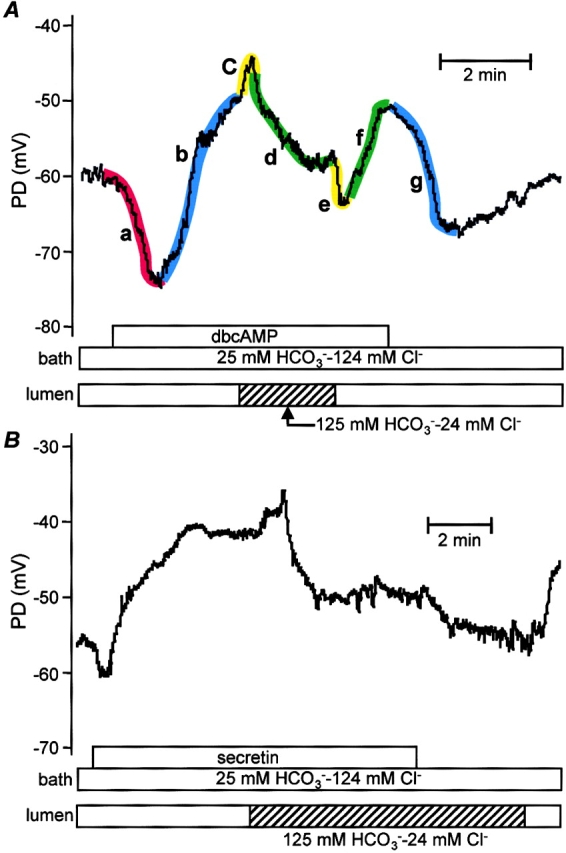
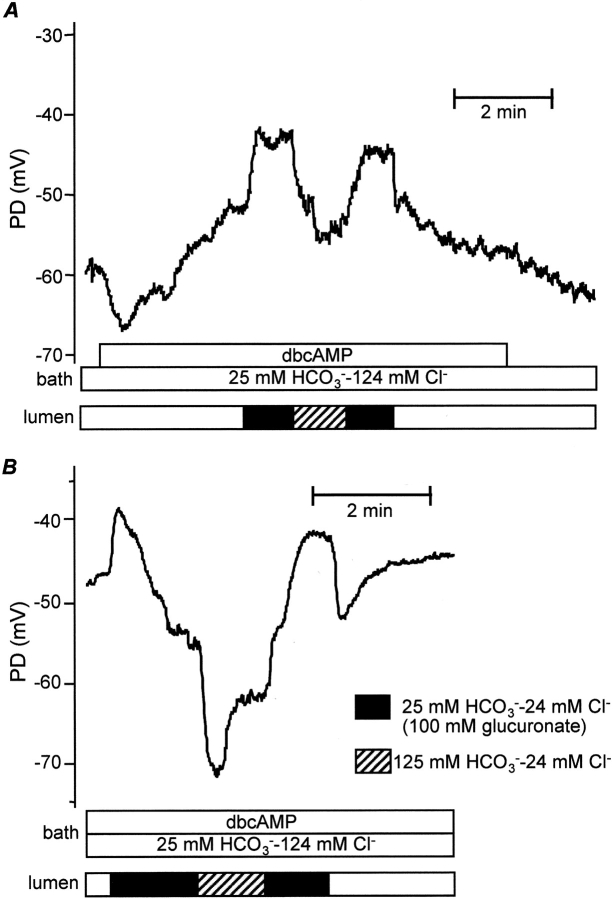
Effects of high luminal HCO3 − concentration during stimulation with dbcAMP (A) and secretin (B). Intracellular potential (PD) was recorded in microperfused interlobular duct segments isolated from guinea-pig pancreas. The bath and lumen of the ducts were perfused initially with the standard HCO3 −-buffered solution containing 124 mM Cl− and 25 mM HCO3 −. The duct was stimulated by addition of dbcAMP (0.1 mM, A) or secretin (1 nM, B) to the bath. The luminal perfusate was then switched to the high HCO3 − solution (24 mM Cl− and 125 mM HCO3 −). Segments labeled a–g are described in the text. Representative of seven (A) and four (B) experiments, respectively.
Stimulation with Dibutyryl cAMP and Secretin
To simulate the effects of maximal stimulation with secretin, 0.1–0.5 mM dibutyryl cyclic AMP (dbcAMP), a membrane-permeable analogue of cAMP, was added to the bath. As shown in Fig. 1 A, Vm underwent a biphasic change consisting of a transient hyperpolarization (Fig. 1 A, a) followed by a sustained depolarization (b). On average, the hyperpolarization was 6.6 ± 1.4 mV in magnitude and its duration varied from 60 to 90 s. The subsequent depolarization took Vm to a mean value of −52.5 ± 2.3 mV after 2–3 min of stimulation. This represents a small but significant depolarization when compared with the prestimulation value (n = 8, P < 0.01). When dbcAMP was later withdrawn from the bath perfusate, Vm returned to its more negative resting value (g).
Stimulation with secretin (1 nM), a physiological secretagogue that acts by elevating intracellular cAMP, evoked a similar pattern of changes in Vm (Fig. 1 B). A transient hyperpolarization of 3.8 ± 0.6 mV (n = 4) and ∼1 min duration was followed by depolarization to a steady-state value of −49.8 ± 3.7 mV after 3–4 min of stimulation. As with dbcAMP, this represents a significant depolarization when compared with the prestimulation value (−57.5 ± 3.0 mV, P < 0.05).
Since cAMP is known to activate CFTR at the luminal membrane, it was anticipated that, under these conditions, stimulation would bring about a depolarization as a result of the efflux of Cl− to the lumen. However, the preceding, transient hyperpolarization was unexpected and the underlying mechanism is less obvious. One possibility, based on our previous observation that Na+- HCO3 − cotransport contributes to HCO3 − uptake at the basolateral membrane (Ishiguro et al., 1996a), is that the hyperpolarization is due to the electrogenicity of the Na+-HCO3 − cotransporter, which becomes activated at the onset of secretion. Alternatively, the hyperpolarization might arise through an increase in the K+ conductance of the basolateral membrane. It therefore seems likely that the steady-state value of Vm that is attained during sustained stimulation (approximately −52 mV) represents a balance between a number of depolarizing and hyperpolarizing influences.
Substitution of Luminal Cl− with HCO3 −
During free-flow secretion in vivo, the concentration of HCO3 − in the lumen rises several fold and the luminal Cl− concentration falls correspondingly. To simulate this more realistic physiological situation and estimate the steady-state value of Vm when the luminal membrane faces a HCO3 −-rich fluid, we switched the luminal perfusate to a high HCO3 − solution containing 125 mM HCO3 − and 24 mM Cl−. The composition of the bath solution remained unchanged.
In the experiment shown in Fig. 1 A, the luminal perfusate was switched to the high HCO3 − solution during sustained stimulation with dbcAMP. The immediate response to the solution change was a small, transient depolarization of 4.0 ± 0.5 mV (n = 8) (Fig. 1 A, c). Vm then hyperpolarized (d) by 9.3 ± 2.4 mV (P < 0.01) to a new steady-state value of −61.8 ± 2.4 mV after 2 min. These changes in Vm were reversed when the luminal perfusate was switched back to the high Cl− solution. In this case there was a transient hyperpolarization (e) followed by a depolarization (f) toward the value obtained previously with high luminal Cl− concentration.
A similar pattern of changes was observed during stimulation with 1 nM secretin (Fig. 1 B). Switching the luminal perfusate to the high HCO3 − solution caused a small, transient depolarization of 3.2 ± 0.7 mV (n = 4) followed by a hyperpolarization of 10.8 ± 2.2 mV (P < 0.05) to −60.6 ± 5.5 mV.
For comparison, we also examined the effect of switching the luminal perfusate to the high HCO3 − solution in unstimulated ducts (Fig. 2, A and B) . In this case, Vm hyperpolarized by 8.8 ± 1.0 mV to −64.3 ± 2.7 mV after 1 min (n = 11, P < 0.01) and the initial, transient depolarization was either absent or very small. Stimulation with dbcAMP (Fig. 2 A) or with secretin (Fig. 2 B) caused a transient hyperpolarization of 5.0 ± 0.9 mV (n = 11) which lasted ∼60–90 s. In contrast to the experiments with high luminal Cl−, the steady-state value of Vm (−62.1 ± 2.6 mV) was only slightly depolarized when compared with the value before stimulation (−64.3 ± 2.7 mV, P < 0.05). This suggests that stimulation does not have as large an effect on steady-state Vm when the luminal membrane faces an HCO3 −-rich fluid.
Figure 2.
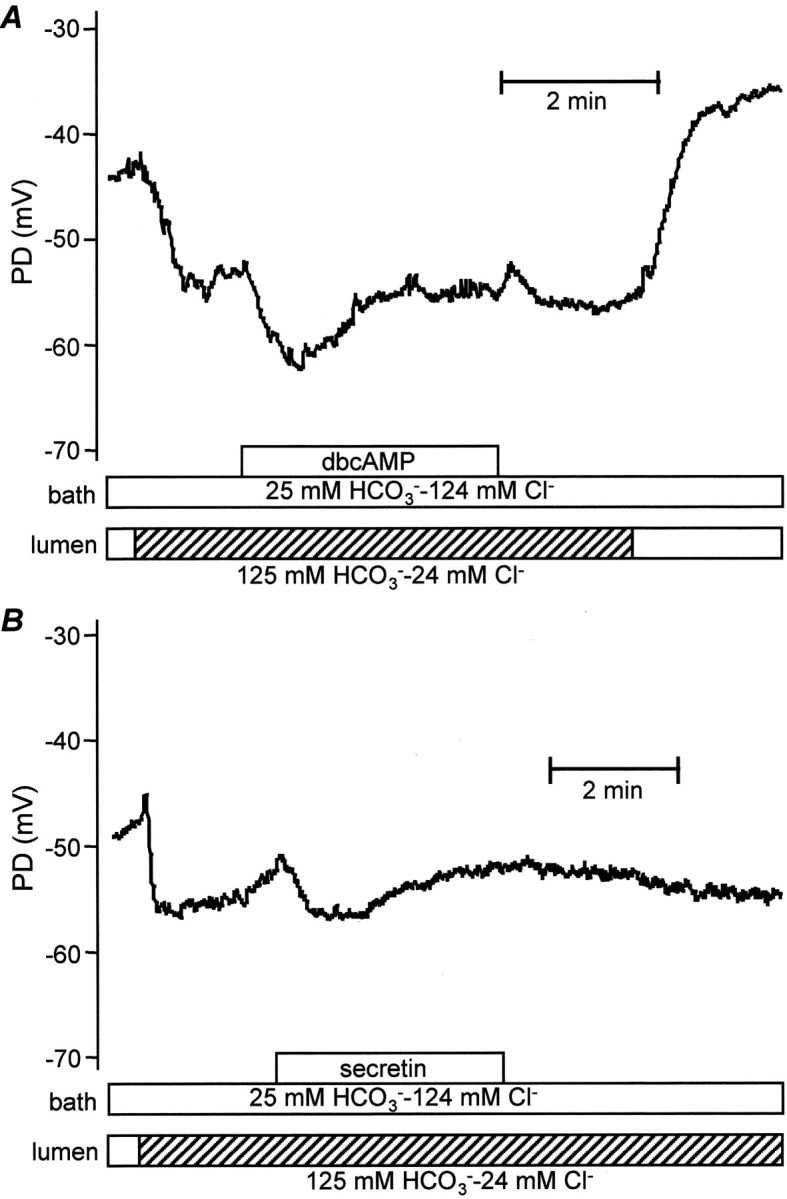
Stimulation with dbcAMP (A) and secretin (B) in the presence of high luminal HCO3 − concentration. The bath and lumen were perfused initially with the standard HCO3 −-buffered solution. The luminal perfusate was then switched to the high HCO3 − solution (24 mM Cl− and 125 mM HCO3 −) and the duct subsequently stimulated by addition of dbcAMP (0.1 mM, A) or secretin (1 nM, B) to the bath. Representative of eight (A) and four (B) experiments, respectively.
Together, the data in Figs. 1 and 2 indicate that switching the luminal anion composition from high Cl− to high HCO3 − causes Vm to hyperpolarize by ∼9 mV in both unstimulated and stimulated ducts. Furthermore, the depolarizing component of the response to stimulation appears to be significantly reduced in the presence of high luminal HCO3 − concentrations.
Contributions of Luminal Cl− and pH
The changes in Vm that followed the switch from high Cl− to high HCO3 − in the lumen could have resulted either from the change in Cl− concentration, or from the change in HCO3 − concentration, or a combination of the two. Therefore, in the experiments shown in Fig. 3 , we attempted to separate these two effects by first reducing the luminal Cl− concentration from 124 to 24 mM by replacement with 100 mM glucuronate, and with luminal HCO3 − remaining constant at 25 mM. The luminal HCO3 − concentration was subsequently raised from 25 to 125 mM, with luminal Cl− remaining constant at 24 mM.
Figure 3.
Effects of separately lowering luminal Cl− and raising luminal HCO3 − during stimulation with dbcAMP. The bath and lumen were perfused initially with the standard HCO3 −-buffered solution and the duct stimulated by addition of dbcAMP (0.1 mM) to the bath. The luminal perfusate was then switched first to the low Cl−-low HCO3 − solution (24 mM Cl−, 25 mM HCO3 −, and 100 mM glucuronate) and then to the high HCO3 − solution (24 mM Cl− and 125 mM HCO3 −). Two examples from six comparable experiments.
When the luminal Cl− concentration was reduced by glucuronate substitution during stimulation with dbcAMP, Vm depolarized from −48.3 ± 1.1 mV to −39.7 ± 1.8 mV within 20 s (n = 6). Since stimulation with dbcAMP would have activated CFTR and raised the Cl− conductance of the luminal membrane, we would predict that a reduction in luminal Cl−, and the subsequent efflux of Cl− would lead to a depolarization as indeed it did. This might therefore account for the transient depolarization (c) seen in Fig. 1 A and it could also explain its absence in the unstimulated ducts (Fig. 2 A) where the luminal membrane Cl− conductance would have been lower.
The subsequent behavior of Vm, after the reduction in luminal Cl− concentration, varied between different ducts. In some, e.g., Fig. 3 A, Vm remained depolarized. In others, e.g., Fig. 3 B, the depolarization was short-lived and Vm proceeded to repolarize and even to hyperpolarize. Combining all of the experiments, the mean value of Vm at the end of this period was −46.3 ± 4.6 mV. One possible explanation for the repolarization that sometimes followed the lowering of luminal Cl− (as in Fig. 3 B) would be the depletion of intracellular Cl−, which we have shown previously to occur quite rapidly under these conditions (Ishiguro et al., 2002).
When the luminal perfusate was next switched to the high HCO3 − solution (with the luminal Cl− concentration remaining fixed at 24 mM) Vm hyperpolarized by 10.3 ± 1.6 mV to a mean value of −56.6 ± 3.5 mV after 1 min (Fig. 3, P < 0.01). A transient overshoot was sometimes observed as seen in Fig. 3 B. From these data we conclude that the sustained hyperpolarization that follows the switch from high luminal Cl− to high luminal HCO3 − (Fig. 1 A, d) is mainly due to the raised luminal HCO3 − concentration.
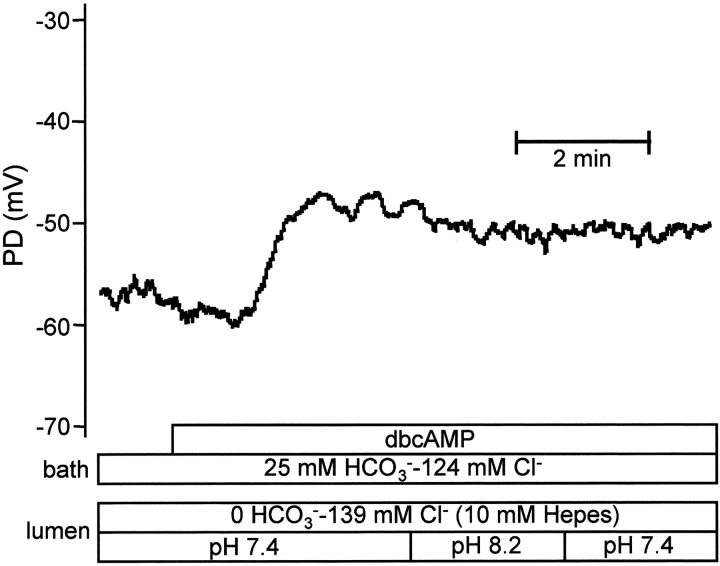
To eliminate the possibility that it was the high luminal pH rather than the high luminal HCO3 − concentration that affected Vm, experiments were performed with the lumen perfused initially with a HCO3 −-free Hepes-buffered solution (pH 7.4) (Fig. 4) . In this case the initial value of Vm was −48.2 ± 1.3 mV (n = 4). In the absence of luminal HCO3 −, stimulation with dbcAMP caused a small hyperpolarization followed by a sustained depolarization. Raising the pH of the luminal perfusate to the value that would normally accompany 125 mM luminal HCO3 − (pH 8.2) had no significant effect on the value of Vm (−49.4 ± 1.4 mV, NS).
Figure 4.
Effects of high luminal pH during stimulation with dbcAMP. The bath was perfused with the standard HCO3 −-buffered solution throughout experiment. The lumen was perfused initially with an HCO3 −-free, Hepes-buffered solution (pH 7.4). Following stimulation with dbcAMP (0.1 mM) the luminal perfusate was switched to the high-pH, Hepes-buffered solution (pH 8.2). Representative of four experiments.
Together, the results shown in Figs. 3 and 4 indicate that it is the high luminal HCO3 − concentration, rather than the reduced Cl− concentration or the elevated luminal pH, that causes the hyperpolarization responsible for segment d in Fig. 1 A.
Contribution of the Basolateral Na+-HCO3 − Cotransporter
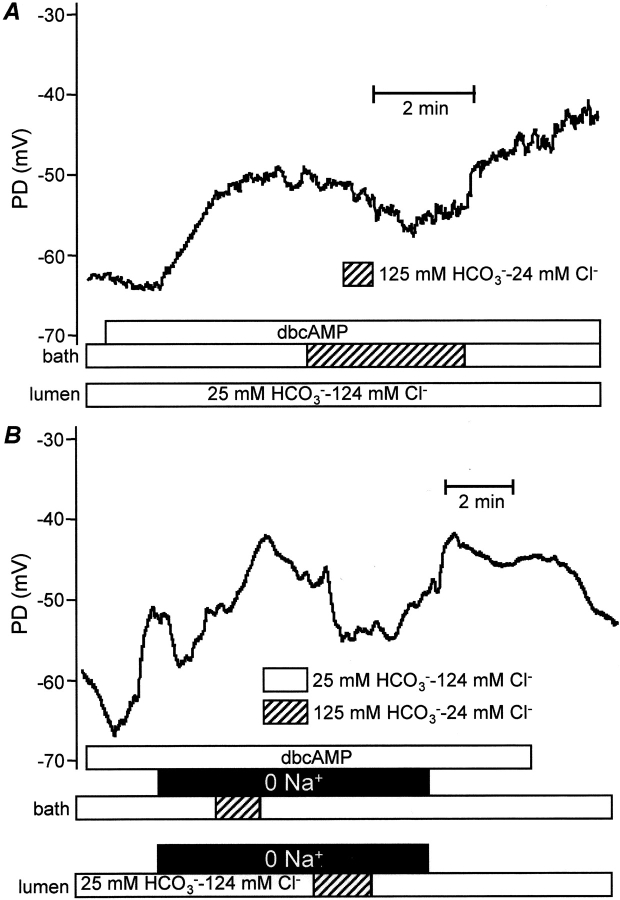
In our previous studies, we have demonstrated that it is Na+-HCO3 − cotransport, rather than Na+-H+ exchange, that plays the dominant role in HCO3 − accumulation across the basolateral membrane during stimulated secretion (Ishiguro et al., 1996a, 1998). Since the pancreatic NBC isoform is believed to be electrogenic, we next examined the effect on Vm of changing the concentrations of HCO3 − and Cl− in the bath. In these experiments, the duct lumen and the bath were perfused initially with the standard HCO3 −-buffered solution containing 124 mM Cl− and 25 mM HCO3 −. The duct was then stimulated with 0.5 mM dbcAMP (Fig. 5 A). When the bath perfusate was switched to the high HCO3 − solution, Vm hyperpolarized from −45.4 ± 3.2 mV to -53.0 ± 2.3 mV (P < 0.05, n = 4). The effect was reversible since Vm returned to a more depolarized value when the bath perfusate was switched back to the high Cl− solution. The hyperpolarization that followed the rise in bath HCO3 − concentration can be explained by increased activity of an electrogenic process either bringing net negative charge into the cell or taking net positive charge out of the cell. Although we cannot exclude other mechanisms, this result is clearly consistent with a significant contribution from the electrogenic Na+-HCO3 − cotransporter at the basolateral membrane.
Figure 5.
Effects of raising bath HCO3 − concentration. The bath and lumen were perfused initially with the standard HCO3 −-buffered solution and the duct stimulated by addition of dbcAMP (0.5 mM) to the bath. The bath perfusate was then switched to the high HCO3 − solution (24 mM Cl− and 125 mM HCO3 −) in the presence (A) or absence (B) of extracellular Na+. B also shows the effect of raising the luminal HCO3 − concentration to 125 mM. Each trace is representative of four experiments.
In support of this conclusion, Fig. 5 B shows the effect of raising the bath HCO3 − concentration in the absence of extracellular Na+. While keeping the Cl− and HCO3 − concentrations unchanged, Na+ was substituted with NMDG and choline in both bath and luminal perfusates to avoid any complications resulting from the imposition of a transepithelial Na+ gradient. The initial response to the Na+-free solutions was a transient hyperpolarization of Vm from −49.9 ± 1.6 mV to −57.3 ± 3.2 mV (P < 0.05, n = 4). This could be due to the efflux of Na+ via various basolateral transporters and channels. However, instead of hyperpolarizing when the bath perfusate was switched to the high-HCO3 − solution, Vm now depolarized by 10.7 ± 1.5 mV to −38.9 ± 3.3 mV (P < 0.01, n = 4), probably as a result of Cl− efflux via the basolateral Cl− conductance that we have reported previously (Ishiguro et al., 2002). These results suggest that the hyperpolarization that follows the rise in bath HCO3 − concentration in Fig. 5 A is dependent on basolateral Na+ and is therefore probably mediated by the Na+-HCO3 − cotransporter. Fig. 5 B also shows that the hyperpolarization associated with an elevated luminal HCO3 − concentration (Fig. 1 A, d) is unaffected by the absence of Na+.
As a further test of our hypothesis, we examined the effect on Vm of basolateral application of H2DIDS, a reversible inhibitor of Na+-HCO3 − cotransport (Fig. 6 A). To simulate physiological conditions, the bath was perfused with the standard HCO3 −-buffered, high Cl− solution and the lumen was perfused with the high HCO3 − solution. The initial changes in Vm after stimulation with dbcAMP were comparable to those already seen in Fig. 2 A. When 0.5 mM H2DIDS was then applied to the bath, Vm gradually depolarized from −63.4 ± 4.3 mV to −52.5 ± 4.9 mV (P < 0.05, n = 4) over a period of 3 min. After H2DIDS was removed, Vm gradually recovered toward its previous value. In addition, pretreatment with H2DIDS (Fig. 6 B) largely abolished the transient hyperpolarization that was associated with the onset of stimulation with dbcAMP (Fig. 2 A). Vm changed by 2.0 ± 0.2 mV (n = 4) in the presence of H2DIDS compared with 6.4 ± 1.9 mV (P < 0.05, n = 4) in its absence (Fig. 6 A), suggesting that stimulation results in rapid activation of the H2DIDS-sensitive Na+-HCO3 − cotransporter.
Figure 6.
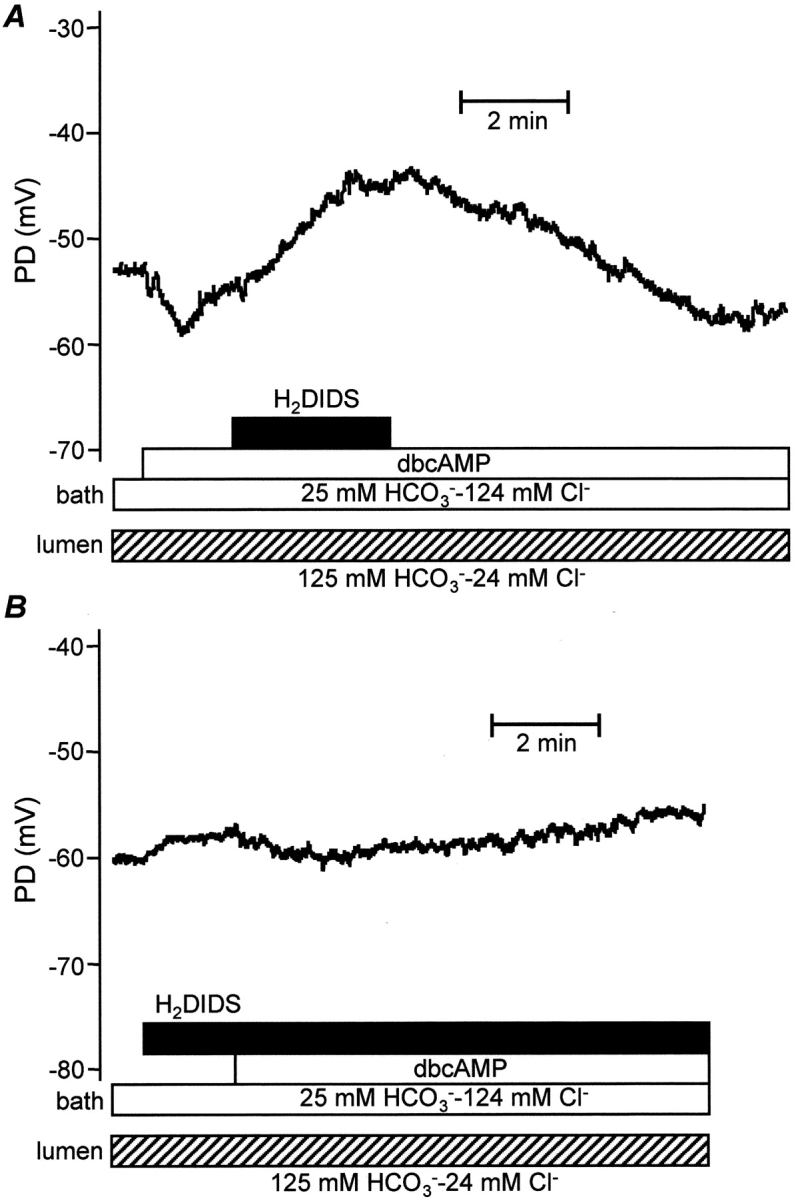
Effects of basolateral application of H2DIDS. The lumen was perfused with the high HCO3 − solution and the duct stimulated by addition of dbcAMP (0.5 mM) to the bath. H2DIDS (0.5 mM) was applied to the bath either before (A) or during stimulation (B) to inhibit basolateral Na+-HCO3 − cotransport. Each trace is representative of four experiments.
Together, these results indicate that the electrogenicity of the basolateral Na+-HCO3 − cotransporter has a significant hyperpolarizing effect on Vm, thus helping to maintain the steady-state value at approximately −60 mV during sustained stimulation under physiological conditions.
DISCUSSION
The electrophysiological properties of pancreatic duct cells have already been extensively studied in microperfused ducts from rat pancreas (Novak and Greger, 1988a,b, 1991; Novak and Pahl, 1993). The major observations from those studies were as follows. (a) Stimulation with cAMP caused Vm to depolarize from the resting value (−60 to −70 mV) to about −35 mV, often preceded by a small, transient hyperpolarization. (b) The depolarization evoked by stimulation was not dependent on the presence of HCO3 −. (c) Luminal application of Cl− channel blockers and inhibitors of Cl−-HCO3 − exchange caused Vm to hyperpolarize. (d) Inhibitors of the basolateral Na+-H+ exchanger caused a depolarization of ∼10 mV as a result of inhibition of a pH-sensitive K+ conductance on the basolateral membrane. (e) Modifying the Na+ concentration in the bath had no effect on Vm, thus signifying little contribution from basolateral Na+-HCO3 − cotransport.
Based on these studies and patch-clamp observations, a model of HCO3 − transport across pancreatic ductal epithelium was proposed in which (a) accumulation of intracellular HCO3 − is achieved by hydration of CO2 and extrusion of H+ by basolateral Na+-H+ exchange, and (b) HCO3 − secretion across the luminal membrane occurs by exchange for luminal Cl− via an anion exchanger working in parallel with the CFTR Cl− channel (Gray et al., 1988; Novak and Greger, 1988b). However, such a mechanism can only satisfactorily account for HCO3 − secretion in those species where the luminal concentration of HCO3 − reaches a maximum value of ∼70 mM (Sohma et al., 2000). Although this appears to be true in the rat (Sewell and Young, 1975), such a value is unusually low compared with other species such as the guinea-pig or dog (Case and Argent, 1993). Membrane potential measurements have never previously been reported in isolated ducts from species where the concentration of HCO3 − in the pancreatic juice reaches 140 mM or more.
In the present study we have examined the steady-state values of Vm in unstimulated and stimulated guinea-pig pancreatic duct cells exposed to luminal solutions of varied anionic composition. Luminal microperfusion has enabled us to simulate the changes in luminal HCO3 − and Cl− concentrations that are expected to occur when the duct cells secrete a HCO3 −-rich fluid following stimulation. These experiments have revealed some interesting differences between the electrophysiological properties of the guinea-pig and rat ducts which point to possible differences in the secretory mechanisms.
Effects of Stimulation on Membrane Potential in Guinea-pig Ducts
In the unstimulated condition, the electrolyte composition of the fluid in the duct lumen is unlikely to differ significantly from that of plasma. We therefore began by perfusing the duct lumen with the same 25 mM HCO3 −-buffered solution as that flowing through the bath. Stimulation with dbcAMP or secretin caused a biphasic change in Vm consisting of a transient hyperpolarization, from approximately −60 to −65 mV, followed by depolarization to a steady-state value of around −50 mV. Although the biphasic pattern was similar to that observed in the rat ducts, the overall depolarization in the guinea-pig ducts (∼10 mV) was considerably smaller than the 25 mV depolarization (to around −35 mV) seen in the rat ducts (Novak and Greger, 1988b).
Transient Hyperpolarization
One possible explanation for the transient hyperpolarization in the guinea-pig ducts is the activation of electrogenic Na+-HCO3 − cotransport at the basolateral membrane. This is supported by observation that basolateral application of H2DIDS significantly reduced the size of the transient (Fig. 6 B). The pancreatic variant of the Na+-HCO3 − cotransporter (pNBC1; Abuladze et al., 1998) is known to be expressed at the basolateral membrane of human pancreatic duct cells (Marino et al., 1999) and its stoichiometry was found to be 2 HCO3 −/1 Na+ in a cell line derived from mouse pancreatic duct cells (Gross et al., 2001a). With Na+ concentrations of 140 and 15 mM in the bath solution and in the cells, respectively (Ishiguro et al., 1996a), and with corresponding HCO3 − concentrations of 25 and 20 mM, the reversal potential of the 2:1 cotransporter would be −69 mV. As the Vm values observed in the present study were always smaller than −69 mV, the basolateral Na+-HCO3 − cotransporter would be expected to transport HCO3 − into the cell, thus contributing a hyperpolarizing effect to Vm as well as supplying HCO3 − to the luminal membrane. However, it is not known how the cotransporter becomes activated after stimulation, given that the Na+ and HCO3 − concentration gradients remain virtually unchanged. One possibility is that there is an increase in transport capacity resulting from phosphorylation by protein kinase A. Another is that there is a phosphorylation-induced switch in the stoichiometry of the NBC from 3:1 to 2:1 as observed with the kidney variant of the cotransporter (Gross et al., 2001b).
An additional source of hyperpolarization may come from the activation of cAMP-regulated maxi-K+ channels. These have been found at the basolateral membrane of rat ducts (Gray et al., 1990) and once activated would tend to drive the membrane potential toward the more negative equilibrium potential of K+. It has been shown in rat ducts that addition of K+ channel blockers to the bath causes a depolarization of Vm (Novak and Greger, 1988a) and a strong inhibition of fluid secretion (Ashton et al., 1991).
Since the depolarization that follows the transient hyperpolarization in the guinea-pig ducts is presumably due to the activation of anion conductances at the luminal membrane, our data indicate that dbcAMP stimulation activates the basolateral NBC, and perhaps a basolateral K+ conductance, more quickly than it does the luminal anion conductances.
Depolarization in the Steady State
Since the steady-state value of Vm after stimulation (approximately −50 mV) represents the balance between these various depolarizing and hyperpolarizing influences, the marked difference between the guinea-pig and rat in the overall magnitude of the depolarization probably reflects species differences in the properties of the transporters at the basolateral and luminal membranes. For example, pHi measurement in rat pancreatic duct cells have not detected any significant contribution of Na+-HCO3 − cotransport to basolateral HCO3 − uptake (Novak and Christoffersen, 2001). The evidence suggests that HCO3 − accumulation in the rat is achieved mainly by Na+-H+ exchange which, being electrically neutral, would not help to offset the depolarizing influence of the activated luminal anion conductances. In the guinea-pig, on the other hand, Na+-HCO3 − cotransport plays a dominant role in basolateral HCO3 − accumulation and its electrogenicity will certainly help to maintain a more negative Vm (Ishiguro et al., 1996a, 1998).
Effects of High Luminal [HCO3 −]
Our present study has, for the first time, examined the effects on Vm of perfusing the duct lumen with a HCO3 −-rich solution that more accurately reflects the physiological conditions that exist during maximal secretion. Substitution of HCO3 − for Cl− in the lumen induced remarkable changes in Vm. In the unstimulated ducts (Fig. 2), the replacement of 100 mM Cl− by 100 mM HCO3 − shifted Vm to a more negative value. If the luminal membrane was predominantly permeable to Cl−, and if nothing else in the system had changed, then Vm would be expected to depolarize since the equilibrium potential for Cl− would shift from around −38 to 6 mV. The size of the depolarization would depend on the relative magnitudes of the luminal Cl− conductance and the basolateral K+ conductance, which would tend to hold Vm at a more negative value, but it would inevitably be a depolarization and this is not what was observed.
In the dbcAMP-stimulated ducts (Fig. 1 A), where the luminal membrane Cl− conductance would be greater as a result of CFTR activation, there was some evidence of an initial depolarization when the luminal Cl− was replaced by HCO3 −, but again this was followed by a significant and sustained hyperpolarization. To separate the effects of reducing the luminal Cl− concentration from those of raising the luminal HCO3 − concentration, the experiment was repeated in two stages (Fig. 3). Lowering the luminal Cl− concentration first, by replacement with glucuronate, induced the expected depolarization, probably reflecting the efflux of Cl− via CFTR. The subsequent repolarization, whose rate varied, was probably due to the resulting depletion of intracellular Cl−. We have shown previously, under the same experimental conditions, that the intracellular Cl− concentration falls from 35 to ∼7 mM as a result of Cl− efflux to the lumen (Ishiguro et al., 2002). It is worth noting that, in rat pancreatic ducts, lowering the luminal Cl− concentration during stimulation with secretin induces a depolarization of ∼20 mV without any significant repolarization (Novak and Pahl, 1993). This is probably because basolateral Na+-K+-2Cl− cotransport continuously replenishes the intracellular Cl− in the rat ducts and therefore the intracellular Cl− concentration would not fall so far.
When the luminal HCO3 − concentration was subsequently raised to 125 mM in the guinea-pig ducts, without any concurrent change in luminal Cl− concentration, Vm underwent a fairly rapid hyperpolarization of ∼10 mV (Fig. 3). This was clearly a consequence of the raised HCO3 − concentration rather than any secondary effect of reducing the Cl− concentration or of raising the luminal pH (Fig. 4). It was also comparable with the hyperpolarization that was observed in unstimulated ducts when the luminal HCO3 − concentration was raised (Fig. 2).
We have two possible explanations for the hyperpolarizing effect of high luminal HCO3 − concentrations. First, a recent patch-clamp study of guinea-pig pancreatic duct cells (O'Reilly et al., 2000) demonstrated that extracellular HCO3 − reduces the luminal membrane Cl− conductance, possibly by direct binding of HCO3 − to a particular site on CFTR. This would hyperpolarize the cells by reducing the depolarizing effect of Cl− efflux to the lumen. Second, if the HCO3 − permeability of the luminal membrane was comparable with the Cl− permeability, Vm would be expected to shift to a more negative value with the high luminal HCO3 − concentration. This is because the equilibrium potential for HCO3 − would be −49 mV (with 125 mM HCO3 − in the lumen) compared with −38 mV for Cl− (with 124 mM Cl− in the lumen). Whatever the mechanism, there is no doubt that the hyperpolarization resulting from the high luminal HCO3 − concentration may have a vital role in maintaining the driving force for HCO3 − efflux across the luminal membrane.
HCO3 − Transport Across the Basolateral Membrane
Raising the bath concentration of HCO3 − from 25 to 125 mM (at constant PCO2) during stimulation with dbcAMP induced a sustained and reversible hyperpolarization of ∼10 mV (Fig. 5 A), which was abolished in the absence of bath Na+ (Fig. 5 B). This finding, together with the observation that Vm declined slowly during basolateral application of H2DIDS (Fig. 6 A), supports our hypothesis that the electrogenicity of the basolateral Na+-HCO3 − cotransporter makes a significant contribution to the membrane potential in guinea-pig duct cells. It is also in agreement with our previous measurements of pHi recovery from acid loading (Ishiguro et al., 1996a) and fluid secretion (Ishiguro et al., 1998) that indicate that Na+-HCO3 − cotransport contributes ∼75% of the basolateral HCO3 − accumulation in this species. In the rat, by contrast, addition of 25 mM HCO3 − to the bath had no effect on Vm (Novak and Greger, 1988a). This is almost certainly because HCO3 − accumulation in the rat ducts is achieved by a combination of intracellular CO2 hydration and electroneutral H+ extrusion via a basolateral Na+-H+ exchanger.
It has been proposed recently (Shumaker et al., 1999) that the crucial role of CFTR in HCO3 − secretion by pancreatic duct cells is to bring about a depolarization which will stimulate basolateral HCO3 − uptake via the Na+-HCO3 − cotransporter. However, the initial hyperpolarization following cAMP stimulation in the present study suggests that activation of the cotransporter may precede and almost completely offset the depolarizing effect of anion efflux across the luminal membrane. As discussed above, the marked activation of the cotransporter at the onset of secretion is most likely due to a change in phosphorylation status rather than a change in the driving force brought about by CFTR activity.
Steady-state Membrane Potential During Secretion
A major aim of our current work is to examine whether the electrochemical potential gradient for HCO3 − is sufficient to account for HCO3 − secretion via a luminal anion conductance, possibly CFTR itself. In the simplified cartoon shown in Fig. 7 we combine the Vm data from the present study with our previous measurements of intracellular Cl− concentration (Ishiguro et al., 2002) and intracellular HCO3 − concentration (Ishiguro et al., 2000). The main difference in the experimental conditions was the use of a lower temperature (30°C) for the intracellular Cl− measurements to avoid dye leakage. Although this may have introduced a small systematic error in the [Cl−]i values, the cells were still clearly responsive to stimulation under those conditions (Ishiguro et al., 2002).
Figure 7.
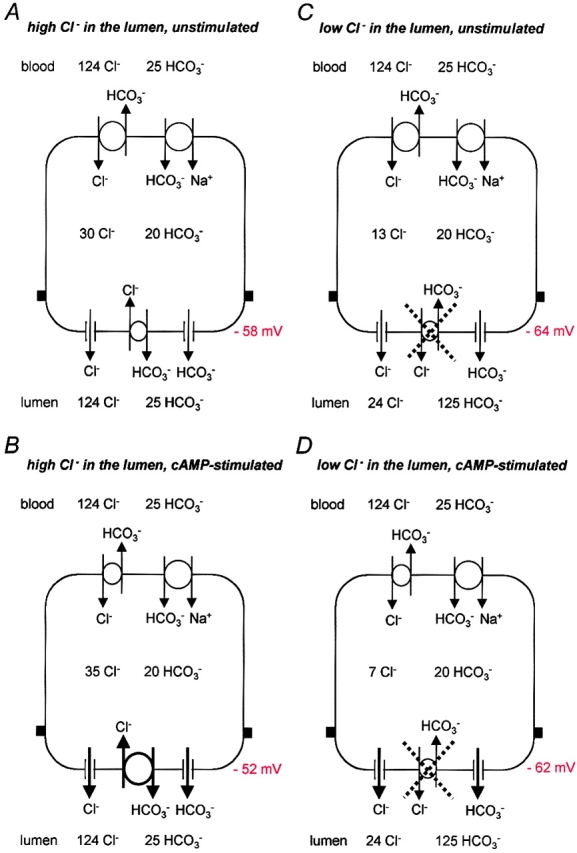
Implications of measured Vm values for anion channel/transporter activity under different conditions. A simplified model of the pancreatic duct cell showing the CFTR Cl− conductance, a putative HCO3 − conductance and the Cl−-HCO3 − exchanger at the luminal membrane, and the Na+-HCO3 − cotransporter and Cl−-HCO3 − exchanger at the basolateral membrane. The basolateral Na+-H+ exchanger and K+ and Cl− conductances are omitted for clarity. Steady-state values for the membrane potential and the intracellular concentrations of Cl− and HCO3 − (Ishiguro et al., 2000, 2002) are shown under four different sets of experimental conditions: A and B represent unstimulated and stimulated ducts perfused luminally with the high Cl− solution; C and D represent unstimulated and stimulated ducts perfused luminally with the high HCO3 − solution. It is anticipated that, in vivo, the conditions progress first from A to B and thence to D as the luminal concentration of HCO3 − rises.
With the high Cl− (124 mM) solution in both the bath and the lumen, the intracellular concentrations of Cl− and HCO3 − are ∼30 and 20 mM, respectively, in the unstimulated duct cell (Fig. 7 A). Under these conditions, Vm is approximately −58 mV. Stimulation with dbcAMP depolarizes Vm to approximately −52 mV, which still favors the efflux of both Cl− and HCO3 − through luminal anion conductances (Fig. 7 B). At this stage, the intracellular Cl− concentration (∼35 mM) is maintained above electrochemical equilibrium by the Cl−-HCO3 − exchanger at the luminal membrane that is driven by a steep inward concentration gradient of Cl−. The intracellular HCO3 − concentration (∼20 mM) is maintained above electrochemical equilibrium by the basolateral Na+-HCO3 − cotransporter which, with a 1:2 stoichiometry, will operate in an inward direction at a membrane potential of −52 mV. HCO3 − efflux across the luminal membrane is thus mediated by the Cl−-HCO3 − exchanger and possibly also a HCO3 − conductance. Cl− recycles across the luminal membrane via CFTR and the mechanism is essentially what was originally proposed for the rat pancreatic duct (Gray et al., 1988; Novak and Greger, 1988b).
In vivo, the concentration of HCO3 − in the duct lumen will gradually rise as a result of this HCO3 −-rich secretion, both with time following stimulation, and with distance along the duct. Consequently, the conditions will shift toward those depicted in Fig. 7 D. Here, with 24 mM Cl− and 125 mM HCO3 − in the lumen, the intracellular Cl− concentration falls to a very low steady-state value (∼7 mM), while intracellular HCO3 − remains at ∼20 mM as a result of continued Na+-HCO3 − cotransport across the basolateral membrane. The fall in intracellular Cl− is the result of continuing Cl− efflux to the lumen via CFTR, which occurs in the guinea-pig without a compensatory increase in Cl− uptake. The lack of Cl− uptake is due partly to the limited capacity of the basolateral membrane for Cl− uptake, and partly to the inhibition of the luminal Cl−-HCO3 − exchanger by the high luminal HCO3 − concentration (Ishiguro et al., 2000). The steady-state value of Vm is now approximately −62 mV. This will still favor Cl− and HCO3 − efflux to the lumen, despite the steep concentration gradient for the latter, since their equilibrium potentials are now −33 and −49 mV, respectively. Because of the low intracellular concentration of Cl− compared with that of HCO3 −, there will be little competition between Cl− and HCO3 − for efflux via an anion conductance that is permeable to both ions. In addition, if luminal HCO3 − has a selective, inhibitory effect on the Cl− conductance, the conditions are clearly favorable for the secretion of a HCO3 −-rich fluid.
HCO3 − Permeability of the Luminal Membrane
Having estimated the electrochemical potential gradient for HCO3 − diffusion across the luminal membrane during maximal secretion, it is reasonable to ask whether the gradient is large enough to drive the observed secretory flux of HCO3 −. This can be done by computing the required HCO3 − permeability, under the conditions shown in Fig. 7 D, and comparing it with the measured anion conductance of isolated pancreatic duct cells that have been stimulated with cAMP. From our previous work, we know that the guinea-pig ducts secrete fluid containing ∼140 mM HCO3 − at a rate of ∼2 nl min−1 mm−2 under comparable conditions (Ishiguro et al., 1998). Therefore, the secretory flux of HCO3 − is ∼0.5 nmol s−1 cm−2. Using the Goldman-Hodgkin-Katz equation and the data summarized in Fig. 7 D, we estimate that a net HCO3 − flux of this magnitude would require a luminal membrane HCO3 − permeability of 0.25 μm s−1.
In the intact pancreas, it is likely that HCO3 − secretion generates a small lumen-negative transepithelial PD and that this, in turn, is responsible for driving the paracellular cation flux. The few available measurements, all from other species, suggest that the PD could be 5–10 mV after stimulation (Schulz, 1980; Novak and Greger, 1991). If so, this would also be reflected in a more negative intracellular potential, and the driving force that we have estimated for HCO3 − diffusion across the luminal membrane in the isolated guinea-pig ducts (∼13 mV) would have to be reduced by perhaps 2–5 mV. In this case the HCO3 − permeability of the luminal membrane would need to be 15–40% greater than the figure that we have calculated above.
Reported values for the Cl− current density in cAMP-stimulated guinea-pig pancreatic duct cells range from ∼60 to 600 pA/pF when a 60 mV driving force is applied (O'Reilly et al., 2000). Using an estimate of the surface area of the cells, based on the whole-cell capacitance (5 pF; O'Reilly et al., 2000) and assuming a specific capacitance of 1 μF cm−2, we obtain a single-cell Cl− conductance of 1–10 mS cm−2. If most of the anion conductance is localized at the luminal membrane, and if this represents 1/15 of the total cell surface (Sohma et al., 2000), then the specific conductance of the luminal membrane would be 15–150 mS cm−2. Allowing for the Cl− concentrations used in the whole-cell current measurements, this conductance range translates to a Cl− permeability coefficient of 0.3–3 μm s−1. Supposing that the PHCO3 −/PCl − permeability ratio of the CFTR channels responsible for the current is ∼0.4 (O'Reilly et al., 2000), the HCO3 − permeability of the luminal membrane would be expected to lie between 0.12 and 1.2 μm s−1.
Estimates of PHCO3 −/PCl − for CFTR in the literature range from ∼0.2 to 0.5 (Poulsen et al., 1994; Linsdell et al., 1997; O'Reilly et al., 2000), so it is possible that we have under- or over-estimated the luminal membrane HCO3 − permeability. Despite this and other uncertainties, it seems clear that the observed luminal HCO3 − conductance is of the correct order of magnitude to account for the secretory flux of HCO3 − in stimulated guinea-pig duct cells. We therefore conclude that, in the guinea-pig, the production of a HCO3 −-rich fluid during maximal stimulation can be achieved mainly by the electrodiffusive efflux of HCO3 − across the luminal membrane. Although the most plausible candidate for the luminal membrane HCO3 − conductance is CFTR, our previous work surprisingly failed to detect any significant inhibition of HCO3 − secretion by luminally applied blockers of CFTR such as glibenclamide and NPPB (Ishiguro et al., 1996b). However, these compounds only partially block CFTR, so it is possible that an increase in the driving force generated by the basolateral transporters compensates for the reduced anion conductance at the luminal membrane.
A complete model for HCO3 − secretion in the pancreatic duct also has to explain why the concentration of Cl− in the secreted fluid is so low. The most likely explanation, as we have suggested previously (Ishiguro et al., 2002), is that during maximal secretion the intracellular Cl− concentration falls to a level that lies very close to the equilibrium value for Cl− at the luminal membrane. In other words, the limited capacity for Cl− uptake at the basolateral membrane, combined with the shut-down of the luminal membrane anion exchanger, results in the loss of any significant driving force for Cl− secretion at the luminal membrane. Although this hypothesis requires further verification, it eliminates the need for any dramatic changes in the Cl− permeability or anion selectivity of the luminal membrane.
To summarize, the present results and our previous studies on guinea-pig duct cells (Ishiguro et al., 2000, 2002) suggest (a) that HCO3 − secretion is supported mainly by Cl−-HCO3 − exchange when the luminal HCO3 − concentration is below ∼70 mM, and (b) that it is probably mediated by a HCO3 −-permeable anion conductance, most likely CFTR, when the luminal HCO3 − concentration is higher. The former mechanism would operate in the proximal ducts where some luminal Cl− is probably supplied continuously by acinar secretion. It would also operate at other points along the ductal system at the onset of secretion when the luminal Cl− concentration is initially high. But since the luminal HCO3 − concentration will quickly rise with time and distance along the ducts, secretion via the conductance will probably predominate in most of the ductal system during sustained stimulation.
In conclusion, the electrochemical potential gradient for HCO3 − is outwardly directed across the luminal membrane of guinea-pig pancreatic duct cells even when the luminal HCO3 − concentration is 125 mM. This finding supports our hypothesis that a HCO3 −-permeable anion conductance mediates HCO3 − secretion across the luminal membrane. The favorable gradient for HCO3 − secretion is achieved as a result of the hyperpolarizing effect of the basolateral Na+-HCO3 − cotransporter and a number of factors that minimize the depolarizing effect of Cl− efflux across the luminal membrane, not the least of which is the depletion of intracellular Cl−.
Acknowledgments
This study was supported by the Ministry of Education, Science, Technology, Sports and Culture (Japan); the Ministry of Health and Welfare (Japan); the Uehara Memorial Foundation; and the Cystic Fibrosis Trust (UK).
Footnotes
Abbreviations used in this paper: CFTR, cystic fibrosis transmembrane conductance regulator.
References
- Abuladze, N., I. Lee, D. Newman, J. Hwang, K. Boorer, A. Pushkin, and I. Kurtz. 1998. Molecular cloning, chromosomal localization, tissue distribution, and functional expression of the human pancreatic sodium bicarbonate cotransporter. J. Biol. Chem. 273:17689–17695. [DOI] [PubMed] [Google Scholar]
- Ashton, N., B.E. Argent, and R. Green. 1991. Characteristics of fluid secretion from isolated rat pancreatic ducts stimulated with secretin and bombesin. J. Physiol. 435:533–546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Case, R.M., and B.E. Argent. 1993. Pancreatic duct cell secretion: control and mechanisms of transport. The Pancreas: Biology, Pathophysiology, and Disease. 2nd ed. V.L.W. Go, E.P. Dimagno, J.D. Gardner, E. Lebenthal, H.A. Reber, and G.A. Scheele, editors. Raven Press, New York. 301–350.
- Choi, J.Y., D. Muallem, K. Kiselyov, M.G. Lee, P.J. Thomas, and S. Muallem. 2001. Aberrant CFTR-dependent HCO3 − transport mutations associated with cystic fibrosis. Nature. 410:94–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawford, I., P.C. Maloney, P.L. Zeitlin, W.B. Guggino, S.C. Hyde, H. Turley, K.C. Gatter, A. Harris, and C.F. Higgins. 1991. Immunocytochemical localization of the cystic fibrosis gene product CFTR. Proc. Natl. Acad. Sci. USA. 88:9262–9266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Domschke, S., W. Domschke, W. Rosch, S.L. Konturek, E. Wunsch, and L. Demling. 1976. Bicarbonate and cyclic AMP content of pure human pancreatic juice in response to graded doses of synthetic secretin. Gastroenterology. 70:533–536. [PubMed] [Google Scholar]
- Gray, M.A., J.R. Greenwell, and B.E. Argent. 1988. Secretin-regulated chloride channels on the apical plasma membrane of pancreatic duct cells. J. Membr. Biol. 105:131–142. [DOI] [PubMed] [Google Scholar]
- Gray, M.A., J.R. Greenwell, J.R. Garton, and B.E. Argent. 1990. Regulation of maxi-K+ channels on pancreatic duct cells by cyclic AMP-dependent phosphorylation. J. Membr. Biol. 115:203–215. [DOI] [PubMed] [Google Scholar]
- Gray, M.A., A. Harris, L. Coleman, J.R. Greenwell, and B.E. Argent. 1989. Two types of chloride channel on duct cells cultured from human fetal pancreas. Am. J. Physiol. 257:C240–C251. [DOI] [PubMed] [Google Scholar]
- Gross, E., N. Abuladze, A. Pushkin, I. Kurtz, and C.U. Cotton. 2001. a. The stoichiometry of the electrogenic sodium bicarbonate cotransporter pNBC1 in mouse pancreatic duct cells is 2 HCO3 −:1 Na+. J. Physiol. 531:375–382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gross, E., K. Hawkins, A. Pushkin, P. Sassani, R. Dukkipati, N. Abuladze, U. Hopfer, and I. Kurtz. 2001. b. Phosphorylation of Ser982 in the sodium bicarbonate cotransporter kNBC1 shifts the HCO3 −: Na+ stoichiometry from 3:1 to 2:1 in murine proximal tubule cells. J. Physiol. 537:659–665. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, H., S. Naruse, M. Kitagawa, T. Hayakawa, R.M. Case, and M.C. Steward. 1999. Luminal ATP stimulates fluid and HCO3 − secretion in guinea-pig pancreatic duct. J. Physiol. 519:551–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, H., S. Naruse, M. Kitagawa, T. Mabuchi, T. Kondo, T. Hayakawa, R.M. Case, and M.C. Steward. 2002. Chloride transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J. Physiol. 539:175–189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, H., S. Naruse, M. Kitagawa, A. Suzuki, A. Yamamoto, T. Hayakawa, R.M. Case, and M.C. Steward. 2000. CO2 permeability and bicarbonate transport in microperfused interlobular ducts isolated from guinea-pig pancreas. J. Physiol. 528:305–315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, H., S. Naruse, J.I. San-Román, R.M. Case, and M.C. Steward. 2001. Pancreatic ductal bicarbonate secretion: past, present and future. JOP. 2:192–197. [PubMed] [Google Scholar]
- Ishiguro, H., S. Naruse, M.C. Steward, M. Kitagawa, S.B.H. Ko, T. Hayakawa, and R.M. Case. 1998. Fluid secretion in interlobular ducts isolated from guinea-pig pancreas. J. Physiol. 511:407–422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, H., M.C. Steward, A.R.G. Lindsay, and R.M. Case. 1996. a. Accumulation of intracellular HCO3 − by Na+-HCO3 − cotransport in interlobular ducts from guinea-pig pancreas. J. Physiol. 495:169–178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ishiguro, H., M.C. Steward, R.W. Wilson, and R.M. Case. 1996. b. Bicarbonate secretion in interlobular ducts from guinea-pig pancreas. J. Physiol. 495:179–191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee, M.G., J.Y. Choi, X. Luo, E. Strickland, P.J. Thomas, and S. Muallem. 1999. Cystic fibrosis transmembrane conductance regulator regulates Cl−/HCO3 − exchange in mouse submandibular and pancreatic ducts. J. Biol. Chem. 274:14670–14677. [DOI] [PubMed] [Google Scholar]
- Linsdell, P., J.A. Tabcharani, J.M. Rommens, Y.X. Hou, X.B. Chang, L.C. Tsui, J.R. Riordan, and J.W. Hanrahan. 1997. Permeability of wild-type and mutant cystic fibrosis transmembrane conductance regulator chloride channels to polyatomic anions. J. Gen. Physiol. 110:355–364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lohi, H., M. Kujala, E. Kerkela, U. Saarialho-Kere, M. Kestila, and J. Kere. 2000. Mapping of five new putative anion transporter genes in human and characterization of SLC26A6, a candidate gene for pancreatic anion exchanger. Genomics. 70:102–112. [DOI] [PubMed] [Google Scholar]
- Marino, C.R., L.M. Matovcik, F.S. Gorelick, and J.A. Cohn. 1991. Localization of the cystic fibrosis transmembrane conductance regulator in pancreas. J. Clin. Invest. 88:712–716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marino, C.R., V. Jeanes, W.F. Boron, and B.M. Schmitt. 1999. Expression and distribution of the Na+-HCO3 − cotransporter in human pancreas. Am. J. Physiol. 277:G487–G494. [DOI] [PubMed] [Google Scholar]
- Novak, I., and B.C. Christoffersen. 2001. Secretin stimulates HCO3 − and acetate efflux but not Na+/HCO3 − uptake in rat pancreatic ducts. Pflugers Arch. 441:761–771. [DOI] [PubMed] [Google Scholar]
- Novak, I., and R. Greger. 1988. a. Electrophysiological study of transport systems in isolated perfused pancreatic ducts: properties of the basolateral membrane. Pflugers Arch. 411:58–68. [DOI] [PubMed] [Google Scholar]
- Novak, I., and R. Greger. 1988. b. Properties of luminal membrane of isolated rat pancreatic ducts: effect of cyclic AMP and blockers of chloride transport. Pflugers Arch. 411:546–553. [DOI] [PubMed] [Google Scholar]
- Novak, I., and R. Greger. 1991. Effect of bicarbonate on potassium conductance of isolated perfused rat pancreatic ducts. Pflugers Arch. 419:76–83. [DOI] [PubMed] [Google Scholar]
- Novak, I., and C. Pahl. 1993. Effect of secretin and inhibitors of HCO3 −/H+ transport on the membrane voltage of rat pancreatic duct cells. Pflugers Arch. 425:272–279. [DOI] [PubMed] [Google Scholar]
- O'Reilly, C.M., J.P. Winpenny, B.E. Argent, and M.A. Gray. 2000. Cystic fibrosis transmembrane conductance regulator in guinea-pig pancreatic duct cells: Inhibition by bicarbonate ions. Gastroenterology. 118:1187–1196. [DOI] [PubMed] [Google Scholar]
- Padfield, P.J., A. Garner, and R.M. Case. 1989. Patterns of pancreatic secretion in the anaesthetised guinea pig following stimulation with secretin, cholecystokinin octapeptide, or bombesin. Pancreas. 4:204–209. [DOI] [PubMed] [Google Scholar]
- Poulsen, J.H., H. Fischer, B. Illek, and T.E. Machen. 1994. Bicarbonate conductance and pH regulatory capability of cystic fibrosis transmembrane conductance regulator. Proc. Natl. Acad. Sci. USA. 91:5340–5344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussa, E., S.L. Alper, and F. Thevenod. 2001. Immunolocalization of anion exchanger AE2, Na+/H+ exchangers NHE1 and NHE4, and vacuolar type H+-ATPase in rat pancreas. J. Histochem. Cytochem. 49:463–474. [DOI] [PubMed] [Google Scholar]
- Schulz, I. 1980. Bicarbonate transport in the exocrine pancreas. Ann. NY Acad. Sci. 341:191–209. [DOI] [PubMed] [Google Scholar]
- Sewell, W.A., and J.A. Young. 1975. Secretion of electrolytes by the pancreas of the anaesthetized rat. J. Physiol. 252:379–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shumaker, H., H. Amlal, R. Frizzell, C.D. Ulrich, and M. Soleimani. 1999. CFTR drives Na+-nHCO3 − cotransport in pancreatic duct cells: a basis for defective HCO3 − secretion in CF. Am. J. Physiol. 45:C16–C25. [DOI] [PubMed] [Google Scholar]
- Sohma, Y., M.A. Gray, Y. Imai, and B.E. Argent. 2000. HCO3 − transport in a mathematical model of the pancreatic duct epithelium. J. Membr. Biol. 176:77–100. [DOI] [PubMed] [Google Scholar]