Abstract
In Kv2.1 potassium channels, changes in external [K+] modulate current magnitude as a result of a K+-dependent interconversion between two outer vestibule conformations. Previous evidence indicated that outer vestibule conformation (and thus current magnitude) is regulated by the occupancy of a selectivity filter binding site by K+. In this paper, we used the change in current magnitude as an assay to study how the interconversion between outer vestibule conformations is controlled. With 100 mM internal K+, rapid elevation of external [K+] from 0 to 10 mM while channels were activated produced no change in current magnitude (outer vestibule conformation did not change). When channels were subsequently closed and reopened in the presence of elevated [K+], current magnitude was increased (outer vestibule conformation had changed). When channels were activated in the presence of low internal [K+], or when K+ flow into conducting channels was transiently interrupted by an internal channel blocker, increasing external [K+] during activation did increase current magnitude (channel conformation did change). These data indicate that, when channels are in the activated state under physiological conditions, the outer vestibule conformation remains fixed despite changes in external [K+]. In contrast, when channel occupancy is lowered, (by channel closing, an internal blocker or low internal [K+]), the outer vestibule can interconvert between the two conformations. We discuss evidence that the ability of the outer vestibule conformation to change is regulated by the occupancy of a nonselectivity filter site by K+. Independent of the outer vestibule-based potentiation mechanism, Kv2.1 was remarkably insensitive to K+-dependent processes that influence current magnitude (current magnitude changed by <7% at membrane potentials between −20 and 30 mV). Replacement of two outer vestibule lysines in Kv2.1 by smaller neutral amino acids made current magnitude dramatically more sensitive to the reduction in K+ driving force (current magnitude changed by as much as 40%). When combined, these outer vestibule properties (fixed conformation during activation and the presence of lysines) all but prevent variation in Kv2.1 current magnitude when [K+] changes during activation. Moreover, the insensitivity of Kv2.1 current magnitude to changes in K+ driving force promotes a more uniform modulation of current over a wide range of membrane potentials by the K+-dependent regulation of outer vestibule conformation.
Keywords: permeation, conductance, selectivity filter, structure
INTRODUCTION
The Kv2.1 potassium channel is a slowly inactivating delayed rectifier responsible for repolarization of the action potential in both brain and peripheral organs. It has widespread distribution in the CNS (Hwang et al., 1993; Murakoshi and Trimmer, 1999), and is perhaps especially important under conditions of high frequency firing (Du et al., 2000). Both current magnitude and activation rate are influenced by changes in external [K+] in the physiological range (Wood and Korn, 2000). As external [K+] is elevated between 0 and 10 mM, current is potentiated and activation rate is increased in a concentration-dependent manner. These functional changes are associated with an interconversion between two different outer vestibule conformations. Channels in these two conformations also display different sensitivity to TEA (Immke et al., 1999; Immke and Korn, 2000). This difference is manifest as a change in TEA efficacy, not potency—either channels are blocked by TEA or they aren't. Consequently, activated channels can be described as in one of two conformations: a TEA-sensitive conformation that produces larger currents and a TEA-insensitive conformation that produces smaller currents. Which of these two states the channel is in appears to depend on the occupancy by K+ of a site associated with the channel selectivity filter (Immke and Korn, 2000). Thus, elevation of external [K+] increases occupancy of this site, channels shift from the TEA-insensitive to TEA-sensitive conformation, and currents are potentiated.
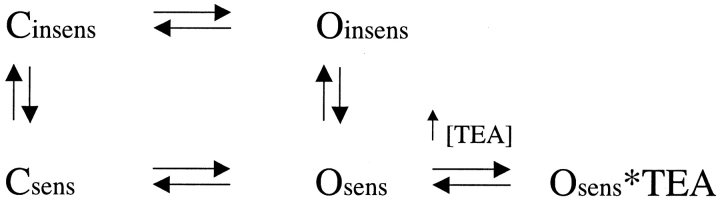
When external [K+] is elevated while channels are held in the closed state, the changes in TEA sensitivity and current characteristics are observed upon channel activation by the next depolarization (Immke and Korn, 2000; Wood and Korn, 2000). This implies that the K+-dependent change in conformation can occur when channels are closed, or very early during the transition from the closed to activated state. (We use the term “activated” rather than “open” merely to indicate that we do not mean to distinguish between nonconducting and conducting states that are typically observed in single channel recordings at depolarized potentials [i.e., the final closed and open states]. Rather, our intention is to distinguish between channels in deeper closed states versus conducting states, as observed macroscopically.) If the transition between TEA-sensitive and TEA-insensitive conformations could also occur while channels are activated, application of high [TEA] would be expected to shift the equilibrium such that TEA-insensitive channels (Oinsens) would become TEA-sensitive (Osens, Scheme I).
SCHEME I.
However, even at saturating [TEA], once channels are activated, the percentage of channels in each conformation remains constant (Immke and Korn, 2000). Consequently, these observations suggest that, once activated, the outer vestibule conformation remains fixed. Alternatively, it could be that activated channels did not redistribute between conformations with saturating [TEA] because the percentage of channels in each conformation was strictly dependent on K+ occupancy, and changing external [TEA] didn't change K+ occupancy. In part I of this manuscript, we tested these hypotheses by rapidly changing external [K+] once channels were activated by depolarization.
Another intriguing observation from previous work was that, with 100 mM internal K+, only a small percentage of channels were TEA insensitive (and thus subject to K+-dependent potentiation), yet elevation of [K+] from 0 to 10 mM potentiated currents by ∼40% (Immke and Korn, 2000; Wood and Korn, 2000). We considered this potentiation to be remarkable in that it was presumably superimposed on a decrease in current magnitude associated with the large decrease in driving force that influenced the entire channel population. In part II of this manuscript, we demonstrate that the change in Kv2.1 current magnitude associated with the change in driving force is extremely small. Indeed, except for the conformational change mechanism, current magnitude in Kv2.1 is remarkably insensitive to changes in external [K+]. We provide evidence for the structural basis of this insensitivity.
MATERIALS AND METHODS
Experiments were done on wild-type Kv2.1 channels, Kv2.1 with two outer vestibule mutations (Kv2.1 K356G K382V), and Shaker H4 Δ6–36 (Hoshi et al., 1990). Details of mutagenesis for Kv2.1 channel mutants are described elsewhere (Immke et al., 1999). K+ channel cDNA was subcloned into the pcDNA3 expression vector and expressed in the human embryonic kidney cell line, HEK293 (American Type Culture Collection). Cells (2 × 106 cells/ml) were cotransfected by electroporation (Invitrogen Electroporator II; 71 μF, 375 V) with K+ channel expression plasmid (0.5–15 μg/0.2 ml) and CD8 antigen (1 μg/0.2 ml). After electroporation, cells were plated on glass coverslips submerged in maintenance media. Electrophysiological recordings were made 18–48 h later. On the day of recording, cells were washed with fresh media and incubated with Dynabeads M450 conjugated with antibody to CD8 (1 μl/ml; Dynal). Cells that expressed CD8 became coated with beads, which allowed visualization of transfected cells (Jurman et al., 1994).
Electrophysiology
Currents were recorded at room temperature using the standard whole cell patch clamp technique. Patch pipets were fabricated from N51A glass (Garner Glass Co.), coated with Sylgard, and firepolished. After formation of the seal, cells were detached from the cover glass to improve external perfusion surrounding the entire cell. Currents were filtered at 2 KHz and sampled at 100 μs/pt. Series resistance ranged from 0.5 to 2.5 MΩ and was compensated 80–90%. The holding potential was −80 mV, and depolarizing stimuli were presented once every 10 s. For macroscopic conductance measurements, 10 mV depolarizing stimuli, 5 ms in duration, were presented at 100 Hz during the recorded current sweep. Data were analyzed with Clampfit 6.0.5 (Axon Instruments, Inc.) and analyzed with SigmaPlot 8.0 (SPSS, Inc.). The Goldman-Hodgkin-Katz current equation used to generate the data in Fig. 5 D was
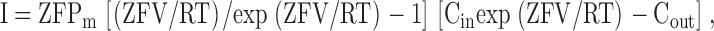 |
(1) |
where Z, F, and R are the usual constants, T is temperature, V is membrane potential, I is membrane current, Pm is the membrane permeability, and Cin and Cout are the internal and external cation concentrations, respectively (Ferreira and Marshall, 1985).
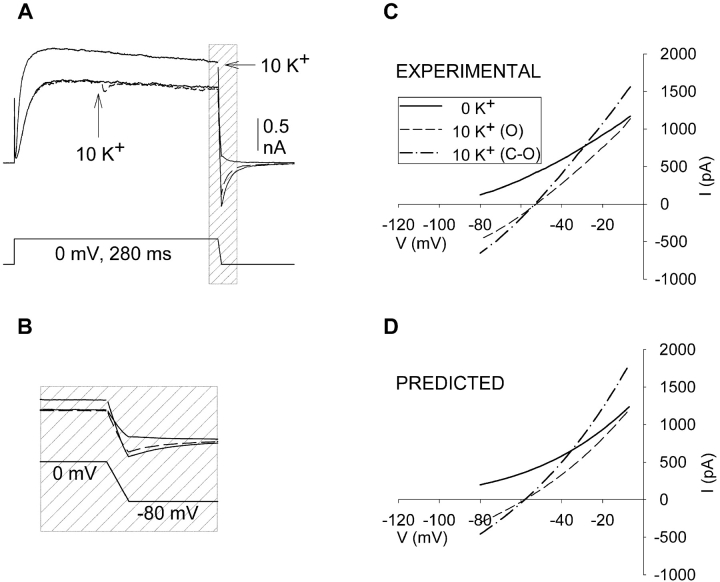
Figure 5.
K+-dependent changes in the I-V curve of Kv2.1 (A) Currents recorded as in Fig. 2, except that during the repolarization, membrane potential was ramped from 0 to −80 mV over a duration of 5 ms. Three superimposed currents are shown, one recorded in 0 external K+ (unlabeled solid line), one in which external [K+] was changed from 0 to 10 mM (at arrow, dashed line) during activation, and one after closing and reopening in 10 mM external K+. 300 μs of data were blanked at the end of the depolarization due to a program error in generating ramps in pClamp. (B) Current and voltage during repolarization illustrated on an expanded time scale. (C) Current plotted as a function of voltage during the repolarization. Similar results were obtained in seven cells. (D) Theoretical curves obtained from the Goldman-Hodgkin-Katz current equation (Eq. 1). Parameter values were obtained as described in the text.
Electrophysiological Solutions
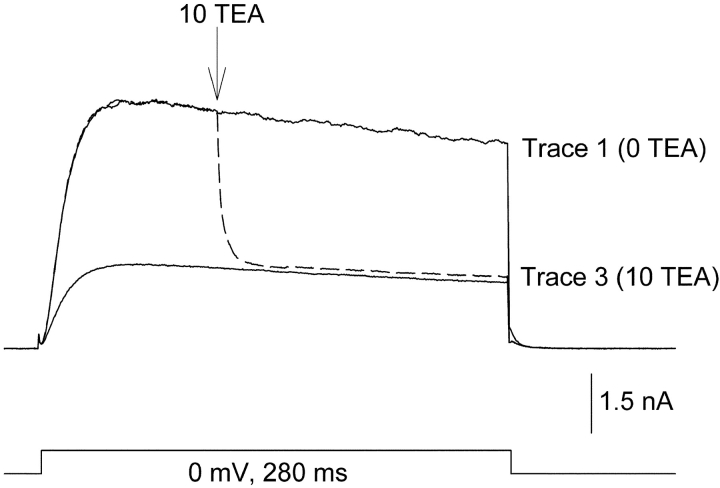
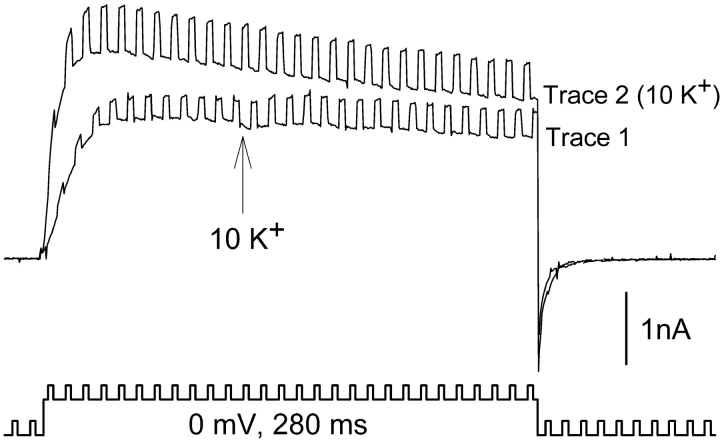
Fast solution changes were made using double barrel theta capillary tubing (the tip of each barrel was ∼30 μM diameter) connected to a stepper motor (Warner Instruments). Solutions were fed into each barrel by gravity. One barrel was placed within 15 μm of the cell being recorded before the start of the experiment. Solution was always flowing through both barrels, and solutions were changed by rapidly stepping from one barrel to the other. The speed of the solution change was tested by recording currents through Kv2.1 channels and switching between barrels containing 0 or 10 mM TEA (Fig. 1) . The protocol used in Fig. 1 was used for almost all experiments (deviations are described in the results section). Three currents were recorded consecutively at 10-s intervals, each evoked by a 280 ms depolarization to a single membrane potential. Trace 1 illustrates a current recorded in the control condition, which in Fig. 1 is the absence of external TEA. In trace 2 (dashed line), recorded 10 s later, the barrels were stepped to change solutions from 0 to 10 mM TEA 100 ms after the start of the depolarization (arrow). After trace 2, the cell was continuously bathed in the second external solution (in this case, the 10 mM TEA solution). Trace 3 was then recorded 10 s after trace 2. With the rapid switch, complete solution change occurred, on average, in 19.3 ± 0.6 ms (n = 9). To test whether mixing occurred between the outflow of the two barrels, we compared the effects of changing external solutions (e.g., changing [TEA] or [K+]), on a slow time scale between this perfusion system and a standard system with only a single flowing solution. Functionally detectable mixing of outflow did not occur. Internal solutions contained (in mM): 100 KCl, 25 N-methyl-D-glucamine (NMG), 10 Hepes, 10 EGTA-NMG, 1 CaCl2, 4 MgCl2; pH to 7.3 with HCl, osmolality 285. Control external solutions contained (in mM): 155 NMG, 10 Hepes, 10 glucose, 2 CaCl2, and 1 MgCl2; pH 7.3 with HCl, osmolality 325. External solutions with TEA or K+ were made by equimolar substitution for NMG.
Figure 1.
Inhibition of K+ current by rapid application of TEA to activated channels. Three superimposed currents, recorded consecutively at 10-s intervals. (Trace 1) Outward current in the absence of TEA. (Trace 2, dashed line) Cells were activated in the absence of TEA. 100 ms after depolarization, the external solution was switched to one containing 10 mM TEA (at arrow). (Trace 3) Channels were closed and reactivated in the presence of 10 mM TEA. Complete solution change, as judged by the time required for 97% inhibition, occurred in 19.3 ± 0.6 ms (n = 9). The data were reasonably well fit by a single exponential, which produced a time constant of 3.47 ± 0.22 ms. There was no external K+ in this experiment.
RESULTS
Part I. Control of Outer Vestibule Dynamics
Current magnitude is insensitive to changes in external K+ when channels are already in the activated state
At [K+] between 0 and 10 mM, the outer vestibule reorientation that is responsible for the change in TEA sensitivity also results in a change in current magnitude (Wood and Korn, 2000). Therefore, we used the change in current magnitude as an assay for the change in outer vestibule conformation.
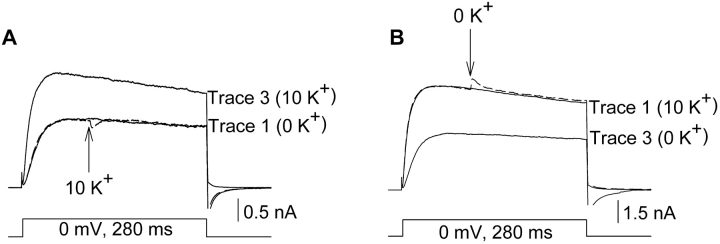
To determine whether the outer vestibule conformation could change when channels were in the activated state, external [K+] was changed during the depolarization that activated the channels (Fig. 2) . In Fig. 2 A, trace 1 (solid line) illustrates an outward K+ current in the recorded absence of external [K+]. Trace 2 (dashed line) illustrates a current in which external [K+] was switched from 0 to 10 mM, 100 ms after the start of the depolarization (arrow). After an immediate small current transient (which followed in direction the change in driving force), there was little effect on current magnitude (in six cells, current magnitude changed by −0.7 ± 0.7%). In contrast to the lack of effect when [K+] was changed during channel activation (trace 2), marked potentiation occurred after channels were closed and reopened in 10 mM [K+] (trace 3; current was potentiated by 38.0 ± 6.0% in six cells). The converse experiment (Fig. 2 B) produced similar results. Changing [K+] from 10 mM to 0 while currents were activated produced essentially no effect on current magnitude (except for the small transient; Fig. 2 B dashed line; n = 4). When channels were closed and reopened in the presence of 0 mM external K+, current magnitude was reduced as expected for the change in outer vestibule conformation.
Figure 2.
Changing external [K+] when channels were activated did not affect current magnitude. All currents were activated by depolarization to 0 mV. (A, trace 1) Current recorded in the absence of external K+. (Trace 2, dashed line) Current was activated in the absence of external K+. 100 ms after depolarization (at the arrow), the external solution was switched to one containing 10 mM K+. Mean potentiation = −0.7 ± 0.7% (n = 6). (Trace 3) Channels were closed and reactivated in the presence of 10 mM external K+. Mean potentiation = 38.0 ± 6.0% (n = 6). (B) Converse experiment to A. (Trace 1) Current recorded in 10 mM K+. (Trace 2, dashed line) Current was activated in 10 mM external K+. 100 ms after depolarization, the external solution was switched to one containing 0 mM K+. Trace 3. Channels were closed and reactivated in the presence of 0 external K+.
The data in Fig. 2 provide strong evidence that a change in outer vestibule conformation did not occur when [K+] was elevated while channels were activated. However, final current magnitude likely represents the summed influence of several different processes. For example, in addition to the outer vestibule-based mechanism, elevation of [K+] might potentiate currents due to an increase in single channel conductance associated with increased saturation of the pore (Hille and Schwartz, 1978; Neyton and Miller, 1988; Yang and Sigworth, 1998). Concommitantly, current magnitude should also be reduced by the significant decrease in driving force. These latter two effects, however, which are not likely to involve a significant change in channel conformation, should occur during activation upon changing [K+]. Consequently, the stable current magnitude after the change in [K+] during channel activation may not simply have reflected a lack of potentiation but may also have involved these other simultaneous events that coincidentally produced no net change in current. To test this, we examined the effect of rapidly changing external [K+] on the macroscopic conductance of activated channels (Fig. 3) .
Figure 3.
Changing external [K+] when channels were activated had little effect on macroscopic conductance. To measure macroscopic conductance, 10 mV, 5-ms depolarizations were superimposed on the depolarization to 0 mV. (Trace 1) Channels were activated in the absence of external K+. At the arrow, external [K+] was changed to 10 mM. In this cell, conductance increased by 21% with essentially no change in current magnitude. In eight cells, the average conductance change was 22.7 ± 2.6%. (Trace 2) Channels were closed and reactivated in the presence of 10 mM external K+. In this cell, conductance increased by 110%. In eight cells, conductance increased by 93.1 ± 7.2%.
Elevation of external [K+] from 0 to 10 mM had just a small effect on macroscopic conductance when [K+] was changed during the depolarization (Fig. 3, trace 1; conductance was increased by 22.7 ± 2.6%, n = 8). When channels were closed and reopened in the presence of 10 mM K+, conductance was almost doubled in association with the increase in current magnitude (Fig. 3, trace 2; in eight cells, conductance was increased by 93.1 ± 7.2%). Thus, the bulk of the conductance change only occurred after channels were closed and reopened. These results provide additional evidence that, after the switch from 0 to 10 mM K+, the outer vestibule conformation did not change until channels were closed and reopened. Moreover, the data in Fig. 3 indicate that the stable current during activation at 0 mV did, indeed, reflect additional opposing effects that produced no net change in current magnitude.
K+-dependent conductance changes in channels that do not display current potentiation associated with a K+-dependent change in outer vestibule conformation
Our interpretation of the results in Figs. 2 and 3 was that the outer vestibule conformation changed only after closing and reopening of the channels, and that this reorientation of the outer vestibule was responsible for the current potentiation and the additional, larger change in conductance. The data in Fig. 3 also indicate that two other effects of [K+] elevation also occurred in these experiments. First, there was an increase in macroscopic conductance that occurred while channels were activated. This would be expected to occur as a result of an increase in pore saturation in these multiion pores (Hille and Schwartz, 1978; Neyton and Miller, 1988; Yang and Sigworth, 1998). Second, elevation of external [K+] substantially decreases the driving force on K+ due to the shift in reversal potential, and should thus decrease current magnitude. In contrast to the outer vestibule-based mechanism, these effects should occur in essentially all K+ channels and be independent of whether [K+] was changed when channels were closed or activated. Consequently, our hypothesis predicts that, in channels that do not display K+-dependent potentiation associated with a change in outer vestibule conformation, only a single level of conductance increase will be observed. Moreover, this increase in conductance should occur when [K+] is changed during activation, and no further change in conductance will occur when the channels are closed and reopened. Any change in current magnitude that may occur will depend on the balance between the increase in conductance and the decrease in driving force.
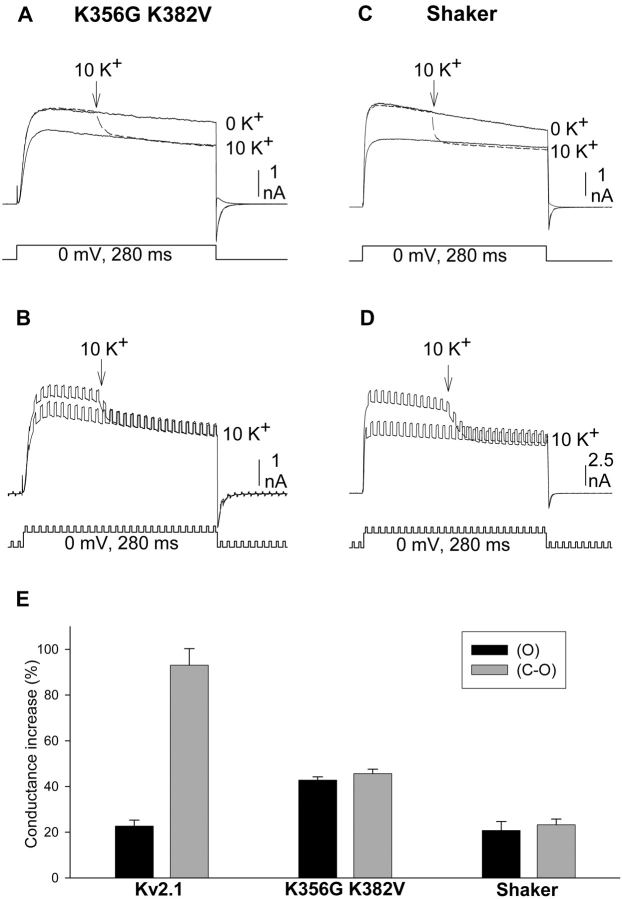
Mutation of two outer vestibule lysines in Kv2.1 to smaller, neutral amino acids eliminates the K+-dependent potentiation of current associated with the change in outer vestibule conformation (Wood and Korn, 2000). However, these mutations do not abolish the K+-dependent conformational change itself (Immke et al., 1999). Consequently, our hypothesis predicts that, in this mutant channel (Kv2.1 K356G K382V), any K+-dependent change in current magnitude and macroscopic conductance that occurs should be observed while channels are activated. In addition, no further change in current magnitude or conductance should occur once channels are closed and reopened.
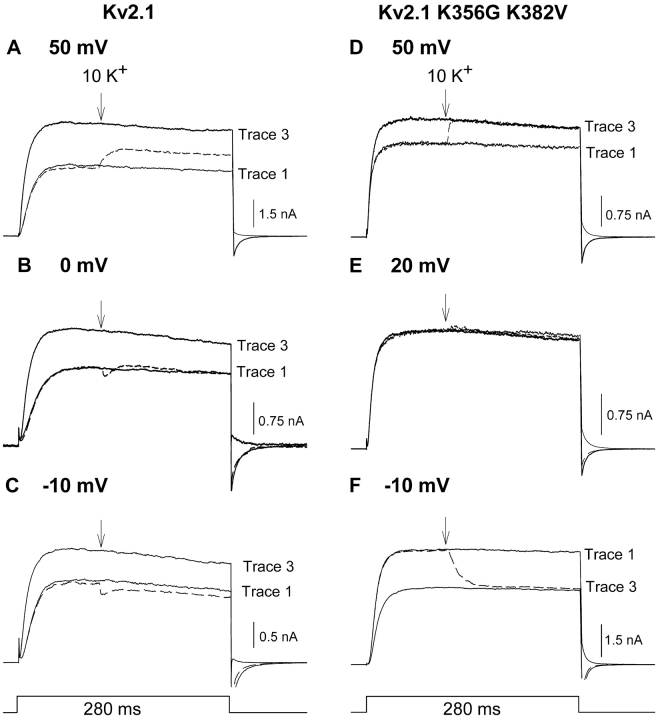
Fig. 4 A illustrates currents from Kv2.1 K356G K382V. Elevation of external [K+] from 0 to 10 mM during channel activation produced an immediate decrease in current magnitude (dashed line). When channels were closed and reopened in the presence of 10 mM K+, current magnitude was identical to that which followed the rapid change in K+ during activation. Fig. 4 B illustrates the effect of [K+] elevation on macroscopic conductance in this channel. Concommitant with the current reduction, elevation of [K+] during activation produced an immediate increase in macroscopic conductance. In contrast to wild-type Kv2.1, however, the conductance through Kv2.1 K356G K382V in 10 mM K+ after closing and reopening of the channels was identical to that produced by switching to 10 mM K+ during activation (Fig. 4, B and E).
Figure 4.
Response to changing external [K+] in channels without outer vestibule lysines. Protocols used were identical to those used in Figs. 2 and 3. (A and B) Recordings from Kv2.1 K356G K382V. (C and D) Recordings from Shaker. Currents were initially recorded in 0 mM K+ (shown in A and C, omitted for clarity in B and D). During the subsequent activation (dashed lines in A and C, larger currents in B and D), external [K+] was changed from 0 to 10 mM K+ (at the arrow). The third activation occurred after closing and reopening the channels in 10 mM K+. (E) The K+-dependent increase in conductance, measured from recordings as in B and D, obtained after the change in [K+] during the activation (black bars, O) and after closing and reopening the channels in 10 mM [K+] (gray bars, C-O). Bars represent seven cells in each group for Kv2.1 and K356G K382V, four cells in each group for Shaker.
Figs. 4, C and D, illustrate similar experiments done on the Shaker K+ channel. The Shaker channel does not display the K+-dependent potentiation mechanism associated with a change in outer vestibule conformation. However, Shaker would still be subject to the change in driving force and increase in conductance associated with increased channel saturation. Consequently, it would also be expected that the complete change in current magnitude and conductance would occur when external [K+] was elevated during activation. Indeed, elevation of external [K+] produced an immediate decrease in current magnitude (Fig. 4 C) and increase in macroscopic conductance (Fig. 4 D). Moreover, the conductance in 10 mM K+ after closing and reopening of the channel was identical to that produced by elevation of [K+] to 10 mM during activation (Fig. 4, D and E). Thus, these two channels that lacked the potentiation mechanism associated with the K+-dependent change in outer vestibule conformation displayed a K+-dependent change in current magnitude and conductance that was independent of channel activation state. It should also be noted that, despite the K+-dependent increase in conductance, elevation of external [K+] substantially reduced current magnitude through Kv2.1 K356G K382V and Shaker. This large (and expected) response to the change in K+ driving force contrasted sharply with the results obtained with Kv2.1. This difference will be addressed in part II of this paper.
I-V relationships
When [K+] was changed during activation in Kv2.1, the change in K+ driving force had little effect on current magnitude at 0 mV. However, inspection of the currents in Fig. 2 revealed that the current magnitude at −80 mV (during the tail) was almost fully responsive to the change in [K+] during activation. This demonstrated that when the barrels containing external solution were flipped during activation, the external [K+] actually did change. However, it also raised the question as to how current magnitude could be so unresponsive to the change in driving force at 0 mV yet almost completely responsive at −80 mV. To examine this further, we examined the effects of changing [K+] over the entire voltage range between 0 and −80 mV. To accomplish this, we used a ramp protocol during the repolarization after channel activation. This allowed us to measure the reversal potential in effect during each of the three experimental conditions (represented by the three superimposed traces in Fig. 2) and to measure the changes in current magnitude as a function of voltage following elevation of [K+].
Fig. 5 A illustrates a set of three currents obtained similarly to those in Fig. 2 A except that, during repolarization, the voltage was ramped over a period of 5 ms to produce a gradual change in membrane potential between 0 and −80 mV. Fig. 5 B illustrates the ramped current traces and voltage protocol (shaded section of Fig. 5 A) on an extended time scale. As observed in Fig. 2, rapidly changing external [K+] from 0 to 10 mM on activated channels did not affect current magnitude (other than the small initial transient; Fig. 5 A, dashed line). Upon closing and reopening the channels, current magnitude was potentiated (10 K+, solid line). Fig. 5 C illustrates the current measured during the voltage ramp. Three observations can be made from this figure. First, currents reversed at identical membrane potentials when [K+] was changed during activation and after closing and reopening of the channels (−54.7 ± 2.9 mV and −56.4 ± 2.9 mV; n = 7; the predicted Vrev was −58 mV). This demonstrates that, in both situations, channels were exposed to identical [K+]. Second, as first demonstrated in Fig. 3, macroscopic conductance increased when external [K+] was elevated during activation (compare 0 K+ curve to 10 K+ dashed line). Third, when channels were closed and reopened, the conductance was increased further (10 K+, dotted line).
We next sought to determine whether the observed effects could be described by the Goldman-Hodgkin-Katz current (GHK) equation, wherein current magnitude can be accounted for simply by internal and external ion concentrations and a single permeability variable (see Eq. 1 in materials and methods).
Fig. 5 D illustrates three theoretical curves generated by the GHK equation. The solid line representing the 0 K+ condition was generated by setting the permeability factor, Pm, to a value that produced a reasonably good fit of the experimental 0 K+ data (we used the presumed actual [K+], which produced the theoretical reversal potential, rather than arbitrarily alter a [K+] in the equation to fit the experimental reversal potential). The theoretical 10 K+ data in Fig. 5 D were obtained by finding the change in Pm that produced reasonably good fits (by eye) of the respective 10 K+ experimental data in Fig. 5 C. The Pm values that produced these fits represented an increase in permeability of 15% when [K+] was elevated on activated channels and 85% once channels were closed and reopened. This compares well with the average increase in conductance obtained from Fig. 3 experiments (22.7 ± 2.6% and 93.1 ± 7.2%), especially in light of the fact that, during the ramp protocol, some channel closure is occurring. (Note that Pm, the permeability variable, is equivalent to conductance in this context, because the only observed membrane current was carried by K+ [Ferreira and Marshall, 1985].)
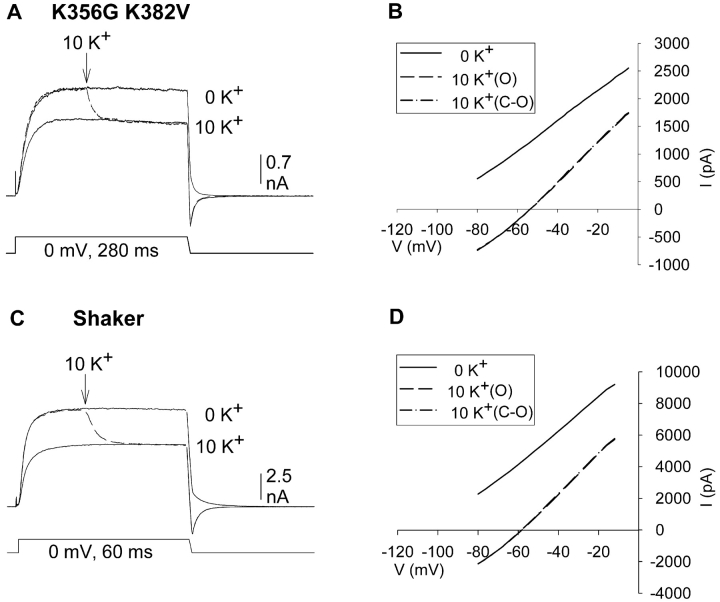
We next examined the I-V relationships in Kv2.1 K356G K382V and Shaker (Fig. 6) . Fig. 4 illustrated that, at 0 mV, this channel displayed only a single K+-dependent change in conductance, which occurred immediately upon changing [K+] during activation. We used the ramp I-V experiments to examine whether channel closure had an effect on current magnitude at any membrane potential between 0 and −80 mV. Our working hypothesis was that the K+-dependent change in wild type Kv2.1 current magnitude was due to three processes (driving force reduction, conductance increase associated with pore saturation and conductance increase associated with outer vestibule reorientation). Because these other two channels did not contain the outer vestibule-based potentiation mechanism, our hypothesis predicted that the I-V relationship in 10 mM external K+ would be unchanged by closing and reopening the channel.
Figure 6.
K+-dependent changes in the I-V curves of channels lacking outer vestibule lysines. (A) Three superimposed currents through Kv2.1 K356G K382V, activated by 280 ms depolarization to 0 mV. Trace 1 was recorded in 0 mM K+. During trace 2, [K+] was changed from 0 to 10 mM (dashed line). Trace 3 was recorded after closing and reopening the channels in 10 mM K+. (B) I-V relationships for the three traces, measured during the repolarizing ramp. Measured Vrev for 10 K+ data: −51.3 ± 1.5 and −51.7 ± 1.5 (n = 3). Slope conductances, measured between −50 and −10 mV for 10 K+ data: 34.4 ± 2.8 and 35.6 ± 2.3 (n = 3). (C) Three superimposed currents recorded from Shaker using the same protocol as in A except that the depolarizing step was 60 ms in duration and the ramp was 2 ms in duration. (D) I-V relationships for the three traces shown in C, measured during the repolarizing ramp. Average measured Vrev for 10 K+ data: −53.8 ± 1.2 and −54.1 ± 1.2 (n = 5). Average slope conductances, measured between −50 and −10 mV for 10 K+ data: 21.8 ± 1.3 and 24.8 ± 1.9 (n = 5).
Fig. 6 A illustrates three superimposed currents through Kv2.1 K356G K382V. Fig. 6 C illustrates three superimposed currents through Shaker, evoked by a 60-ms depolarization to 0 mV. For Shaker, the duration of the depolarizing stimulus was shortened, and solution switch was made earlier during the depolarization, to minimize effects of slow inactivation on current magnitude. In addition, membrane potential during the repolarization phase was ramped from 0 to −80 mV over a 2-ms period in Shaker. As in Fig. 4 experiments, elevation of external [K+] resulted in a complete and immediate change in current magnitude in both of these channels (Fig. 6, A and C). The I-V relationships, obtained during the voltage ramps for each of the three conditions, are illustrated in Fig. 6, B and D (K356G K382V and Shaker, respectively). As observed for Kv2.1, the reversal potential in 10 mM K+ was the same for currents immediately upon changing [K+] during activation (dashed line) and after closing and reopening the channels (dotted line). This indicated that [K+] changed completely during the activation period. As expected from Fig. 4, a conductance increase was observed when external [K+] was elevated during activation. Furthermore, as predicted by our hypothesis, there was no additional increase in conductance when channels were subsequently closed and reopened.
In contrast to Kv2.1, the I-V relationships in these two channels were not well modeled by the simple GHK equation. However, the nearly linear I-V curves allowed us to calculate a slope conductance between −50 and −10 mV. Conductance increased by 34.4 ± 2.8% (n = 3) in K356G K382V and 21.8 ± 1.3% (n = 5) in Shaker. These values are in good agreement with the conductance measurements at 0 mV (Fig. 4). Thus, these results are consistent with the hypothesis that a single voltage-independent process was responsible for the conductance increase that occurred upon elevation of external [K+].
The ability to change outer vestibule conformation depends on channel occupancy, not state of activation.
The results thus far indicated that the outer vestibule conformation could change when channels were closed, but not when they were activated. Two different mechanisms could account for this result. First, it was possible that the outer vestibule could only change conformation when channels were in the closed state, and that it was locked in position when channels were in the activated state. This possibility might reflect, for example, different positions of the S4 channel domain relative to the pore, or some other structural difference, between the two states. A second possibility was that differences in the ability of closed and activated channels to change outer vestibule conformation reflected a difference in occupancy of the channel by K+. When channels are activated, they are constantly occupied by K+. In contrast, when channels are closed, many or all sites in the conduction pathway are likely to be unoccupied. Consequently, the results are also consistent with a mechanism whereby the outer vestibule could reorient when the pore (or a K+ site in the pore) was unoccupied, but was locked in position when the pore (or a K+ site in the pore) was occupied. We did two different experiments to test these two hypotheses. First, we used TEA as an internal channel blocker to transiently interrupt entry of K+ into the pore of activated channels (Immke et al., 1999). Second, we examined the ability of the outer vestibule to change conformation when current through activated K+ channels was carried by a lower internal [K+].
Internal TEA.
When TEA binds to its internal blocking site, no K+ enters the pore and the pore becomes unoccupied. When the channel becomes unblocked, either because of the normal off kinetics of the blocker or because the empty pore becomes insensitive to internal TEA (Immke et al., 1999), K+ reenters and occupies the pore. We demonstrated previously that this technique could be used to modulate the percentage of channels in each outer vestibule conformation by lowering K+ occupancy of a selectivity filter binding site (Immke et al., 1999; Immke and Korn, 2000; Wood and Korn, 2000). In this experiment, we asked a different question: Does transient interruption of K+ flow into the pore, which transiently reduces pore occupancy, allow the outer vestibule to change conformation when in the activated state?
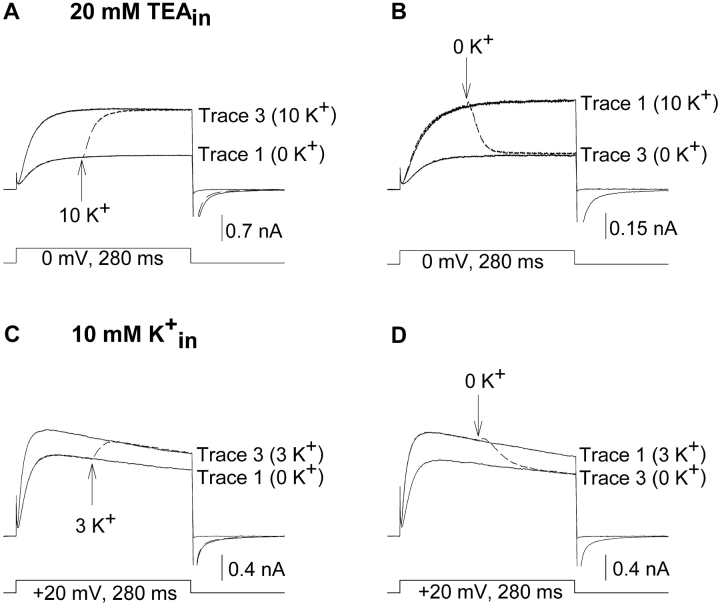
In the presence of 20 mM internal TEA, elevation of external [K+] from 0 to 10 mM during activation resulted in an immediate potentiation of outward current (Fig. 7 A). Upon closing and reopening the channels in the presence of 10 mM K+, current magnitude was identical to that obtained when [K+] was elevated during activation. The complete (97%) change in current magnitude during activation required 61.8 ± 1.8 ms (n = 4), ∼3 times longer than was required to change external solutions (see Fig. 1). Qualitatively similar results were obtained with 1 mM internal TEA (n = 3; unpublished data) and when currents were evoked by depolarization to +50 mV (n = 3; unpublished data). This potentiation was not due to a trans-ion knockoff effect, because (a) maximal potentiation was observed when [K+] was elevated on activated channels (i.e., there was no additional change in current magnitude when channels were closed and reopened), (b) changes in current magnitude were much slower than would be expected for a trans-ion knock off effect (∼3–4 times slower than time required for solution change), and (c) currents through Kv2.1 K356G K382V, which are blocked identically by internal TEA, but do not display potentiation associated with the change in outer vestibule conformation, were not potentiated when external [K+] was elevated in the presence of internal TEA (Wood and Korn, 2000). In the converse experiment, reduction of external [K+] during activation resulted in an immediate decrease in current magnitude (Fig. 7 B; 97% complete in 62.3 ± 3.3 ms, n = 4). Thus, even with high internal [K+], K+-dependent changes in current magnitude (and outer vestibule conformation) could occur when channels were activated, if an internal channel blocker was present to transiently interrupt the flow of K+ into the pore.
Figure 7.
K+-dependent changes in current magnitude during activation at low K+ occupancy. (A and B) The protocol was identical to that of Fig. 2 except that 20 mM TEA was included in the recording pipet. 20 mM internal TEA blocked the channel by ∼90% (Immke et al., 1999). (A) Elevation of [K+] from 0 to 10 mM, 100 ms after channels were activated, resulted in potentiation during activation (dashed line). Potentiation was 97% complete in 61.8 ± 1.8 ms (n = 4; the data were reasonably well fit by a single exponential, which produced a time constant of 18.4 ± 1.4 ms.). (B) Reduction of [K+] from 10 mM to 0 mM, 100 ms after channels were activated, reduced current magnitude during activation (dashed line). The change in current magnitude was 97% complete in 62.3 ± 3.3 ms (n = 4; the data were reasonably well fit by a single exponential, which produced a time constant of 16.2 ± 1.2 ms). (C and D) The protocol was identical to that of Fig. 2 except that internal [K+] was 10 mM, and currents were recorded at 20 mV. (C) Trace 1 was recorded in 0 external K+. In trace 2 (dashed line), external [K+] was changed from 0 to 3 mM at the arrow. Trace 3 was recorded after closing and reopening the channels in 3 mM external K+. (D) Similar experiment as in C, except that currents were initially recorded in 3 mM external K+, and in trace 2 (dashed line), external [K+] was switched from 3 to 0 mM during activation (at arrow). Essentially identical results were obtained on three cells each for experiments in C and D.
Low internal [K+].
As a second test of the hypothesis, we lowered pore occupancy by lowering internal [K+]. Fig. 7, C and D, illustrate currents carried by 10 mM internal K+. Trace 1 in Fig. 7 C illustrates a current recorded in 0 mM external K+. The dashed line illustrates the current response to changing external [K+] from 0 to 3 mM during activation. Trace 3 illustrates the current after closing and reopening of the channel in 3 mM external K+. Two points are to be made from this figure. First, in contrast to the results obtained with 100 mM internal K+ (Figs. 2 and 5 A), currents were immediately potentiated when [K+] was elevated during activation. Second, after closing and reopening of the channel in 3 mM external K+, current magnitude was identical to that attained on the previous stimulation. Similar results were obtained when external [K+] was reduced during activation (Fig. 7 D). With 10 mM internal K+, reduction of external [K+] from 3 to 0 mM during activation caused an immediate and complete reduction in current magnitude to the 0 K+ level. These results indicate that, with low internal [K+], outer vestibule conformation could change during activation with a change of external [K+]. Thus, the experiments of Fig. 7 indicate that the ability of the outer vestibule to change conformation did not depend directly on channel activation state but, rather, on occupancy of the pore by K+.
Part II. Changes in Current Magnitude due to Changes in Channel Saturation and Driving Force
Our goal in this section of the paper was to better understand why current magnitude in Kv2.1 changed so little when external [K+] was changed during activation (c.f. Figs. 2 and 5 A). In the absence of the special potentiation mechanism that involved changes in the outer vestibule conformation, changing external [K+] influenced current magnitude in Kv2.1 (and other channels) by two opposing processes. Elevation of external [K+] decreased current magnitude due to the shift in reversal potential (and consequent reduction in driving force) and increased current magnitude due to an increased macroscopic conductance associated with greater saturation of the pore. At some membrane potential, the current reduction due to decreased driving force would have to exactly balance the current increase due to increased conductance. This reversal point, at which there is no net change in current magnitude, occurs because the change in reversal potential shifts the I-V curve to the right, and the increased conductance steepens the slope of the I-V curve (this type of effect can be observed in Fig. 5 C). Moreover, the balance between these two processes requires that at potentials more negative than this reversal point, elevation of external [K+] will reduce current magnitude, whereas at potentials more positive than this reversal point, elevation of external [K+] will increase current magnitude. When external [K+] was switched between 0 and 10 mM on activated Kv2.1 channels, currents evoked by depolarization to 0 mV did not change size (Figs. 2 and 5 A). Thus, it appeared that 0 mV was the voltage at which this reversal point occurred in these experiments.
Despite the close fit to the prediction of the GHK equation (Fig. 5), the Kv2.1 results were surprising to us. The K+-dependent increase in conductance during activation was small (Fig. 3), and we expected a substantial reduction in current magnitude due to the large decrease in driving force. Indeed, the results obtained in the mutant Kv2.1 channel and in Shaker (Fig. 4, A and B) were more in line with our expectations. In Kv2.1 K356G K382V, for example, elevation of external [K+] markedly reduced current magnitude at 0 mV even though the increase in macroscopic conductance was twice that observed with Kv2.1. To more carefully examine the changes in Kv2.1 current magnitude associated with the combined change in driving force and conductance, we examined the effects of rapidly changing [K+] at different activation potentials (Fig. 8) .
Figure 8.
K+-dependent changes in current magnitude during activation at different membrane potentials. The protocol used in all panels was the same as in Fig. 2, except that currents were recorded at different voltages. (A–C) Data from wild type Kv2.1, recorded at the membrane potentials shown. In all panels, trace 1 was recorded in 0 mM K+. In trace 2 (dashed line), current was activated in the absence of external K+. 100 ms after de-polarization (at the arrow), the external solution was switched to one containing 10 mM K+. In trace 3, channels were closed and reactivated in the presence of 10 mM external K+. (D–F) Data from Kv2.1 K356G K382V, recorded at the membrane potentials shown. Data represent essentially identical results obtained from 3–9 cells at each membrane potential.
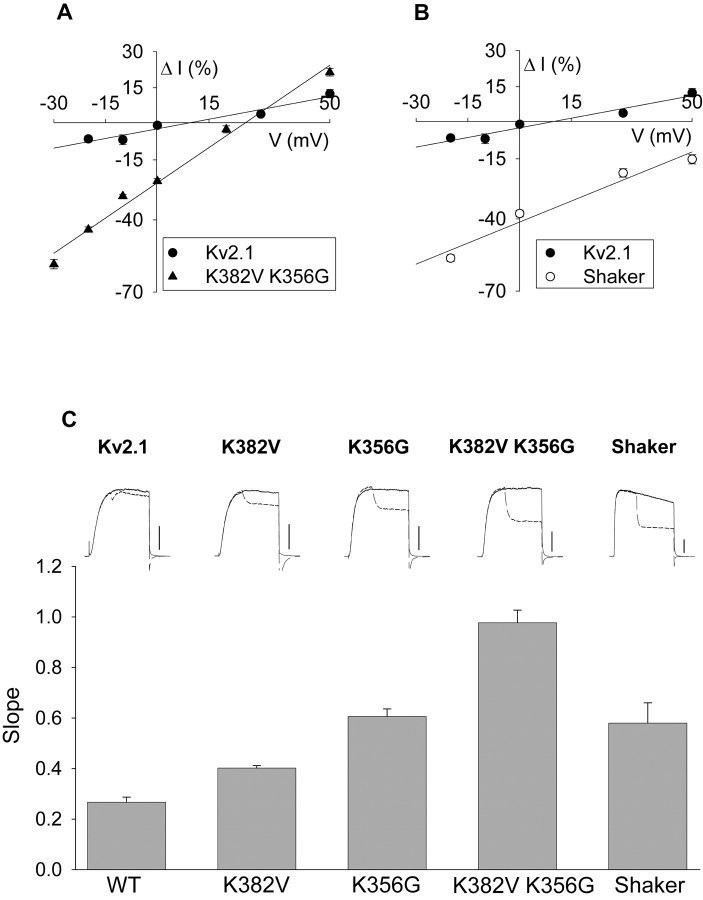
Fig. 8, A–C, illustrates currents recorded from Kv2.1 at 50, 0, and −10 mV, respectively. As described in Figs. 2 and 5 A, changing external [K+] when currents were evoked by stepping to 0 mV resulted in no change in current magnitude (Fig. 7 B, dashed trace). At 50 mV, the change in external [K+] on activated channels produced a small increase in current magnitude (Fig. 8 A, dashed line). Conversely, at −10 mV, the change in external [K+] on activated channels produced a small decrease in current magnitude (Fig. 8 C, dashed line). Fig. 9 A (circles) illustrates the change in current magnitude produced by the change in external [K+] on activated channels at several different potentials. Comparison of the data in Fig. 9 A (circles) with the theoretical curves from the GHK equation (Fig. 5 D) suggests that the balance between driving force reduction and conductance increase account for the observed results. The calculated curves in Fig. 5 D predict a K+-dependent increase in current magnitude of 12.7% at 50 mV and an 11.1% decrease in current magnitude at −20 mV. These values compare well with the experimentally observed 12.5% increase and 7% decrease in current magnitude at 50 mV and −20 mV, respectively (Fig. 9 A, circles). What is most relevant, however, is the remarkable insensitivity of Kv2.1 to these two processes. Between −20 and 30 mV, current through Kv2.1 changed by <7% when external [K+] was elevated from 0 to 10 mM.
Figure 9.
Sensitivity of current magnitude in different channels to changes in external [K+]. (A–B) Data were derived from experiments in Fig. 8, where the percentage change in current was measured by comparing the current magnitude in trace 2 (dashed lines) to that in trace 1. Data for Kv2.1 in A and B are identical and were duplicated for ease of comparison to other channels. Straight lines are linear regression fits to the data. Each data point represents 3–9 cells tested. (C, top) Currents recorded from channels indicated at a membrane potential of −20 mV. Solid line is current recorded in 0 mM external K+. The dashed line illustrates current recorded when external [K+] was switched from 0 to 10 mM 100 ms after the start of the depolarization. Calibration bars, 0.5 nA for wild-type Kv2.1; 1 nA for all other channels. (C, bottom) Data were collected and linear regression fits obtained for all channels as in A and B. The slope of the regression is plotted on the ordinate.
We demonstrated in part I of this paper that changes in outer vestibule conformation did not contribute to K+-dependent changes in current magnitude (or conductance) that occurred while channels were activated at 0 mV. Two additional observations indicate that the outer vestibule-based mechanism did not contribute to the change in current magnitude at positive and negative voltages. First, in Kv2.1, an additional change in current magnitude occurred upon closing and reopening the channels that was consistent with the occurrence of the conformational change. At 50 mV, upon closing and reopening the channels, current magnitude was increased further (Fig. 8 A, trace 3). At −10 mV, upon closing and reopening the channels, current was potentiated (Fig. 8 C, trace 3). This potentiation, which was expected of the outer vestibule-based mechanism, was opposite of the K+-dependent effect that occurred immediately during activation (Fig. 8 C, dashed line). Second, currents through the mutant channel, Kv2.1 K356G K382V, which are not influenced by the K+-dependent change in outer vestibule conformation, responded similarly to Kv2.1 when external [K+] was elevated during channel activation (Fig. 8, D–F). Current magnitude at 50 mV was increased (Fig. 8 D, dashed line), and current magnitude at −10 mV was decreased (Fig. 8 F, dashed line). The potential at which there was no net change in current shifted to 20 mV (Fig. 8 E). As illustrated in Fig. 9 A (filled triangles), this reflects a shift in the balance between the conductance increase and driving force reduction that accompanied the elevation in [K+]. Consistent with (a) the lack of outer vestibule-based potentiation mechanism in these channels and (b) the results of voltage ramp experiments (Fig. 6), current magnitude upon closing and reopening these channels in 10 mM K+ was identical to that observed immediately after [K+] was elevated during activation (Fig. 8, D–F, trace 3).
Outer vestibule lysines in Kv2.1 dampen the channel response to changes in external [K+].
The changes in current magnitude in Kv2.1 with changes in external [K+] were small over the entire range of voltages between −20 and 50 mV (Figs. 8, A–C, and 9 A, circles). Indeed, between −20 and 30 mV, current magnitude changed by 7% or less due to the combined effects of conductance increase and driving force reduction. Results with the Kv2.1 K356G K382V channel hinted at the fact that the two outer vestibule lysines may have been involved in this insensitivity. The lysines at positions 356 and 382 are both exposed to the conduction pathway (Gross et al., 1994; Kurz et al., 1995; Immke et al., 1999) and can influence the flow of K+ current through the chan-nel (Wood and Korn, 2000). Consequently, we asked whether the presence of these lysines in the outer vestibule reduced the sensitivity of Kv2.1 current to changes in external [K+]. To address this, we examined the K+-dependent changes in magnitude of current through activated channels in three mutant Kv2.1 channels that had outer vestibule lysines replaced by smaller, neutral amino acids.
Fig. 9 A (triangles) plots the change in current magnitude that resulted from changing external [K+] during activation of Kv2.1 K356G K382V, at voltages ranging from −30 to 50 mV. As in wild-type Kv2.1, the change in current magnitude was a linear function of voltage. Consequently, the slope of the line that describes this relationship can be considered a measure of the sensitivity of current magnitude to the change in external [K+]. Clearly, this mutant Kv2.1 channel that lacked outer vestibule lysines was significantly more sensitive to the change in [K+]. For example, at −20 mV, current was reduced by >40% in the mutant channel, compared with ∼7% in Kv2.1. Note also that, depending on what voltage channels were at, current responses to the change in external [K+] were much more wide-ranging in the absence of outer vestibule lysines. Upon elevation of external [K+] to 10 mM, current in the mutant channel could be unchanged (20 mV), reduced by >40% (−20 mV) or increased by ∼20% (50 mV). Over this same voltage range, current magnitude in wild-type Kv2.1 remained within 12% of control, regardless of membrane potential.
We then asked whether the lysines at position 382 or position 356 were more important for this change in sensitivity. Fig. 9 C illustrates currents evoked at −20 mV in five different channels (four derived from Kv2.1 and one derived from Shaker), arranged in order according to the magnitude of current change produced by the switch from 0 to 10 mM K+. The first four bars in Fig. 9 C indicate the slope of the curve, generated as in Fig. 9 A, for each of the channels derived from Kv2.1. Current magnitude in both Kv2.1 K382V and Kv2.1 K356G was significantly more sensitive to the change in external [K+] than the wild-type channel, with K356G being slightly but significantly more sensitive than K382V. The slope of the curve for Kv2.1 K356G K382V was equal to the sum of the slopes generated from the two single mutants. This additivity suggests that all of the lysines in the outer vestibule contributed linearly (albeit unequally) to the decrease in K+ sensitivity observed in the wild-type channel. Importantly, this additivity also suggests that neither mutation produced an increase in sensitivity due to an allosteric effect of either of the mutations.
Sensitivity of the Shaker potassium channel to the change in driving force.
We examined the K+-dependent change in current magnitude on Shaker in a similar type of experiment. The currents illustrated in Fig. 9 C, top right, represent the response of Shaker to the change in external [K+], recorded at −20 mV. As observed for currents recorded at 0 mV (Fig. 6 A), elevation of external [K+] during activation resulted in an immediate decrease in current magnitude. Fig. 9 B (open circles) and the fifth bar in Fig. 9 C illustrate the effect of elevating [K+] from 0 to 10 mM on activated Shaker channels over a range of membrane potentials. As with the Kv2.1 channel without lysines, Shaker was dramatically more sensitive to the change in external [K+] than Kv2.1. In Shaker, however, this increased sensitivity was manifest somewhat differently. The slope of the curve in Fig. 9 B is about twice that of Kv2.1. However, at all voltages between −20 and 50 mV, there was a large decrease in current magnitude.
DISCUSSION
Current magnitude through Kv2.1 is modulated by external [K+] via three different mechanisms. First, the outer vestibule of the channel can be in two different conformations (Immke and Korn, 2000), each of which is associated with different macroscopic current magnitudes (Wood and Korn, 2000). As external [K+] is elevated, the percentage of channels in each of these two conformations changes, such that at higher [K+], more channels are in the conformation that produces larger currents. This modulation mechanism, as far as we know, is unique to Kv2.x channels within the family of voltage-gated K+ channels. The second two mechanisms produce opposing changes in current magnitude that are common to all ion channels. Outward current will be reduced upon elevation of external [K+] due to the reduction in driving force. Conversely, to the extent that channels are not saturated by K+, outward current will increase upon [K+] elevation due to an increase in pore saturation (Hille and Schwartz; 1978; Neyton and Miller, 1988) (Note that increasing K+ occupancy of the pore can also influence current magnitude via an effect on gating. For example, elevation of external [K+] slows inactivation in Shaker Shaker [Lopez-Barneo et al., 1993][Baukrowitz and Yellen, 1996] and shifts the voltage dependence of inactivation in Kv2.1 [Wood and Korn, 2000]. These effects were not at issue in this paper.)
The findings in this paper demonstrate that, while activated, Kv2.1 channels are remarkably insensitive to changes in external [K+]. While channels are activated, the outer vestibule does not change conformation even if [K+] changes. Moreover, structural attributes of the Kv2.1 outer vestibule minimize the response of Kv2.1 channels to changes in K+ driving force, and reduce the K+-dependent change in macroscopic conductance. As a consequence of this insensitivity, when channels are in the activated state between −20 and 30 mV, current magnitude varies by <7% when external [K+] changes between 0 and 10 mM.
In contrast, when channels close and reopen in the presence of different external [K+], current magnitude through Kv2.1 can vary quite markedly. Because of the small increase in macroscopic conductance associated with increased channel saturation, and the relative insensitivity of Kv2.1 to the change in K+ driving force, the K+-dependent change in current magnitude under these conditions is primarily due to the change in outer vestibule conformation.
Control of Outer Vestibule Conformation
Previous results indicated that K+ occupancy of a specific selectivity filter binding site determined which conformation the outer vestibule was in (Immke et al., 1999; Immke and Korn, 2000). When this site was occupied, the conformation allowed both internal and external TEA to block the channel and resulted in larger macroscopic currents. When this site was unoccupied, the channel was completely insensitive to both inter-nal or external TEA and macroscopic currents were smaller. However, previous results suggested that changes in occupancy of this site might not be responsible for regulating whether or not the transition between the two conformations can occur. First, given the rapid flow of ions through the conduction pathway, it seemed unlikely that the channel was able to interconvert between the two conformations as ions traversed the pore. Second, channels could initially activate into one conformation or the other. Consequently, when activated, some channels initially had this site occupied and some did not. However, regardless of the initial occupancy state, application of high [TEA] did not cause channels to convert from one conformation to the other. Thus, occupancy of this particular site had no impact on the ability of the channels to change conformation when in the activated state.
The inability of high [TEA] to cause an interconversion between the two outer vestibule conformations was consistent with two possible mechanisms. First, the outer vestibule could have been locked in position when channels were in the activated state. In this case, once channels were activated in one or the other conformation, the conformation was fixed until channels closed. An alternative possibility was that the outer vestibule conformation could change when channels were activated, but only if K+ occupancy of the pore (or a site in the pore) changed. However, this latter possibility could only hold if application of external TEA didn't influence K+ occupancy of the pore (or the relevant site in the pore). The primary goal of experiments in part I of this paper was to determine how the K+-dependent change in outer vestibule conformation was regulated. We sought to determine whether the ability of the outer vestibule to change conformation was state dependent, whether the outer vestibule was ever locked in one or the other conformation, and what permitted or prevented the transition between locked and unlocked conformations.
The results in part I of this paper demonstrate that under normal conditions, (physiological internal [K+] and no internal channel blocker), the outer vestibule is locked when channels are activated. Consequently, once activated, the percentage of channels in each conformation remains fixed, even if external [K+] changes. If external [K+] changes during activation, a change in conformation is observed only after channels close and reopen. Similarly, if [K+] changes while channels are closed, channels open with a different distribution between the two outer vestibule conformations (Immke and Korn, 2000; Wood and Korn, 2000). Thus, under normal physiological conditions, channels must close in order for outer vestibule to change conformation.
We have no way to determine with currently available techniques whether (a) the outer vestibule can undergo transitions between conformations freely while channels are closed or (b) the outer vestibule in closed channels is fixed in one conformation or other and the redistribution between conformations occurs during the transition between closed and open states. Nonetheless, our results demonstrate that, under normal conditions, it is the [K+] present in the closed state that determines what percentage of channels will open into each of the two outer vestibule conformations.
Requirement for Pore Occupancy
Our data also demonstrate, however, that “conformational rigidity” is not associated directly with the activation state of the channel. The outer vestibule conformation remained fixed whether channels were activated at −20 or 50 mV. Because Kv2.1 channels are not maximally activated until ∼20 mV (Korn and Ikeda, 1995; Wood and Korn, 2000), these data suggest that the outer vestibule conformation could not change either when fully activated or with transitions into shallow closed or closed-inactivated states (e.g., see Klemic et al., 1998). In contrast, when K+ occupancy of the channel was held quite low, either with a low internal [K+] or by transiently interrupting K+ flow into activated channels by an internal channel blocker, outer vestibule conformation could change, regardless of membrane potential or activation state (Fig. 7). These data indicate that it is occupancy of a site in the channel by K+ that prevents activated channels from changing conformation. This dependence on occupancy would explain the ability of the channel to change conformation when the channel is closed but not when activated. When closed, occupancy of the pore is dramatically reduced, perhaps to the point of being empty of K+. When activated, the channel is always heavily occupied by K+.
Where Is the Site that Regulates Outer Vestibule Dynamics?
Occupancy of a particular selectivity filter site determines which of two conformations the channel is in (Immke and Korn, 2000). At low external [K+], some activated channels have this site occupied, whereas some do not. At higher [K+], this site is fully occupied in activated channels. Regardless of whether this site was fully occupied or not, the outer vestibule could not change conformations when external [K+] was changed during activation. Consequently, we conclude that the site that determines whether channels are able to change conformation is different from the site responsible for modulating which conformation the channel is in.
We cannot know unequivocally which K+ site in the pore is the site that regulates whether or not the outer vestibule can change conformations. However, several lines of evidence suggest that the central cavity may be the relevant site. We previously proposed a simple model of the K+-dependent conformational change, which postulated a simultaneous reorientation of the turret and internal TEA binding site (Immke et al., 1999). Structural information gleaned from the KcsA potassium channel (Doyle et al., 1998) suggested that such a simultaneous movement would also involve movement of the pore helices. The presence of K+ in the central cavity has been proposed to interact electrostatically with the pore helices (Doyle et al., 1998; Roux and MacKinnon, 1999; Zhou et al., 2001). In addition, the apparent affinity for this site in Shaker has been estimated to be near 60 mM (Thompson and Begenisich, 2001). This is consistent with our findings that the relevant site is largely unoccupied at an internal [K+] of 10 mM yet significantly occupied with an internal [K+] of 100 mM. This site would also be transiently unoccupied in the presence of internal TEA, and presumably empties when the channel closes (Miller, 1987; Demo and Yellen, 1992; Holmgren et al., 1997). Together, these experimental observations are consistent with the central cavity being the location of the K+ binding site associated with stabilizing outer vestibule conformation. Recent models of permeation through K+ channels support this hypothesis. According to these models, at low internal [K+], ions occupy the selectivity filter preferentially over the central cavity (Chung et al., 1999). This results from a combination of two influences. The selectivity filter sites have a higher affinity for K+ than the central cavity and a significant energy barrier appears to slow entry of K+ from the cytoplasm into the central cavity (Chung et al., 1999, 2002).
Finally, structural data from the KcsA potassium channel also support this view. Zhou et al. (2001) suggested that the selectivity filter region of KcsA could change conformation at low [K+] but not at high [K+]. Moreover, Zhou et al. (2001) suggested that in the presence of low [K+] and high [Na+], the selectivity filter was occupied by [K+] but the central cavity was not. While not completely analogous to our experiments, these data support the interpretation that in KcsA, the region associated with the selectivity filter can change conformations when selectivity filter sites are occupied. In contrast, at higher [K+], when the central cavity also becomes occupied, the KcsA selectivity filter is proposed to become rigid (Zhou et al., 2001). The change in Kv2.1 outer vestibule conformation appears to involve a subtle change in selectivity filter conformation (Immke et al., 1999). Thus, it seems reasonable to speculate that occupancy of the same site in the pore determines whether the selectivity filter and outer vestibule are rigid or can interconvert between different conformations.
Insensitivity of Kv2.1 Channels to Conformation-independent Effects of External K+
An enigmatic observation in previous work was that the outer vestibule conformation mechanism could potentiate currents by as much as 40% with elevation of external [K+] from 0 to 10 mM (Wood and Korn, 2000), even though only a small percentage (estimated to be ∼20%) of channels were apparently involved (see Immke and Korn, 2000). This was surprising because we would have expected the outer vestibule potentiation effect to be superimposed on a significant decrease in current magnitude associated with the reduction in K+ driving force.
The results of part II of this paper demonstrated that currents through Kv2.1 channels are remarkably insensitive to the change in K+ driving force. Currents through Kv2.1 displayed a small (20%) K+-dependent increase in macroscopic conductance associated with the increase in pore saturation. This effect, which would increase outward current magnitude, was well within the range observed in other channels (Fig. 4; Yang and Sigworth, 1998). Because of the relative insensitivity of Kv2.1 currents to the change in driving force, at ∼0 mV, the current reduction associated with the change in driving force exactly balanced the increase associated with the increase in conductance (Figs. 2, 5 A, 8, and 9).
There are several functional ramifications of this insensitivity to the change in driving force. First, given the fact that the K+-dependent change in outer vestibule conformation doesn't occur when channels are activated, currents through Kv2.1 are almost insensitive to changes in external [K+] when channels are activated. Second, the insensitivity of Kv2.1 to the change in driving force permits significant macroscopic current potentiation due to a mechanism that occurs in only a small percentage of channels. The importance of this factor is most easily appreciated by comparison of Kv2.1 with the Shaker potassium channel. When external [K+] is elevated from 0 to 10 mM, Kv2.1 currents at membrane potentials between −20 and 30 mV are changed by <7% (Fig. 9 B). In contrast, this same elevation of [K+] reduces current magnitude in Shaker by 20–55%. Indeed, a potentiation mechanism such as that described in Kv2.1 could not work in a channel with Shaker's response to the change in driving force. Third, the insensitivity of Kv2.1 to the change in driving force allows the outer vestibule potentiation mechanism to influence current magnitude relatively uniformly at different membrane potentials. This point is illustrated by comparison of Kv2.1 with either Shaker or the mutant Kv2.1 channel (Kv2.1 K356G K382V). In Kv2.1, currents recorded at potentials between −20 and 30 mV varied by ±7% when [K+] was elevated from 0 to 10 mM (Fig. 9). In contrast, elevation of [K+] from 0 to 10 mM changed current magnitude in these other channels by anywhere from 0 to 55%, depending on membrane potential (Fig. 9, A and B). The significance of these Kv2.1 channel properties is underscored by the possibility that this channel may have particular functional importance under conditions where external [K+] can change (discussed below).
The Structural Basis for the Insensitivity of Kv2.1 to the Change in Driving Force
This insensitivity of Kv2.1 to changes in K+ driving force appears to be due in large part to the presence of lysines at two positions in the outer vestibule that are unique to Kv2.1. Removal of these lysines resulted in a fourfold increase in channel sensitivity to changes in [K+] (Fig. 9, A and C). Moreover, as described above, mutation of these outer vestibule lysines resulted in a nonuniform K+-dependent change in current magnitude as a function of voltage. Thus, these outer vestibule lysines not only reduced the absolute change in current magnitude associated with the change in external [K+], but over a physiologically relevant range of membrane potentials, the small variation that did occur was more nearly uniform.
Although the precision of the fourfold symmetry of the Kv channels is not understood with certainty, the structure of KcsA suggests that the outer vestibule of the pore is quite symmetric (Doyle et al., 1998). Consequently, we assume that the lysines on all four channel subunits are similarly located in the outer vestibule. Mutation of just the position 382 lysines, or just the position 356 lysines, each produced a partial increase in sensitivity to [K+] (Fig. 9 C). The K356G mutation produced a slightly greater increase in sensitivity to changes in [K+] than the K382V mutation. However, the increase in sensitivity produced by each of these mutations summed to the increased sensitivity of the double mutation. This suggests that all outer vestibule lysines contributed linearly to the reduction in channel sensitivity to variation in external [K+], with the lysines at position 356 assuming a slightly more significant role. Moreover, the summing effect of these mutations argue against the possibility that either mutation produced a nonspecific, allosteric change in channel function.
Significance of These Findings
The Kv2.1 channel may have particular functional significance under conditions of high frequency firing (Du et al., 2000), which can cause significant elevations of extracellular [K+] (Somjen and Giacchino, 1985; Xiong and Stringer, 1999). Consequently, the [K+]-dependent change in outer vestibule conformation and associated properties could be of important functional significance under conditions of prolonged or high frequency firing. The findings in this paper specifically address the control of current magnitude by external [K+]. At the simplest level of analysis, changes in external [K+] during activation have essentially no effect on current magnitude. However, once channels enter deep closed states and reopen in the presence of higher external [K+], macroscopic current will be larger and activate more quickly (Wood and Korn, 2000). Under conditions of high frequency firing, channels may open and close rapidly, and significant numbers of channels may enter closed inactivated states (Klemic et al., 1998). This latter observation suggests that under high frequency firing conditions, channels occupy nonconducting states that are different in some way from the deep closed states obtained with prolonged hyperpolarization. Channels maintained at −20 mV also enter shallow closed and perhaps closed-inactivated states. Elevation of [K+] at −20 mV does not produce a change in outer vestibule conformation (Figs. 8 and 9). Thus, as discussed above, the ability of the outer vestibule to change conformation does not depend directly on channel gating state. However, one might imagine that during rapidly fluctuating membrane potentials, K+ occupancy of the pore might change. Consequently, it will be of interest to determine whether current magnitude in channels that participate in high frequency firing undergo the K+-dependent conformational change, and consequent change in current magnitude.
Acknowledgments
We thank Joseph Consiglio, Kathleen Galle, and Josef Trapani for insightful comments on the manuscript.
Supported by the National Science Foundation and National Institutes of Health NS41090.
References
- Baukrowitz, T., and G. Yellen. 1996. Use-dependent blockers and exit rate of the last ion from the multi-ion pore of a K+ channel. Science. 271:653–656. [DOI] [PubMed] [Google Scholar]
- Chung, S.-H., T.W. Allen, M. Hoyles, and S. Kuyucak. 1999. Permeation of ions across the potassium channel: Brownian dynamics studies. Biophys. J. 77:2517–2533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung, S.-H., T.W. Allen, and S. Kuyucak. 2002. Modeling diverse range of potassium channels with Brownian dynamics. Biophys. J. 83:26–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demo, S.D., and G. Yellen. 1992. Ion effects on gating of the Ca2+-activated K+ channel correlate with occupancy of the pore. Biophys. J. 61:639–648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyle, D.A., J.M. Cabral, R.A. Pfuetzner, A. Kuo, J.M. Gulbis, S.L. Cohen, B.T. Chait, and R. MacKinnon. 1998. The structure of the potassium channel: molecular basis of K+ conduction and selectivity. Science. 280:69–77. [DOI] [PubMed] [Google Scholar]
- Du, J., L.L. Haak, E. Phillips-Tansey, J.T. Russell, and C.J. McBain. 2000. Frequency-dependent regulation of rat hippocampal somato-dendritic excitability by the K+ channel subunit Kv2.1. J. Physiol. 522:19–31. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira, H.G., and M.W. Marshall. 1985. The Biophysical Basis of Excitability. Cambridge University Press.
- Gross, A., R. Abramson, and R. MacKinnon. 1994. Transfer of the scorpion toxin receptor to an insensitive potassium channel. Neuron. 13:961–966. [DOI] [PubMed] [Google Scholar]
- Hille, B., and W. Schwartz. 1978. Potassium channels as multi-ion pores. J. Gen. Physiol. 72:409–442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holmgren, M., P.L. Smith, and G. Yellen. 1997. Trapping of organic blockers by closing of voltage-dependent K+ channels. Evidence for a trap door mechanism of activation gating. J. Gen. Physiol. 109:527–535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoshi, T., W.N. Zagotta, and R.W. Aldrich. 1990. Biophysical and molecular mechanisms of Shaker potassium channel inactivation. Science. 250:533–538. [DOI] [PubMed] [Google Scholar]
- Hwang, P.M., M. Fotuhi, D.S. Bredt, A.M. Cunningham, and S.H. Snyder. 1993. Contrasting immunohistochemical localizations in rat brain of two novel K+ channels of the Shab subfamily. J. Neurosci. 13:1569–1576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke, D., M.J. Wood, L. Kiss, and S.J. Korn. 1999. Potassium-dependent changes in the conformation of the Kv2.1 potassium channel pore. J. Gen. Physiol. 113:819–836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Immke, D., and S.J. Korn. 2000. Ion-ion interactions at the selectivity filter: evidence from K+-dependent modulation of tetraethylammonium efficacy in Kv2.1 potassium channels. J. Gen. Physiol. 115:509–518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jurman, M.E., L.M. Boland, Y. Liu, and G. Yellen. 1994. Visual identification of individual transfected cells for electrophysiology using antibody-coated beads. Biotechniques. 17:876–881. [PubMed] [Google Scholar]
- Klemic, K.G., C.-C. Shieh, G.E. Kirsch, and S.W. Jones. 1998. Inactivation of Kv2.1 potassium channels. Biophys. J. 74:1779–1789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korn, S.J., and S.R. Ikeda. 1995. Permeation selectivity by competition in a delayed rectifier potassium channel. Science. 269:410–412. [DOI] [PubMed] [Google Scholar]
- Kurz, L.L., R.D. Zuhlke, H.-J. Zhang, and R.H. Joho. 1995. Side chain accessibilities in the pore of a K+ channel probed by sulfhydryl-specific reagents after cysteine-scanning mutagenesis. Biophys. J. 68:900–905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lopez-Barneo, J., T. Hoshi, S.H. Heinemann, and R.W. Aldrich. 1993. Effects of external cations and mutations in the pore region on C-type inactivation of Shaker potassium channels. Receptors Channels. 1:61–71. [PubMed] [Google Scholar]
- Miller, C. 1987. Trapping single ions inside single ion channels. Biophys. J. 52:123–126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murakoshi, H., and J.S. Trimmer. 1999. Identification of the Kv2.1 K+ channel as a major component of the delayed rectifier K+ current in rat hippocampal neurons. J. Neurosci. 19:1728–1735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neyton, J., and C. Miller. 1988. Discrete Ba2+ block as a probe of ion occupancy and pore structure in the high-conductance Ca2+-activated K+ channel. J. Gen. Physiol. 92:569–586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux, B., and R. MacKinnon. 1999. The cavity and pore helices in the KcsA K+ channel: electrostatic stabilization of monovalent cations. Science. 285:100–102. [DOI] [PubMed] [Google Scholar]
- Somjen, G.G., and J.L. Giacchino. 1985. Potassium and calcium concentrations in interstitial fluid of hippocampal formation during paroxysmal responses. J. Neurophysiol. 53:1098–1108. [DOI] [PubMed] [Google Scholar]
- Thompson, J., and T. Begenisich. 2001. Affinity and location of an internal K+ ion binding site in Shake K channels. J. Gen. Physiol. 117:373–383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wood, M.J., and S.J. Korn. 2000. Two mechanisms of K+-dependent potentiation in Kv2.1 potassium channels. Biophys. J. 79:2535–2546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiong, Z.-Q., and J.L. Stringer. 1999. Astrocyte regulation of the recovery of extracellular potassium after seizures in vivo Eur. J. Neuro. 11:1677–1684. [DOI] [PubMed] [Google Scholar]
- Yang, Y., and F.J. Sigworth. 1998. Single channel properties of IKs potassium channels. J. Gen. Physiol. 112:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou, Y., J.H. Morais-Cabral, A. Kaufman, and R. MacKinnon. 2001. Chemistry of ion coordination and hydration revealed by a K+ channel - Fab complex at 2.0 A resolution. Nature. 414:43–48. [DOI] [PubMed] [Google Scholar]