Abstract
Chronic hepatitis C virus (HCV) infections with genotype 2 or 3 are associated with favourable sustained virologic response (SVR) rates. However, genotype 3 may respond less well. We reassessed all treatment-naive patients with genotype 2 and 3 participating in a large expanded-access, non-randomized, open-label trial, evaluating 180μg pegylated interferon (peg-IFN) alpha-2a (40kD) once weekly and 800 mg/day ribavirin for 24–48 weeks. Factors measured prior to initiation of antiviral therapy were considered in the multiple logistic regression model for predicting SVR. In total, 180 patients were analysed of which 72 (40%) were infected by genotype 2 and 108 (60%) genotype 3. The baseline characteristics between patients infected by genotype 2 or 3 were no different including the distribution of hepatic fibrosis stages by METAVIR score. Overall SVR was lower in those patients infected with genotype 3. The significant multivariate predictors of lack of SVR were hepatic fibrosis (P = 0.014) and genotype 3 (P = 0.030). The negative impact of cirrhosis (METAVIR score F4) on treatment response was more evident among subjects with genotype 3 than those with genotype 2 (P = 0.027). There is significant interaction between cirrhosis and genotype 3 leading to a poor antiviral response in such patients requiring an alternate management strategy. This finding should be confirmed in a larger population.
Keywords: cirrhosis, genotype, hepatic fibrosis, hepatitis C virus, interferon therapy, outcomes, sustained viral response
Introduction
Antiviral therapy for the treatment of hepatitis C virus (HCV) infection has evolved over time from interferon monotherapy to combination therapy with interferon and ribavirin (RBV), to the present standard of care, namely pegylated interferon (peg-IFN) and ribavirin [1–7]. Several factors have become evident as predictors of response to antiviral therapies; however, none are more powerful than genotype. Genotype 2 and 3 infections are consistently associated with significantly higher rates of sustained virological response (SVR) compared with genotype 1 infections [8–12]. Although the precise biological explanation for the difference in sustained virological response (SVR) rates among genotype 1 vs genotype 2 and 3 remains elusive, it has been clearly demonstrated that viral kinetics in response to interferon therapy differ between the two groups in both the first and second phase of viral decline. The viral decline among genotype 2 and 3 infections is up to eight times faster than that of genotype 1 [13, 14]. This rapid virological response to therapy has prompted the development of shorter courses of therapy for genotype 2 and 3 from the traditional 48 weeks to as few as 12–16 weeks in investigative protocols, whenever a rapid virological response (RVR) is achieved with HCV polymerase chain reaction (PCR) negativity after 4 weeks of therapy [9, 11, 12].
As a result of their favourable antiviral response, genotype 2 and 3 infections are frequently grouped together when the results of clinical trials are evaluated and hence also in treatment guidelines [9, 11, 12]. More recent studies, however, have reported that genotype 3 infections are associated with lower rates of SVR than genotype 2 infections in clinical trials of treatment with pegylated interferon and ribavirin [8, 9, 11, 12]. Although genotype has been a consistent factor associated with treatment response among genotype 2 and 3 patients, there is little consensus regarding other factors associated with differential response between patients with genotype 2 and genotype 3. Dalgard et al. [9] in a pilot study of a short course (14 weeks) of therapy with peg-IFN alpha-2b and RBV demonstrated that the lack of bridging fibrosis/cirrhosis was an important factor associated with SVR in a mixed genotype 2/3 cohort of patients. Von Wagner et al. [12] and Zeuzem et al. [8] found that a high baseline viral load but not fibrosis was associated with a lack of SVR, especially among patients with genotype 3 [8, 12]. Finally, a study by Mangia et al. [11] did not identify any factors other than genotype that predicted response among the study population consisting primarily of patients with genotype 2.
Response to antiviral therapy is not the only differentiating characteristic between the two genotypes. Genotype 3 has long been known to be associated with hepatic steatosis independent of other factors such as body mass index (BMI) and alcohol intake, whereas no such relationship exists between hepatic steatosis and genotype 2 [15–18]. Genotype 3 has also been demonstrated to have considerable differences in E2 glycoprotein compared with genotype 1, which appears to preferentially induce apoptosis, possibly promoting hepatic fibrogenesis [19, 20]. The most striking evidence demonstrating the direct impact of genotype 3 infection on hepatocytes is the reversal of steatosis with successful antiviral therapy [21, 22].
Thus genotype 2 and 3 appear to behave differently in the host as well as in their response to antiviral therapy. The aim of our study was to confirm the difference in SVR among genotype 2- and genotype 3-infected HCV patients and determine factors associated with antiviral response to peg-IFN alpha-2a and RBV, as well as exploring interactions between these factors and genotype.
Patients and methods
Study population
This study is a re-analysis of a large Canadian expanded-access, multi-centred, non-randomized, open-label phase III-B trial evaluating 180 μg 40-kDa peg-IFN (Pegasys®; F. Hoffman-La Roche Ltd, Pharamaceuticals Division, Granzacherstrasse, Basel, Switzerland) once weekly and 800 mg/day RBV (Copegus®; F. Hoffman-La Roche Ltd, Pharamaceuticals Division, Granzacherstrasse, Basel, Switzerland) for 24 or 48 weeks for the therapy of chronic hepatitis C [23, 24]. The patients were allocated at the discretion of the site investigator, to either a planned 24 or 48 weeks with peg-IFN and RBV. At the time of the Canadian study, the results of Hadziyannis et al. regarding the equivalence of SVR by 24- or 48-week therapy in genotype 2 and 3 had not been published [10]. Our study specifically examined the differential treatment effect of genotype 2 and 3 infections in those naïve to antiviral therapy who underwent at least 12 weeks of therapy. The protocol has been described in detail elsewhere [23, 24]. Briefly, between April 2001 and June 2004, patients were recruited from 18 sites across Canada according to standard eligibility criteria which included positive HCV antibody, positive qualitative HCV-RNA, lack of co-infection with hepatitis B or human immunodeficiency virus (HIV), absence of another cause of liver disease and a liver biopsy within the past year. Liver biopsy samples were analysed by local expert pathologists and staged according to the previously validated METAVIR fibrosis score [25]. In this study, we used stringent definition of cirrhosis requiring histological finding of regenerative nodule surrounded by fibrosis (F4 fibrosis score).
Sustained virological response was based on a negative qualitative HCV PCR result (COBAS Amplicor HCV Test v 2.0; F. Hoffman-La Roche Ltd, Pharmaceuticals Division, Grenzacherstrasse, Basel, Switzerland) 24 weeks after the end of the prescribed treatment duration (i.e. 48 weeks in the 24-week treatment arm and 72 weeks in the 48-week treatment arm). If HCV PCR testing was not available 24 weeks after completion of treatment, the patient was declared a treatment failure.
Genotype was determined by the Bayer VERSANT HCV Genotype Assay (Inno Lipa, Innogenetics, Technologiepark, Gent, Belgium). All HCV virology analyses were performed at the British Columbia Centre of Disease Control Virology Laboratory, Vancouver, BC, Canada.
Statistical analysis
Only factors available prior to the initiation of antiviral therapy were considered as potential predictors in the analysis (age, gender, BMI, baseline viral load, genotype and hepatic fibrosis). Univariate associations between SVR and categorical predictors were assessed with Fisher’s exact test (Wilcoxon’s rank-sum test for numeric predictors). Unlike previous studies, the variables entered into the multiple logistic regression (MLR) model were analysed as either continuous or ordinal variables and not grouped into dichotomous variables. This approach was used, as cut-off points for variables such as viral load and METAVIR fibrosis score are not known a priori. Genotype and factors potentially associated with SVR on univariate analysis (P < 0.15) were then entered into the MLR model. Thereafter, the differential influence of predictor variables among genotype was assessed graphically and if required statistically by modelling the cross-product of the variable of interest with genotype in an MLR model. The alpha level of significance for a two-tailed test was considered to be 0.05. All statistical analyses were performed using SAS v. 9.1 (SAS Institute, Cary, NC, USA).
Results
One hundred and eighty patients met the eligibility criteria. Baseline data were complete for all patients except for one (genotype 2) who did not have data for BMI. Baseline characteristics of the 180 patients are presented in Table 1. There were 126 (70%) patients assigned to the 24-week treatment group and 54 (30%) to the 48-week group. Treatment was discontinued before week 12 in 12 (7%) patients, five (7%) with genotype 2 and seven (6%) with genotype 3, because of either poor tolerance or adverse events. There was no difference among patients infected with genotype 2 or 3.
Table 1.
Characteristics of study population according to genotype
| Characteristics | Genotype 2 (n = 72) | Genotype 3 (n = 108) | Genotype 2 and 3 (n = 180) |
|---|---|---|---|
| Female | 30 (42) | 37 (34) | 67 (37) |
| Age (years)a | 48 | 40 | 44 |
| BMI (kg/m2)a, b | 28 | 27 | 27 |
| log10 viral load (IU/L)a | 5.51 | 5.82 | 5.70 |
| METAVIR score | |||
| 0 | 5 (7) | 14 (13) | 19 (11) |
| 1 | 18 (25) | 26 (24) | 44 (24) |
| 2 | 27 (38) | 37 (34) | 64 (36) |
| 3 | 13 (18) | 19 (18) | 32 (18) |
| 4 | 9 (12) | 12 (11) | 21 (11) |
| Treatment duration | |||
| 24 weeks | 45 (62) | 81 (75) | 126 (70) |
| 48 weeks | 27 (38) | 27 (25) | 54 (30) |
| Early withdrawalc | 5 (7) | 7 (6) | 12 (7) |
| Late drop outd | 3 (4) | 9 (9) | 12 (7) |
Values in parentheses are percentages.
Mean.
One patient (genotype 2) did not have data of pretreatment BMI available.
These patients withdrew before week 12 either due to poor tolerance or adverse events.
These patients dropped out or did not submit blood sample for SVR assessment 24 weeks after completing therapy.
Another 12 (7%) patients, three (4%) infected with genotype 2 and nine (9%) infected with genotype 3, either dropped out or did not submit a blood sample to the central laboratory for the final determination of SVR 24 weeks after completion of therapy. These patients were all considered treatment failures and assigned to the ‘no SVR’ category.
Among the 67 genotype 2 patients who had undergone at least 12 weeks of therapy, 54 (81%) achieved an SVR compared with 71 of 101 (70%) genotype 3 patients. Factors significantly associated with SVR in the whole cohort of 180 patients on univariate analysis included METAVIR fibrosis score (P = 0.004), BMI (P = 0.018) and treatment group (P = 0.033). As shown in Table 2, age, gender and baseline log viral load were not significant on univariate analysis.
Table 2.
Univariate analysis of factors at baseline potentially associated with sustained virological response
| SVR | No SVR | p-value | |
|---|---|---|---|
| Age (years)a | 44 | 43 | 0.595c |
| Gender | 0.315d | ||
| Female | 50 (40) | 17 (31) | |
| Male | 75 (60) | 38 (69) | |
| log10 viral load (IU/L)a | 5.68 | 5.75 | 0.861c |
| Genotype | 0.248d | ||
| 2 | 54 (43) | 18 (33) | |
| 3 | 71 (57) | 37 (67) | |
| METAVIR fibrosis score | 0.004d | ||
| 0 | 18 (14) | 1 (2) | |
| 1 | 33 (26) | 11 (20) | |
| 2 | 46 (37) | 18 (33) | |
| 3 | 19 (15) | 13 (24) | |
| 4 | 9 (7) | 12 (22) | |
| BMI (kg/m2)a, b | 27 | 28 | 0.018c |
| Assigned treatment duratione | 0.033d | ||
| 24 weeks | 94 (75) | 32 (58) | |
| 48 weeks | 31 (25) | 23 (42) |
Values given in parentheses are percentages.
Mean.
One patient did not have data available for BMI.
Wilcoxon rank-sum test.
Fisher’s exact test.
When the 12 patients (five from 24-week therapy and seven from 48-week therapy) who withdrew early (before week 12) due to poor tolerance or adverse events are excluded, this factor loses its significance (P = 0.167).
Genotype, METAVIR fibrosis score, treatment group and BMI were then assessed in an MLR model with fibrosis score considered as a categorical variable with values from 0 to 4. In this initial model, genotype and hepatic fibrosis were the only independent variables significantly associated with SVR. The odds ratios associated with lack of SVR are presented in Table 3. Genotype 3 infections had an overall 2.29 greater odds for lack of SVR compared with genotype 2 infections, while a fibrosis score of F3 or F4 compared with F0 had 10.90 and 27.90 greater odds for lack of SVR, respectively. Those assigned to the 48-week treatment duration also had lower SVR; however, when the 12 subjects who withdrew early (before 12 weeks of therapy) were removed from the analysis this factor lost significance (P = 0.167).
Table 3.
Multivariate analysis of factors associated with lack of SVR
| Variable | Odds ratio (95% CI) | p-value |
|---|---|---|
| Genotype | ||
| 2 | 1 | |
| 3 | 2.29 (1.08–4.83) | 0.030 |
| METAVIR fibrosis score | 0.014 | |
| 0 | 1 | |
| 1 | 5.50 (0.63–48.32) | 0.124 |
| 2 | 7.58 (0.91–63.19) | 0.061 |
| 3 | 10.90 (1.23–96.40) | 0.032 |
| 4 | 27.90 (2.93–265.73) | 0.004 |
| BMIa | 1.07 (0.99–1.16) | 0.102 |
One patient did not have data on BMI available and was not included in the MLR model.
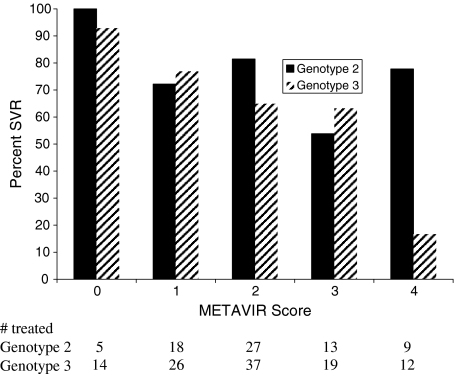
In order to further explore the possibility of an interaction between genotype and fibrosis, SVR rates were plotted according to genotype and METAVIR fibrosis score. Figure 1 demonstrates that patients with genotype 3 and cirrhosis had lower rates of SVR compared with genotype 2 patients with cirrhosis. Patients with genotype 3 and cirrhosis had an SVR rate of 17% (2/12) compared with 78% (7/9) among those with genotype 2 and cirrhosis (P < 0.001; 95% CI for difference 0.27–0.95). This was assessed statistically by constructing an MLR model including genotype, cirrhosis (presence/absence), treatment group and BMI as well as the cross-product of genotype and cirrhosis. This MLR model demonstrated that there is a significant interaction between cirrhosis and genotype, where genotype is an important effect modifier of the negative impact of cirrhosis on response (P = 0.027). Those patients with genotype 3 and cirrhosis do worse than those with genotype 2 and cirrhosis. This difference was not due to adherence as the latter finding remained intact even when data were reanalysed after excluding the 12 patients who withdrew early (before 12 weeks of therapy) or the 12 patients who did not submit a blood sample for determination of SVR 24 weeks after completing the treatment course.
Fig. 1.
Sustained virological rates according to genotype and METAVIR fibrosis score There was a significant interaction between genotype and cirrhosis (F4) (P = 0.027). P-value was derived from the cross-product of genotype and cirrhosis in a multivariate logistic regression model of SVR including cirrhosis (presence/absence), treatment group, BMI and genotype.
Discussion
Therapy with peg-IFN alpha 2a and ribavirin 800 mg/day achieves SVR rates in over 70% when those with genotype 2 and 3 infections are treated as a homogenous group [10]. The remainder either does not respond to antiviral therapy or relapses after cessation of therapy. This study demonstrates that there was a significant interaction between cirrhosis and genotype 3 infections. The SVR rate among genotype 2 infections with cirrhosis was 78% compared with 17% among those patients with genotype 3 and cirrhosis. Our findings show the negative effect on treatment response of advanced fibrosis, especially cirrhosis, is limited to genotype 3 infections with no demonstrable effect on genotype 2.
The influence of hepatic fibrosis on responsiveness to antiviral therapy has been previously demonstrated in one other clinical trial involving patients with genotype 2 and 3 infections [9]. Other investigators, however, have not found the same association between hepatic fibrosis and SVR among genotypes 2 and 3 [8, 11, 12].
There are several possible explanations for the discrepancy between our results and those of other trials of peg-IFN for the treatment of genotype 2 and 3. Similar to the study by Dalgard et al. [9], more than a quarter of our patients had advanced fibrosis (17% F3 and 11% F4); this compares well with the 18% F3 plus only 6% F4, and 18% of patients with METAVIR fibrosis score of ≥3 in the studies of Zeuzem et al. [8] and Mangia et al. [11], respectively. In these two studies, demonstrating an association between baseline viral load and SVR among genotype 3, the focus was on courses of therapy of ≤24 weeks. In the study by Von Wagner et al. [12], the group treated for 16 weeks with genotype 3 and a high baseline viral load, had a poorer response.
Most importantly, during the development of our MLR analysis we did not dichotomize variables such as fibrosis score and baseline viral load as was done in previously mentioned studies of patients with genotype 2 and 3. This analytical approach allowed for improved statistical power and data-driven exploratory analysis rather than arbitrarily choosing non-data-driven cut-off points to dichotomize variables.
Our finding of an interaction between genotype and cirrhosis is limited by the sample size of 21 patients with cirrhosis in our study. Of course there are problems with inter-observer variability for the histological scoring of liver biopsies. This is less of a problem in staging of fibrosis compared with grading of activity especially with expert pathologists [26]. However, one would expect that any variability in identifying cirrhosis would occur equally in patients with genotype 2 or 3. As the power was robust, it is unlikely that this represents a type 1 statistical error. Although our sample size is small, it is larger than the number of patients with advanced fibrosis in the trials listed above specifically addressing the treatment of genotype 2 and 3 infections. Because of the published recommendations suggesting that a pretreatment liver biopsy does not need to be performed in those patients with genotype 2 and 3 there is a paucity of data available from other clinical studies to address the interaction between genotype 3 and fibrosis [27]. Regardless of this, our findings will need to be confirmed in subsequent studies with a larger population.
The precise pathophysiological mechanism explaining the difference in SVR among patients with cirrhosis and genotype 2 vs genotype 3 is uncertain. Yet this may be explained in part by the different early viral dynamics between the two genotypes in response to antiviral therapy, especially among those with advanced fibrosis. Early decreases in HCV viral load have been shown by several investigators to be an independent predictor of subsequent SVR to antiviral agents [9, 28–30]. Hepatic fibrosis has been demonstrated to be associated with a slowing in the decline in HCV-RNA within the first 24 h of interferon therapy [31], and lack of fibrosis is an independent predictor of rapid virological response [9]. Genotype 2 infections are associated with a more rapid, free virion clearance rate, better inhibition of viral replication and enhanced killing of HCV-infected cells in response to IFN therapy [14, 31]. Even though a genotype 2-infected patient may have advanced hepatic fibrosis, the high IFN sensitivity of genotype 2 may be able to overcome the negative impact of fibrosis. In comparison, among genotype 3 infections the viral killing in association with antiviral therapy may not be able to overcome the influence of hepatic fibrosis.
Current recommendations for the treatment of genotype 2 and 3 infections do not include pretreatment liver biopsy. The most recent NIH recommendations state that as the favourable response to current antiviral therapy that occurs in more than 70% of patients infected with genotype 2 or 3, it may not always be necessary to perform a liver biopsy [27]. Our data would support this recommendation for those with genotype 2 infections, yet in genotype 3 infections a pretreatment liver biopsy would yield important information on the patient’s subsequent response to antiviral therapy and perhaps question their appropriateness for shorter courses of therapy. Although rapid virologic response is also a valuable tool predicting ultimate SVR among genotype 3 patients, it may not alleviate the need for pretreatment liver biopsy. In order to provide patients with subsequent information about the likelihood of achieving a clinical cure, they need to endure a minimum of 4 weeks of therapy [9, 11, 12].
In conclusion this study demonstrates an interaction between genotype and cirrhosis, where patients with genotype 3 and cirrhosis respond less well to therapy than those with genotype 2 and cirrhosis. If these findings are confirmed in a larger population, patients with genotype 3 infections may benefit from pretreatment liver biopsies in order to determine their degree of hepatic fibrosis and likelihood of subsequent response to antiviral therapy. Whether patients with genotype 3 and advanced fibrosis may benefit from more prolonged courses of peg-IFN and ribavirin also needs further examination.
Acknowledgments
J.E.P. is supported by a research fellowship through the Canadian Institutes for Health Research sponsored National Canadian Research Training Program in Hepatitis C.
Glossary
Abbreviations
- BMI
body mass index
- HCV
hepatitis C virus
- MLR
multiple logistic regression
- PCR
polymerase chain reaction
- peg-IFN
pegylated interferon
- RBV
ribavirin
- SVR
sustained virological response
Appendix
The Canadian Pegasys Study Group also includes: Frank Anderson MD, The Liver & Intestinal Research Centre, Vancouver, BC; Robert Baily MD, Royal Alexandra Hospital, Edmonton, AB; Curtis Cooper, MD, University of Ottawa, Ottawa, ON; Marc Deschenes, MD, McGill University, Montreal, QC; Victor Feinman MD, Mount Sinai Hospital, Toronto ON; Susan Greenbloom MD, Toronto Digestive Disease Associates Inc., Toronto ON; Nir Hilzenrat MD, Montreal Jewish General Hospital, Montreal QC; Kelly Kaita MD, John Buhler Research Centre, Winnipeg MB; Paul Marotta MD, London Health Sciences Centre, London ON; Linda Scully MD, The Ottawa Civic Hospital, Ottawa ON; Bernard Willems MD, CHUM Hôpital Saint. Luc, Montreal QC; Helga Witt-Sullivan MD, Hamilton Health Sciences Corp-General Site, Hamilton ON; Lawrence Worobetz MD, Royal University Hospital, Saskatoon, SK.
References
- 1.Lai MY, et al. Long-term efficacy of ribavirin plus interferon alfa in the treatment of chronic hepatitis C. Gastroenterology. 1996;111(5):1307–1312. doi: 10.1053/gast.1996.v111.pm8898645. [DOI] [PubMed] [Google Scholar]
- 2.McHutchison JG, et al. Interferon alfa-2b alone or in combination with ribavirin as initial treatment for chronic hepatitis C. Hepatitis Interventional Therapy Group. N Engl J Med. 1998;339(21):1485–1492. doi: 10.1056/NEJM199811193392101. [DOI] [PubMed] [Google Scholar]
- 3.Zeuzem S, et al. Peginterferon alfa-2a in patients with chronic hepatitis C. N Engl J Med. 2000;343(23):1666–1672. doi: 10.1056/NEJM200012073432301. [DOI] [PubMed] [Google Scholar]
- 4.Lindsay KL, et al. A randomized, double-blind trial comparing pegylated interferon alfa-2b to interferon alfa-2b as initial treatment for chronic hepatitis C. Hepatology. 2001;34(2):395–403. doi: 10.1053/jhep.2001.26371. [DOI] [PubMed] [Google Scholar]
- 5.Manns MP, et al. Peginterferon alfa-2b plus ribavirin compared with interferon alfa-2b plus ribavirin for initial treatment of chronic hepatitis C: a randomised trial. Lancet. 2001;358(9286):958–965. doi: 10.1016/s0140-6736(01)06102-5. [DOI] [PubMed] [Google Scholar]
- 6.Fried MW, et al. Peginterferon alfa-2a plus ribavirin for chronic hepatitis C virus infection. N Engl J Med. 2002;347(13):975–982. doi: 10.1056/NEJMoa020047. [DOI] [PubMed] [Google Scholar]
- 7.Poynard T, et al. Randomised trial of interferon alpha2b plus ribavirin for 48 weeks or for 24 weeks versus interferon alpha2b plus placebo for 48 weeks for treatment of chronic infection with hepatitis C virus. International Hepatitis Interventional Therapy Group (IHIT) Lancet. 1998;352(9138):1426–1432. doi: 10.1016/s0140-6736(98)07124-4. [DOI] [PubMed] [Google Scholar]
- 8.Zeuzem S, et al. Peginterferon alfa-2b plus ribavirin for treatment of chronic hepatitis C in previously untreated patients infected with HCV genotypes 2 or 3. J Hepatol. 2004;40(6):993–999. doi: 10.1016/j.jhep.2004.02.007. [DOI] [PubMed] [Google Scholar]
- 9.Dalgard O, et al. Treatment with pegylated interferon and ribavarin in HCV infection with genotype 2 or 3 for 14 weeks: a pilot study. Hepatology. 2004;40(6):1260–1265. doi: 10.1002/hep.20467. [DOI] [PubMed] [Google Scholar]
- 10.Hadziyannis SJ, et al. Peginterferon-alpha2a and ribavirin combination therapy in chronic hepatitis C: a randomized study of treatment duration and ribavirin dose. Ann Intern Med. 2004;140(5):346–355. doi: 10.7326/0003-4819-140-5-200403020-00010. [DOI] [PubMed] [Google Scholar]
- 11.Mangia A, et al. Peginterferon alfa-2b and ribavirin for 12 vs. 24 weeks in HCV genotype 2 or 3. New Engl J Med. 2005;352(25):2609–2617. doi: 10.1056/NEJMoa042608. [DOI] [PubMed] [Google Scholar]
- 12.Von Wagner M, et al. Peginterferon-alpha-2a (40KD) and ribavirin for 16 or 24 weeks in patients with genotype 2 or 3 chronic hepatitis C. Gastroenterology. 2005;129(2):522–527. doi: 10.1016/j.gastro.2005.05.008. [DOI] [PubMed] [Google Scholar]
- 13.Zeuzem S, et al. Viral kinetics in patients with chronic hepatitis C treated with standard or peginterferon alpha2a. Gastroenterology. 2001;120(6):1438–1447. doi: 10.1053/gast.2001.24006. [DOI] [PubMed] [Google Scholar]
- 14.Neumann AU, et al. Differences in viral dynamics between genotypes 1 and 2 of hepatitis C virus. J Infect Dis. 2000;182(1):28–35. doi: 10.1086/315661. [DOI] [PubMed] [Google Scholar]
- 15.Rubbia-Brandt L, et al. Hepatocyte steatosis is a cytopathic effect of hepatitis C virus genotype 3. J Hepatol. 2000;33(1):106–115. doi: 10.1016/s0168-8278(00)80166-x. [DOI] [PubMed] [Google Scholar]
- 16.Sharma P, et al. Hepatic steatosis in hepatitis C virus genotype 3 infection: does it correlate with body mass index, fibrosis, and HCV risk factors? Dig Dis Sci. 2004;49(1):25–29. doi: 10.1023/b:ddas.0000011597.92851.56. [DOI] [PubMed] [Google Scholar]
- 17.Hofer H, et al. Hepatocellular fat accumulation and low serum cholesterol in patients infected with HCV-3a. Am J Gastroenterol. 2002;97(11):2880–2885. doi: 10.1111/j.1572-0241.2002.07056.x. [DOI] [PubMed] [Google Scholar]
- 18.Monto A, et al. Steatosis in chronic hepatitis C: relative contributions of obesity, diabetes mellitus, and alcohol. Hepatology. 2002;36(3):729–736. doi: 10.1053/jhep.2002.35064. [DOI] [PubMed] [Google Scholar]
- 19.Walsh MJ, et al. Steatosis and liver cell apoptosis in chronic hepatitis C: a mechanism for increased liver injury. Hepatology. 2004;39(5):1230–1238. doi: 10.1002/hep.20179. [DOI] [PubMed] [Google Scholar]
- 20.Shaw ML, et al. Characterisation of the differences between hepatitis C virus genotype 3 and 1 glycoproteins. J Med Virol. 2003;70(3):361–372. doi: 10.1002/jmv.10404. [DOI] [PubMed] [Google Scholar]
- 21.Kumar D, et al. Hepatitis C virus genotype 3 is cytopathic to hepatocytes: Reversal of hepatic steatosis after sustained therapeutic response. Hepatology. 2002;36(5):1266–1272. doi: 10.1053/jhep.2002.36370. [DOI] [PubMed] [Google Scholar]
- 22.Poynard T, et al. Effect of treatment with peginterferon or interferon alfa-2b and ribavirin on steatosis in patients infected with hepatitis C. Hepatology. 2003;38(1):75–85. doi: 10.1053/jhep.2003.50267. [DOI] [PubMed] [Google Scholar]
- 23.Lee SS, et al. Treating chronic hepatitis C with pegylated interferon alfa-2a (40 KD) and ribavirin in clinical practice. Aliment Pharmacol Ther. 2006;23(3):397–408. doi: 10.1111/j.1365-2036.2006.02748.x. [DOI] [PubMed] [Google Scholar]
- 24.Sherman M, et al. Peginterferon alfa-2a (40KD) plus ribavirin in chronic hepatitis C patients who failed previous interferon therapy. Gut. 2006;55(11):1631–1638. doi: 10.1136/gut.2005.083113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.The French METAVIR Cooperative Study Group. Intraobserver and interobserver variations in liver biopsy interpretation in patients with chronic hepatitis C. Hepatology. 1994;1(1 Pt 1):15–20. [PubMed] [Google Scholar]
- 26.Rousselet MC. Sources of variability in hitological scoring of chronic viral hepatitis. Hepatology. 2005;41(2):257–264. doi: 10.1002/hep.20535. [DOI] [PubMed] [Google Scholar]
- 27.NIH. National Institutes of Health Consensus Development Conference Statement. Management of hepatitis C: June 10-12, 2002. Hepatology. 2002;36(5) Suppl 1:S3–S20. doi: 10.1053/jhep.2002.37117. [DOI] [PubMed] [Google Scholar]
- 28.Orito E, et al. Loss of serum HCV RNA at week 4 of interferon-alpha therapy is associated with more favorable long-term response in patients with chronic hepatitis C. J Med Virol. 1995;46(2):109–115. doi: 10.1002/jmv.1890460205. [DOI] [PubMed] [Google Scholar]
- 29.Hino K, et al. Serial assay of hepatitis C virus RNA in serum for predicting response to interferon-alpha therapy. Dig Dis Sci. 1995;40(1):14–20. doi: 10.1007/BF02063935. [DOI] [PubMed] [Google Scholar]
- 30.Karino Y, et al. Early loss of serum hepatitis C virus RNA can predict a sustained response to interferon therapy in patients with chronic hepatitis C. Am J Gastroenterol. 1997;92(1):61–65. [PubMed] [Google Scholar]
- 31.Karino Y, et al. Hepatitis C virus genotypes and hepatic fibrosis regulate 24-h decline of serum hepatitis C virus RNA during interferon therapy in patients with chronic hepatitis C. J Gastroenterol Hepatol. 2003;18(4):404–410. doi: 10.1046/j.1440-1746.2003.03009.x. [DOI] [PubMed] [Google Scholar]