Abstract
Cilia are small organelles protruding from the cell surface that beat synchronously, producing biological transport. Despite intense research for over a century, the mechanisms underlying ciliary beating are still not well understood. Even the nature of the cytosolic molecules required for spontaneous and stimulated beating is debatable. In an effort to resolve fundamental questions related to cilia beating, we developed a method that integrates the whole-cell mode of the patch-clamp technique with ciliary beat frequency measurements on a single cell. This method enables to control the composition of the intracellular solution while the cilia remain intact, thus providing a unique tool to simultaneously investigate the biochemical and physiological mechanism of ciliary beating. Thus far, we investigated whether the spontaneous and stimulated states of cilia beating are controlled by the same intracellular molecular mechanisms. It was found that: (a) MgATP was sufficient to support spontaneous beating. (b) Ca2+ alone or Ca2+-calmodulin at concentrations as high as 1 μM could not alter ciliary beating. (c) In the absence of Ca2+, cyclic nucleotides produced a moderate rise in ciliary beating while in the presence of Ca2+ robust enhancement was observed. These results suggest that the axonemal machinery can function in at least two different modes.
Keywords: calcium signaling, cilia, molecular motors, cyclic nucleotides, patch-clamp techniques
INTRODUCTION
Cilia and flagella are organelles capable of producing repetitive motion that serves to transport a mucus layer over the mucociliary epithelium or to propel single cells through water. The general structure of the ciliary machinery, the axoneme, appears to be conserved throughout evolution. This macrocomplex assembly of >200 different peptides produces motion through the activity of dynein molecules (Gibbons, 1995). Despite the apparent similarity in the axonemal structure, the pattern of ciliary beating varies considerably among flagella, single cell organisms, and mucociliary epithelium, reflecting the unique physiological function of each group. Differences in beat pattern also exist among single cell organisms, indicating that differences in the mechanisms controlling the motor probably exist (Tamm, 1994). Much progress has been made in the last few decades in understanding the mechanism of ciliary beating (Gibbons, 1995; Porter and Sale, 2000). Nevertheless, fundamental questions remain unresolved due to the complexity of the axoneme and the variability in mechanisms regulating ciliary beat pattern. Consequently, it is difficult to extrapolate from one ciliary system to another.
Due to the physiological importance of mucociliary function in respiratory, digestive, and reproductive systems, mucociliary epithelium has been intensively investigated over the passed three decades. A number of signal transduction pathways leading to mucociliary beat frequency (CBF)* enhancement have been discovered as well as interplay between these pathways (Lansley et al., 1992; Salathe et al., 1993; Geary et al., 1995; Yang et al., 1996; Guo et al., 1997; Uzlaner and Priel, 1999; Braiman et al., 2000, 2001; Zagoory et al., 2001, 2002). Nevertheless, it remains to be unequivocally established whether the activated signal transduction pathways directly interact with the axoneme or whether additional molecular intermediates are involved. Furthermore, since mucociliary cells beat continuously at a low frequency and can robustly enhance beating in response to various physiological stimuli, it remains to be resolved whether the stimulated state is achieved simply by modifying the concentrations of molecules that also control the basal (spontaneous) state of beating or whether these are two distinct processes.
These fundamental questions remain open partly due to technical limitations. Biochemical methods can provide valuable information regarding the phosphorylation of axonemal proteins by various second messengers, yet correlating the biochemical findings with the physiological processes involved in mucociliary beating are not often straightforward. In addition, in physiological experiments aimed to decipher the signal transduction pathways involved in mucociliary stimulation, it is difficult to differentiate processes directly related to ciliary beating from processes required for the basic function of the cell. Moreover, the recently reported cross talk between the signaling pathways of Ca2+, cGMP, and cAMP (Zagoory et al., 2002) further complicates the interpretation of results in intact cells and tissues. To add to these difficulties, most of the available information on mucociliary cells was obtained from studies on tissue cultures, which do not necessarily reflect the physiological processes occurring in native cells.
To resolve direct interactions between transduction pathways and mucociliary motility, we have combined the patch-clamp technique (Hamill et al., 1981) with CBF measurements in the same cell. With the patch-clamp technique, a tight seal can be formed between the tip of a glass pipette and the basolateral membrane of a single native dissociated ciliated cell (Korngreen et al., 1998; Ma et al., 1999). By applying suction to the shank of the pipette the membrane underlying the pipette can be ruptured, exposing the intracellular milieu to the content of the patch pipette (whole-cell configuration of the patch clamp technique). Due to the considerably larger volume of the pipette relative to the cell, the cytosol is replaced by the content of the patch pipette, providing a means to manipulate the composition and relative concentrations of the intracellular elements (Marty and Neher, 1995). Thus, the whole-cell configuration of the patch-clamp technique was used to directly control the composition of the intracellular environment of single ciliated cells while monitoring the beating of the cilia. This approach enables one to circumvent the intracellular events, which are indirectly involved in regulating the cilia, and to identify intracellular components that directly interact with the axoneme to enhance ciliary beating. Obviously, this simplistic and rather naive approach cannot integrate the complex spatial–temporal interactions between various biochemical events that may be crucial for intricate control of ciliary beating. Nevertheless, it enables to directly address fundamental open questions.
The idea to bypass the signal transduction pathways involved in ciliary function in order to identify control molecules interacting directly with the axoneme is not new. To achieve this goal the ciliated cells were permeabilized using detergents, thereby exposing the inside of the cell to the extracellular solution. This method was extensively used in flagella and single-cell organism research providing valuable information (Porter and Sale, 2000). The few studies that have taken advantage of this experimental approach in mucociliary systems have reached conflicting conclusions. While Rases and Verdugo (1982) and Kakuta et al. (1985) concluded that Ca2+ or Ca2+–calmodulin complex can directly activate the cilia in the presence of MgATP, Lansley et al. (1992) reported that Ca2+ alone does not alter ciliary beating in permeabilized cells. Moreover, all the above studies did not demonstrate robust mucociliary stimulation or address the issue of whether similar mechanisms control spontaneous and stimulated ciliary activity. These limitations may have resulted from the loss of essential intracellular components or of factors within the axoneme following cell permeabilization, as suggested by Lansley et al. (1992), or may reflect the importance of the membrane surrounding the axoneme for proper axonemal function.
Thus, using a different approach (the patch clamp technique), the aim of the present study was to determine whether distinct axonemal processes underlie spontaneous and stimulated ciliary beating or whether beat frequency modulation is achieved simply by graded changes in the concentration of interacting molecules. It was found that spontaneous beating is supported by a basic intracellular salt solution containing ATP. Varying Ca2+ in this basic solution between <10−9 and 10−6 M failed to affect ciliary beating. On the other hand, including Ca2+, cAMP, and cGMP in the intracellular solution could significantly enhance ciliary beating. In the absence of Ca2+, the effect of the cyclic nucleotides on ciliary beating was greatly attenuated.
MATERIALS AND METHODS
Isolation of Single Ciliated Cells
New Zealand white rabbits were killed by gradual exposure to carbon dioxide. The trachea was removed and the epithelium was surgically separated from the cartilage and cut into pieces. The epithelium was then enzymatically dissociated with papain (13 U/ml) for 25 min in the standard extracellular solution that contained: (in mM) 137 NaCl, 2.7 KCl, 0.9 CaCl2, 0.5 MgCl2, 8 Na2HPO4, 1.47 KH2PO4, 5 D-glucose, pH 7.4. The tissue was then gently titurated with a fire-polished pipette. The cells were concentrated by two consecutive centrifugation steps, which also removed the enzyme. The isolated cells were transferred to 35-mm tissue culture dishes and used on the same day. Part of the tissue was maintained overnight in the standard extracellular solution at 4°C and used on the next day. No systematic differences in the experimental results, in cell viability, or in sensitivity to extracellular ATP were observed when cells were used on the next day.
CBF Measurement from Isolated Cells
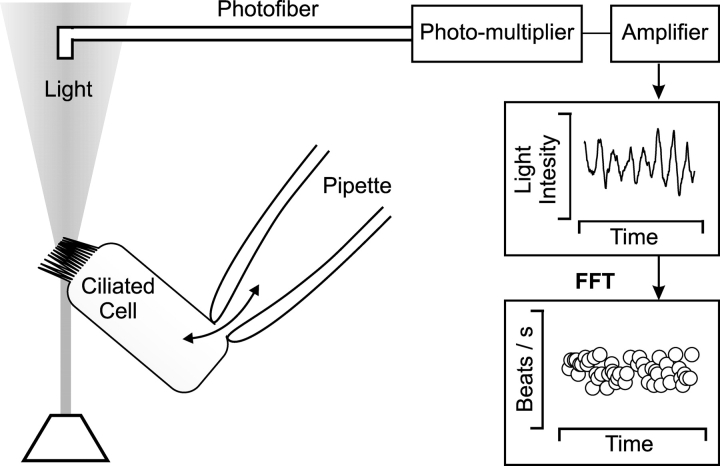
To measure CBF, the cell under investigation was trans-illuminated using a 100 W halogen lamp. Light scattering, caused by the beating cilia, gives rise to variations in the light intensity. This modulated light was collected by a 50 μm diameter optic fiber mounted at the focal plane of the microscope eyepiece. At 40× magnification, the fiber collected light from an area 1.25 μm in diameter (Eshel et al., 1985; Korngreen and Priel, 1994). The collected light was transformed to electrical signals by a photo multiplier. The signals were amplified, low-pass filtered, digitized, and then analyzed by Fast Fourier Transform on a personal computer (Fig. 1) . The cell under investigation was continuously superfused with the desired extracellular solutions via a gravity-fed perfusion system. The outlet of the perfusion tube was placed ∼300 μm away from the cell under investigation to assure rapid and complete exchange of the extracellular solution. A water jacket surrounding the perfusion tube was used to adjust the temperature of the solution reaching the cell.
Figure 1.
An experimental tool to simultaneously investigate the biochemical and physiological mechanisms of ciliary beating. The experimental tool is based on the combination of the whole-cell configuration of patch-clamp technique and light scattering measurements from a single native beating ciliary cell. The whole-cell configuration of the patch clamp technique is used to control the composition of the intracellular environment while maintaining the ciliary machinery (the axoneme and its surrounding membrane) intact. This enables one to disrupt and then reconstruct the biochemical pathways involved in muco-ciliary activation. The light scattering measurements simultaneously follow the corresponding changes in CBF (see materials and methods for details).
Patch-clamp Recording
The standard whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) was used to control the composition of the intracellular solution (Fig. 1), as described previously (Korngreen et al., 1998). Patch pipettes (1–3 MΩ, when filled with intracellular solution) were fabricated from borosilicate glass (GC150F-7.5; Clark Electromedical Instruments), coated with Sylgard 184 (Dow Corning), and fire polished shortly before use. Tight seals (>10 GΩ) were obtained by pressing the pipette against a cilia-free patch of membrane until the resistance of the pipette doubled, followed by gentle suction. Occasionally, tight seal formation was facilitated by applying −40 mV to the patch pipette. The membrane underlying the pipette was then ruptured by further suction. The standard pipette solution contained (mM): 148 CsCl, 5 TES, 1 EGTA, 0.89 CaCl2, 5 MgATP, 0.5 K2ATP, pH 7.2 with CsOH. The concentration of Ca2+ in this solution was estimated to be 1 μM at 32°C (with WinMAXC 1.8; http://www.stanford.edu/~cpatton/maxc.html). Deviations from this solution are noted in the figure legends. Once the whole-cell configuration was established, the cell was continuously superfused with the standard extracellular solution. The continuous perfusion of the cell enabled control of the temperature of the cell under investigation, as discussed above. Stable recordings were typically maintained for 5–10 min. The membrane potential of the cell was clamped to −40 mV.
Solutions and Drugs
Solutions were made with highly purified water (NANOpure; Barnstead), using chemicals of analytical grade. All chemicals were purchased from Sigma-Aldrich. Solutions containing ATP or other nucleotides were prepared freshly each day and the pH of the solution was readjusted.
RESULTS
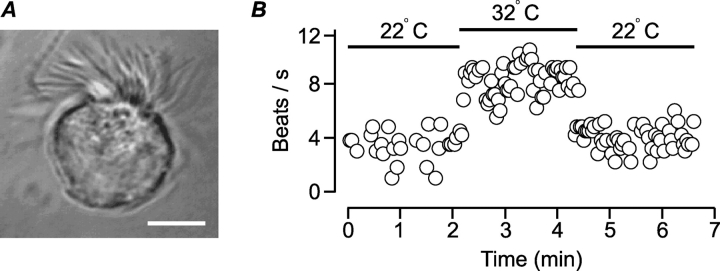
The whole-cell configuration of the patch-clamp technique (Hamill et al., 1981) was used as a means to investigate the regulation of ciliary activity in mucociliary cells. To this end, ciliated cells were enzymatically dissociated from rabbit airway epithelium and maintained at room temperature for use on the same day. Viable cells were visually identified by the spontaneous beating of their cilia and by the acceleration of beating upon warming to 32°C. To measure CBF, the cell under investigation was transilluminated, the light was collected from an area 1.25 μm in diameter, transformed to electrical signals by a photo multiplier, amplified, low-pass filtered, and digitized (see materials and methods and Fig. 1). Variations in the light intensity caused by the beating cilia were analyzed by Fast Fourier Transform to determine the dominant frequency, which was taken to be the CBF. An example of a dissociated ciliated cell is presented in Fig. 2 A. Fig. 2 B shows the time course of CBF measured from an isolated cell. Initially the cell was bathed and perfused at room temperature (22°C). At the time indicated by the bar, the cell was heated to 32°C by perfusing heated solution over the cell, resulting in CBF enhancement. In 22 experiments of this type, CBF increased from 4.2 ± 0.3 to 8.0 ± 0.4 beats/s (mean ± SEM) upon raising the temperature to 32°C. The rise in CBF was not due to the perfusion per se since perfusing the cells with solution at room temperature did not enhance CBF. Experiments could not be performed at 37°C, however, due to the vigorous motion of the cilia, which destabilized the tight-seal during patch-clamp recording. Thus, all CBF measurements were performed at 32°C. In a total of 262 cells, the spontaneous (basal) beat frequency at 32°C ranged from 4 to 15 beats/s (mean 7.7 ± 0.2 beats/s).
Figure 2.
Isolated rabbit-ciliated cells beat spontaneously. (A) Isolated rabbit ciliated cell. Bar, 5 μm. (B) CBF as a function of time measured from a single dissociated ciliated cell. Raising the temperature from 22°C to 32°C enhanced CBF.
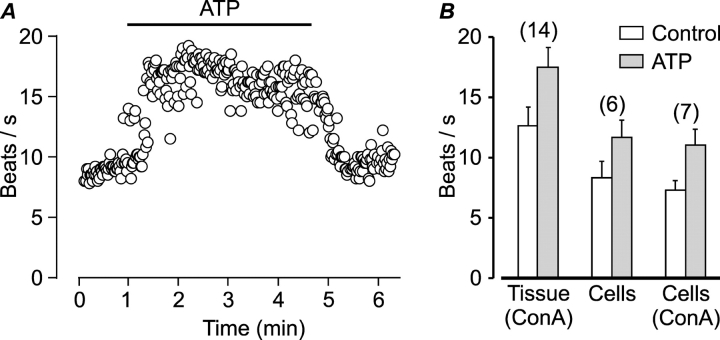
The isolated cells were relatively round in comparison to ciliated cells in situ (Fig. 2 A), indicating changes in cytoskeletal organization. Since intracellular cytoskeletal rearrangements might impact on cell function, we compared the spontaneous and stimulated beating of isolated cells to that of cells in freshly excised pieces of tissue. Extracellular ATP, which strongly stimulates mucociliary activity by activating purinergic receptors (for review see Wanner et al., 1996), was used to probe the ability of the isolated cells to respond to extracellular stimuli. The response of a native dissociated ciliated cell to extracellular ATP is demonstrated in Fig. 3 A. The addition of 200 μM ATP to the extracellular solution (indicated by bar) reversibly enhanced CBF from 9 beats/s to ∼17 beats/s. In most isolated cells, however, both the spontaneous beating and the beating in response to ATP were lower than the beating measured from cells in nondissociated pieces of tissue (Fig. 3 B). The observed difference was not due to the concanavalin A that was used to attach the pieces of tissue to the bottom of the dish since CBF of isolated cells in the presence and absence of concanavalin A was similar (Fig. 3 B). Since all measurements were from the rim of the tissue due to the opaqueness of the isolated pieces of tissue, one possible explanation for the greater activity in the tissue is that the cells may have been partially stimulated by their proximity to damaged cells. It is also possible that synchronization of activity among cells is necessary for effective beating. Alternatively, the enzymatic treatment and/or the rounding of the cells following dissociation (Fig. 2 A) may affect beating. Regardless, while the beating of isolated cells might be somewhat attenuated, it is also evident that the isolated cells retain the ability to beat spontaneously and to enhance beating in respond to extracellular ATP, and thus could be used to address the question in hand.
Figure 3.
Single isolated airway ciliated cells enhance beating in response to extracellular ATP. (A) CBF as a function of time measured from a single dissociated ciliated cell. Extracellular ATP reversibly enhanced CBF. (B) Mean (±SEM) CBF before (empty bars) and following stimulation by 200 μM ATP (full bars) measured from cells in freshly dissected pieces of airway tissue attached to the bottom of the dish with concanavalin A (ConA), or from freshly dissociated ciliated cells attached to the bottom of the dish with or without concanavalin A. The numbers of experiments are indicated above the bars.
Tight-seal Formation Does Not Mechanically Activate the Cilia
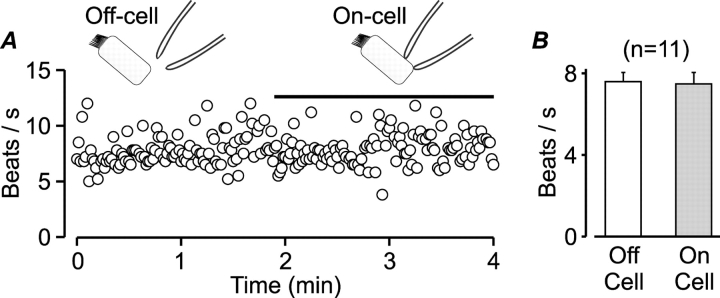
Mechanical stimulation of cultured ciliated cells with a microprobe rapidly enhances both intracellular Ca2+ and CBF (Sanderson et al., 1990). Thus, we first investigated whether establishing a tight seal between the patch pipette and the basolateral membrane of the isolated ciliated cells would mechanically activate the cilia, limiting the usefulness of this experimental approach. Fig. 4 A shows the time course of CBF measured from an isolated cell before, during, and after tight seal formation with the patch pipette. In contrast to the high mechanical sensitivity of ciliated cells in culture monolayers (Sanderson et al., 1990), the dissociated ciliated cell was not mechanically activated by the pipette. In 11 experiments of this type, ciliary activity before (off-cell) and immediately following tight-seal formation (on-cell) was similar (Fig. 4 B). Since the freshly dissociated ciliated cells were not stimulated by tight seal formation, we could next test whether the patch-clamp technique could be used to control the intracellular milieu while monitoring ciliary activity.
Figure 4.
Tight-seal formation with the patch pipette does not mechanically activate the cilia. (A) CBF as a function of time measured from a single dissociated ciliated cell before (off-cell) and following (on-cell, indicated by bar) tight seal formation with the patch pipette. (B) Mean (±SEM) CBF measured from single isolated ciliated cells just before (open bar) and immediately after establishing a tight seal (filled bar). There is no significant difference in CBF between groups (Student's t test). The number of experiments is indicated above the bars.
The Whole-cell Configuration of the Patch-clamp Technique Can be Used to Control Ciliary Activity
It is generally accepted that during whole-cell recording the content of small cells is replaced by the solution in the pipette within <2 min due to diffusion (Marty and Neher, 1995). However, since the cilia of mucociliary cells are thin elongated structures, we investigated whether the content of these limited structures can also be effectively replaced within the same timeframe. To this end, CBF was first measured as a function of time following whole-cell formation without ATP in the pipette solution. CBF was expected to decline with time if a pipette solution without MgATP replaces the content of the cilia since it is well established that intracellular MgATP is an essential energy source to drive the axonemal motors. Fig. 5 A shows an example of the time course of CBF measured from a single ciliated cell. At time zero, the patch of membrane underlying the pipette was ruptured by negative pressure applied to the shank of the pipette, thus establishing the whole-cell configuration of the patch clamp technique. Ciliary activity declined monotonically as a function of time and ceased within 4.5 min. On average, without ATP in the pipette solution, cilia activity ceased within 4.1 ± 0.3 min (n = 11). These results show that although complete replacement of the cytosol appears to be slower in the ciliated cells relative to round cells (Marty and Neher, 1995), it can still be achieved within the time frame of the experiment. To investigate whether the content of the patch pipette can effectively replace the content of the cilia and maintain cilia beating, MgATP was included in the pipette solution. Several studies using permeabilized cells have shown that millimoler concentrations of ATP are required to restore proper ciliary function (Gibbons and Gibbons, 1972; Rases and Verdugo, 1982; Kakuta et al., 1985; Lansley et al., 1992). Thus, 5 mM MgATP and 0.5 mM K2ATP were included in the pipette solution and the whole-cell experiments repeated. Fig. 5 B shows an example of one such experiment. In contrast to the decline in CBF in the absence of intracellular ATP, ciliary activity persisted almost unchanged for >20 min when ATP was included in the pipette solution. In 15 experiments with ATP in the pipette solution, ciliary activity persisted for the duration of the recording (10–20 min). These results show that the molecular machinery underlying ciliary movement remains functional during whole-cell recording, and that the patch-clamp technique can be used as an effective means to control the composition of the intracellular solution. Hence, the patch clamp technique can be used to investigate the effects of defined intracellular solutions on the activity of the cilia.
Figure 5.
Sustained spontaneous ciliary activity requires intracellular ATP. CBF measurements as a function of time, following the establishment of the whole-cell configuration of the patch clamp technique (time zero). CBF measurements from two different cells are presented. In A, the intracellular (pipette) solution contained (in mM): CsCl 155, EGTA 1, TES 5, adjusted to pH 7.2 with CsOH. In B, the pipette solution contained (in mM): CsCl 148, EGTA 1, TES 5, MgATP 5, K2ATP 0.5, adjusted to pH 7.2 with CsOH. Ca2+ was adjusted to 100 nM in both solutions.
The experiments presented in Fig. 5 were performed using CsCl as the major salt in the pipette solution (see materials and methods). Cesium was chosen as the preferred cation since it improved the quality of the patch-clamp recording. No difference in ciliary beating was observed when the intracellular solution contained either K+ or Na+ in place of Cs+. Consequently, all subsequent experiments were performed with 5 mM MgATP and 0.5 mM K2ATP in a CsCl pipette solution.
Ca2+ Does Not Directly Activate the Axoneme
It is well established that Ca2+ is an important mediator in mucociliary activation (see discussion). It is not known, however, whether Ca2+ is required for spontaneous beating or only for ciliary stimulation. Furthermore, there is a controversy regarding the question whether Ca2+ alone in the presence of MgATP can stimulate the cilia by directly interacting with the axoneme of mucociliary cells, or whether Ca2+ serves as a cofactor for biochemical intermediates, or both (Verdugo, 1980; Salathe and Bookman, 1999; Uzlaner and Priel, 1999). In permeabilized mucociliary cell preparations it was shown that in the presence of MgATP a low level of ciliary beating persists without Ca2+ (Kakuta et al., 1985; Lansley et al., 1992). These studies differ, however, in the effect of Ca2+ on ciliary activation. While raising Ca2+ increased ciliary activity in skinned dog tracheal epithelium (Kakuta et al., 1985), Ca2+ was without effect on permeabilized cultured rabbit airway epithelium (Lansley et al., 1992). To unequivocally establish whether Ca2+ can directly activate the axoneme in intact cilia, it is necessary to determine the relationship between the intracellular concentration of Ca2+ ([Ca2+]i) and CBF in cells in which potential biochemical intermediates have been either removed or inhibited without damaging the ciliary membrane. Combining the whole-cell mode of the patch-clamp technique with CBF measurements on single ciliated cells provides a direct means to achieve this goal. To this end, intracellular Ca2+ was clamped to a range of submicromolar concentrations by including different concentrations of CaCl2 and EGTA (1 mM) in the pipette solution, while monitoring CBF. A single Ca2+ concentration was tested in each cell. Fig. 6 A presents examples of CBF measurements from two different cells before and following whole-cell formation (indicated by arrow). Ca2+ in the pipette solution was clamped to either 500 nM (left) or to <1 nM (right). Clearly, neither low nor high intracellular Ca2+ had any obvious affect on CBF for the duration of the recording. In 25 experiments of this type, no apparent change in CBF occurred after whole-cell formation. CBF measured 10 min after whole-cell formation was not significantly different from CBF just before whole-cell formation in either low or high Ca2+ (Fig. 6 B).
Figure 6.
Spontaneous cilia activity is not dependent on Ca2+ or on Ca2+–calmodulin complex. (A) CBF measurements from two different cells before and following whole-cell formation (indicated by arrow). The cells were superfused with the standard extracellular solution at 32°C and voltage clamped at −40 mV. The pipette solution contained (in mM): CsCl 148, EGTA 1, TES 5, MgATP 5, K2ATP 0.5, adjusted to pH 7.2 with CsOH. In addition, intracellular Ca2+ was adjusted to either 500 nM (left) or <1 nM (right). (B) Mean (±SEM) CBF measured from single isolated ciliated cells before (open boxes) and 10 min after establishing the whole-cell configuration (full boxes). Intracellular Ca2+ was adjusted to: <1, 100, or 500 nM, as indicated (left). There is no significant difference in CBF before and after establishing the whole-cell configuration (Student's t test). The numbers of experiments are indicated above the bars. The addition of 100 μg/ml calmodulin to the intracellular solution adjusted to contain 1 μM Ca2+ failed to enhance cilia beating (middle). In some experiments, both the bath and pipette solutions contained less than 1 nM Ca2+, yet spontaneous beating was unaffected (right).
Intracellular Ca2+ controls many cellular processes by binding to calmodulin, and calmodulin was shown to increase the effect of Ca2+ on ciliary beating in permeabilized mucociliary cells (Rases and Verdugo, 1982; Kakuta et al., 1985). Therefore, we investigated whether the addition of 100 μg/ml calmodulin to the ATP-containing pipette solution in the presence of 1 μM Ca2+ will enhance ciliary activity. The average ciliary activity with and without calmodulin in the pipette solution was not significantly different (Fig. 6 B). Together, these results indicate that neither Ca2+ alone nor Ca2+–calmodulin complex directly enhance mucociliary beating.
Spontaneous Beating of Mucociliary Cells Does Not Require Ca2+
The results described above also suggest that spontaneous ciliary beating does not require intracellular Ca2+. However, since the experiments were performed in the presence of 0.9 mM CaCl2 in the extracellular solution, sufficient Ca2+ to support spontaneous activity may have entered the cilia from the extracellular solution. To rule out this possibility, we performed experiments in which the intracellular (pipette) salt solution contained 1 mM EGTA and no added Ca2+ while the cells were perfused with a modified extracellular solution in which the 0.9 mM CaCl2 in the standard extracellular solution was replaced by 10 mM MgCl2, 1 mM EGTA was added, and NaCl was reduced from 137 to122 mM in order to maintain the same osmolarity as in the standard solution. The Ca2+ concentration in this extracellular solution was estimated to be <1 nM. As shown in Fig. 6 B, cilia activity persisted even after both extracellular and intracellular Ca2+ were buffered to <1 nM.
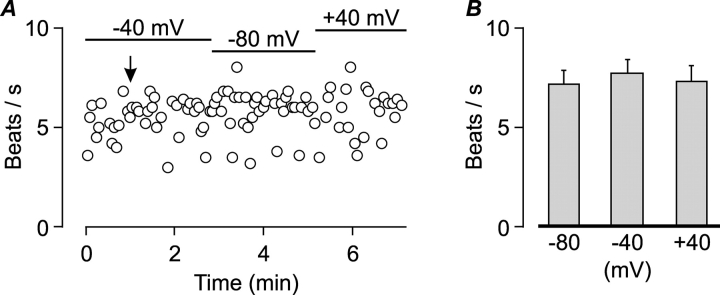
The patch clamp technique can also be used to control the voltage across the membrane of the cell (Hamill et al., 1981). We took advantage of this property to examine whether the membrane potential can modulate CBF when the cells are bathed in the standard extracellular solution. The voltage across the membrane was clamped to various voltages between −80 and 40 mV while monitoring CBF. CBF was found to be insensitive to changes in membrane potential (Fig. 7) .
Figure 7.
Spontaneous beating is not modulated by the membrane potential. (A) An example of CBF measurement from a single isolated cell voltage clamped at either −40, −80, or 40 mV as indicated by bars. The arrow indicates the time of whole-cell formation. Standard extracellular and pipette solutions. (B) Mean (±SEM) CBF of seven ciliated cells voltage-clamped at −80, −40, and 40 mV from experiments of the type shown in A. There was no significant difference between groups (Student's t test).
The results, thus far, strongly suggest that the spontaneous activity of the isolated mucociliary cells is independent of Ca2+ and that Ca2+ alone does not enhance ciliary beating. To substantiate this unorthodox conclusion pertaining to mucociliary cells, it is necessary to demonstrate that mucociliary activity can be enhanced by the whole-cell configuration of the patch-clamp technique under appropriate cytosolic conditions.
Ciliary Activity Can Be Robustly Enhanced in Whole-cell Recording by Including Cyclic Nucleotides and Ca2+ in the Intracellular Solution
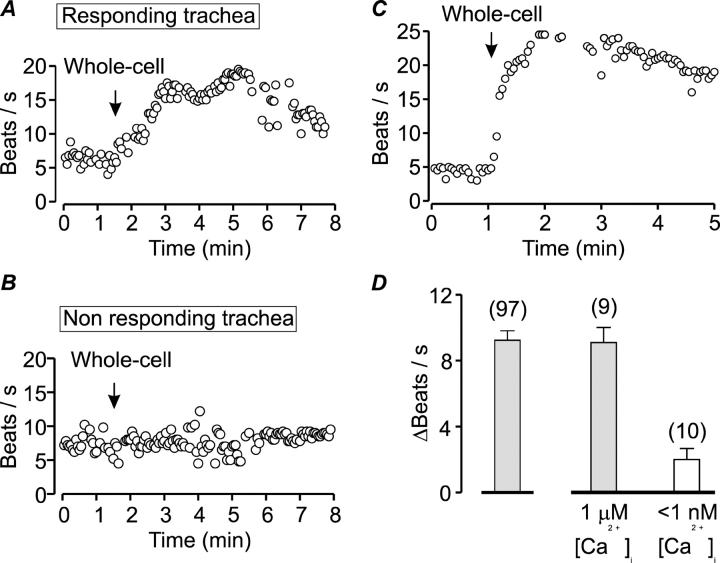
It has been shown recently that in order to induce robust mucociliary stimulation the concentrations of all three second messengers, cAMP, cGMP, and Ca2+, need to be elevated (Braiman et al., 2001; Zagoory et al., 2002). Based on these observations, we investigated whether the ciliary beating can be enhanced by whole-cell formation when 10 μM cAMP, 10 μM cGMP, and 1 μM Ca2+ are collectively included in the pipette solution. The rational for choosing the above mentioned concentrations of second messengers was as follows: using confocal microscopy, it was shown recently that strong and prolonged CBF stimulation is accompanied by a sustained rise in [Ca2+]i in close proximity to the cilia, ranging between 0.6 to 1 μM, (Braiman and Priel, 2001). Therefore, 1 μM [Ca2+]i was chosen. The basal or stimulated concentrations of cyclic nucleotides in airway ciliated cells are not known. Nevertheless, it was shown that extracellular application of membrane permeable analogs of cAMP or cGMP in the concentration range of 100 to 1,000 μM induces CBF enhancement (Tamaoki et al., 1989; Geary et al., 1995; Braiman et al., 1998, Uzlaner and Priel, 1999). It is commonly thought that the concentration of permeable analogs that actually enter the cell is one order of magnitude lower than their respective extracellular concentration. Hence, the lower value of 10 μM of each cyclic nucleotide was used. Indeed, as shown in Fig. 8 , it was possible to enhance cilia activity by forming the whole-cell configuration when the pipette solution was supplemented with the three second messengers. Fig. 8 A shows a typical example of one such experiment. CBF was measured as a function of time from a single isolated cell after establishing a tight seal with a patch-pipette. The spontaneous activity of the cell was ∼7 beats/s. At the time indicated by the arrow, the patch of membrane underlying the pipette was ruptured by negative pressure applied to the shank of the pipette. As a result, CBF gradually increased to ∼20 beats/s and thereafter gradually declined. The progressive increase in CBF may reflect the gradual accumulation of the second messengers in close proximity to their sites of action. The mechanism underlying the decline in CBF despite the continuous presence of the second messengers was consistently observed but not further investigated. These results clearly show that it is possible to enhance cilia activity by forming the whole-cell configuration, and thus the inability of Ca2+ alone or Ca2+-calmodulin to enhance ciliary beating (Fig. 6) was not due to limitations of the experimental approach.
Figure 8.
Ciliary activity can be enhanced by including Ca2+ and cyclic nucleotides in the pipette solution. (A and B) Examples of CBF measurements from cells isolated from two different tracheas on the same day. The arrows indicate the time of whole-cell formation. The standard pipette solution (that contains 1 μM Ca2+) was supplemented with 10 μM cAMP and 10 μM cGMP (stimulating solution). In responsive trachea the stimulating solution activated the cilia (A) while in nonresponding trachea the stimulating solution failed to enhance CBF (B). (C) CBF measurement from a highly responsive cilia cell in which the stimulating solution enhanced CBF from 5 to 25 beats/s. The arrow indicates the time of whole-cell formation. (D) The role of Ca2+ in ciliary stimulation was determined in responsive trachea. With the stimulating solution CBF increased by 9.3 ± 0.5 beats/s (mean ± SEM, middle bar). Reducing Ca2+ in this solution to <1 nM strongly attenuated the ability of the cyclic nucleotides to enhance CBF (2.0 ± 0.7 beats/s, right bar). The mean (±SEM) CBF enhancement by the stimulating solution in all responsive tracheas is shown for comparison (left bar). The numbers of experiments are indicated above the bars.
Despite the apparent effect of the second messengers on ciliary activation, the initial results were perplexing. It became apparent over time that there is a large variability in the response among individual trachea. For example, of nine tracheas investigated in 1 mo, ciliary activation by the stimulating pipette solution was observed only in five tracheas. Fig. 8 B shows an example of CBF measurement from a cell isolated from a different trachea on the same day as that shown in Fig. 8 A. In all three cells from this trachea, the stimulating pipette solution failed to enhance cilia activity. In contrast, five of six cells from the trachea exemplified by the cell in Fig. 8 A responded, enhancing CBF from 5.6 ± 0.4 to 16 ± 3.5 beats/s, on average. While the cause for this heterogeneity remains unresolved, the implications of this finding were twofold: First, for each trachea, it was necessary to determine to what extent the cilia could be activated. Second, comparisons among cells were limited to each trachea, a requirement that greatly slowed progress. Thus, an intracellular solution comprised of the standard pipette solution supplemented with 10 μM cAMP and 10 μM cGMP and adjusted to contain 1 μM Ca2+ was selected as the stimulating solution for comparing among trachea. Only trachea in which the average increase in CBF induced by this intracellular solution was at least five beats/s were used. On average, in 97 cells from 35 responsive tracheas, CBF increased by 9.3 ± 0.5 beats/s (Fig. 8 D). These results clearly demonstrate that a substantial increase in CBF can be induced by the whole-cell configuration. It is noteworthy that in a small number of cells the increase in CBF was substantially greater than the average response. One such experiment is shown in Fig. 8 C in which CBF increased from 5 to 25 beats/s after whole-cell formation. These highly responsive cells demonstrate the inherent ability of the isolated cilia cell to vigorously increase beating frequency.
Robust Ciliary Activation by Cyclic Nucleotides Requires Ca2+
Numerous experiments have shown the pivotal role of Ca2+ in ciliary activation. The results presented in Fig. 6 reveal, however, that Ca2+ alone cannot enhance ciliary beating. This raises the question whether a direct interaction of Ca2+ with the axoneme is at all needed for ciliary stimulation or whether Ca2+ simply serves to stimulate the cascade of events that lead to the formation of cAMP and cGMP. To address this question we examined whether the enhancement in ciliary activity induced by the addition of cyclic nucleotides to the intracellular solution is dependent on Ca2+. In the absence of Ca2+ in the intracellular solution, ciliary activation by the cyclic nucleotides was greatly diminished (Fig. 8 D). In 10 experiments in which the intracellular solution contained 10 μM cGMP, 10 μM cAMP, 1 mM EGTA, and no added Ca2+, CBF increased by only 2.0 ± 0.7 beats/s, whereas the standard stimulating solution enhanced CBF in cells from the same tracheas by 9.3 ± 0.5 beats/s. Thus, both cyclic nucleotides and Ca2+ are needed to induce robust ciliary stimulation. In addition to reiterating the pivotal role of Ca2+ in ciliary activation, the experiments presented in Fig. 8 D also serve as an important control. It might be argued that the apparent inability of Ca2+ alone to enhance ciliary beating (Fig. 6) is due to diffusion limitations of Ca2+ from the pipette solution to the Ca2+ binding sites in the axoneme. This is unlikely to be the case since the results presented in Fig. 8 D clearly demonstrate that sufficient Ca2+ reaches the axoneme to support robust ciliary stimulation in the presence of cyclic nucleotides.
Together, these observations strongly suggest that in addition to Ca2+, cyclic nucleotides are required for cilia activation, and that the molecular mechanisms underlying spontaneous and stimulated ciliary activity are distinct.
DISCUSSION
The main task of mucociliary cells is to transport particles over the epithelium. To rapidly convey large heavy loads, the mucociliary cells must beat coordinately at a high rate. The cilia then return to a low rate of beating when the task is complete. Thus, the ability to enhance beating in response to various physiological cues is a hallmark of mucociliary cells. The question whether the same molecular mechanisms control the wide range of beat frequencies exhibited by the mucociliary cells was addressed in the present study. We revealed two distinct stages in ciliary beating: the first stage is a spontaneous beating that requires only MgATP, whereas the stimulated stage requires in addition to MgATP also Ca2+ and cyclic nucleotides.
The present study shows that all necessary components for spontaneous beating, aside from ATP, are contained within the cilium (the axoneme and its surrounding membrane) and that calcium is not required. Obviously, ciliary motility could not be maintained without the addition of ATP, which is needed as an energy source to drive the axonemal motor. However, the possibility that ATP or its degradation products have additional functions within the cilium remains to be resolved. A similar dependence on MgATP alone has been reported for demembranated sea urchin flagella (Gibbons and Gibbons, 1972). The ability of both sperm flagella and cilia from mucociliary cells to beat in the presence of MgATP alone is consistent with current thoughts that the axonemal motors are similar.
Existing information on nonstimulated (basal) mucociliary activity was gathered primarily from experiments performed on intact cells in which the basal activity served as a reference (control) to investigate the mechanism of mucociliary enhancement by various factors. It is not possible to determine to what extent this basal activity reflects the spontaneous state defined in the present study since the cytosol of intact cells contains Ca2+ and cyclic nucleotides that are needed for cell functions other than cilia beating. Thus, attempting to remove these second messengers from intact cells might alter cell function or lead to cell damage that ultimately will also affect cilia beating. Nevertheless, simultaneous recording of the average [Ca2+]i and ciliary activity under nonstimulated basal conditions revealed a complete lack of correlation between ciliary beating and [Ca2+]i (Korngreen and Priel, 1996; Mao and Wong, 1998). These findings are consistent with the idea that nonstimulated mucociliary cells are driven by MgATP alone, and thus are in the spontaneous state.
The cellular event most implicated in mucociliary stimulation is a change in [Ca2+]i (Girard and Kennedy, 1986; Verdugo, 1980; Villalon et al., 1989; Di Benedetto et al., 1991; Lansley et al., 1992; Korngreen and Priel, 1994, 1996; Paradiso et al., 1995; Levin et al., 1997; Mao and Wong, 1998; Salathe and Bookman, 1999). It is also well known that cAMP and/or cGMP are important modulators of ciliary activity in a variety of ciliary systems (Tamaoki et al., 1989; Lansley et al., 1992; Geary et al., 1993, 1995; Salathe et al., 1993; Yang et al., 1996; Braiman et al., 1998; Uzlaner and Priel, 1999; Zagoory et al., 2002). Since a rise in [Ca2+]i can also lead to the formation of cyclic nucleotides via Ca2+-calmodulin–dependent pathways, characterization of the correlation between [Ca2+]i and CBF in intact cells may be misleading. In the present study, increasing the concentration of Ca2+ in the pipette solution up to 1 μM failed to enhance CBF (Fig. 6), indicating that Ca2+ alone does not control ciliary beating. Consistent with this observation, uncoupling between [Ca2+]i and CBF enhancement was recently demonstrated. It was shown that Ca2+ by itself is insufficient to stimulate CBF and that in addition to elevated [Ca2+]i activated PKG and/or PKA are required (Uzlaner and Priel, 1999; Braiman et al., 2001; Zagoory et al., 2001, 2002). In a recent study it was shown that Ca2+, cGMP, and cAMP signaling pathways are intimately interconnected in the process of ciliary stimulation, and that strong CBF enhancement is achieved when the concentrations of these three well-known second messengers are significantly enhanced (Zagoory et al., 2002). This synergism between Ca2+ and cyclic nucleotides was confirmed by the present results, which show that cyclic nucleotides have only a moderate effect on ciliary beating in the absence of Ca2+, while in the presence of Ca2+ robust ciliary activation is observed (Fig. 8).
Based on these results, we suggest that cytosolic Ca2+ interacts with yet unknown elements of the axoneme, one of which is most likely calmodulin (Yang et al., 2001). This interaction by itself is insufficient to produce stimulation, requiring in addition the presence of cyclic nucleotides. Whether the cyclic nucleotides directly influence the axonemal machinery and/or serve to activate protein kinases constitutively present in the axoneme remains to be resolved. Thus, further work is needed to elucidate the elements of the axoneme that interact with Ca2+ and the exact roles of cGMP and cAMP in this process.
In conclusion, this study shows for the first time that the axonemal machinery can function in at least two different modes: a low rate of beating that requires only ATP and most probably involves only the axonemal motor, and a high rate of beating that involves, in addition, a controlling device regulated by second messengers. It appears that the axonemal motor is well guarded from accidental stimulation since multiple keys are needed to unlatch the controlling device. Differences in the controlling devices and/or keys may underlie the unique physiological requirements of flagella, single cell organisms, and mucociliary epithelium.
Acknowledgments
We thank Alex Braiman, Dan Eshel, and Alon Korngreen for helpful comments on the manuscript.
This research was supported by The Israel Science Foundation (grant No. 262/00).
Footnotes
Abbreviations used in this paper: CBF, cilia beat frequency; TES, 5 N-tris [hydroxymethyl]methyl-2-aminoethanesulfonic acid.
References
- Braiman, A., O. Zagoory, and Z. Priel. 1998. PKA induces Ca2+ release and enhances ciliary beat frequency in a Ca2+-dependent and independent manner. Am. J. Physiol. 275:C790–C797. [DOI] [PubMed] [Google Scholar]
- Braiman, A., N. Uzlaner, and Z. Priel. 2000. The role of cyclic nucleotide pathways and calmodolin in ciliary stimulation. In Computational Modeling in Biological Fluid Dynamics. L.J. Fanci and S. Goren, editors. Springer-Verlag, New York. 124:53–64.
- Braiman, A., N. Uzlaner, and Z. Priel. 2001. Enhancement of CBF is dominantly controlled by PKG and/or PKA. In Cilia and Mucus. M. Salathe, editor. Marcel Dekker Inc. New York. 67–79.
- Braiman, A., and Z. Priel. 2001. Intracellular stores maintain stable cytosolic Ca2+ gradient in epithelial cells by active Ca2+ redistribution. Cell Calcium. 30:361–371. [DOI] [PubMed] [Google Scholar]
- Di Benedetto, G., C.J. Magnus, P.T. Gray, and A. Mehta. 1991. Calcium regulation of ciliary beat frequency in human respiratory epithelium in vitro. J. Physiol. 439:103–113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eshel, D., Y. Grossman, and Z. Priel. 1985. Spectral characterization of ciliary beating: variations of frequency with time. Am. J. Physiol. 249:C160–C165. [DOI] [PubMed] [Google Scholar]
- Geary, C.A., M.F. Goy, and R.C. Boucher. 1993. Synthesis and vectorial export of cGMP in airways epithelium: expression of soluble CNP-specific guanylate cyclases. Am. J. Physiol. 265:L598–L605. [DOI] [PubMed] [Google Scholar]
- Geary, C.A., C.W. Davis, A.M. Pardiso, and R.C. Boucher. 1995. Role of CNP in human airways: cGMP-mediated stimulation of ciliary beat frequency. Am. J. Physiol. 268:L1021–L1028. [DOI] [PubMed] [Google Scholar]
- Gibbons, B.H., and I.R. Gibbons. 1972. Flagellar movement and adenosine triphosphatase activity in sea urchin sperm extracted with Triton X-100. J. Cell Biol. 51:75–97. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibbons, I.R. 1995. Dynein family of motor proteins - present status and future questions. Cell Motil. Cytoskeleton. 32:136–144. [DOI] [PubMed] [Google Scholar]
- Girard, P.R., and J.R. Kennedy. 1986. Calcium regulation of ciliary activity in rabbit tracheal epithelia explants and outgrowth. Eur. J. Cell Biol. 40:203–209. [PubMed] [Google Scholar]
- Guo, F.H., K. Uetani, S.J. Haque, B.R. Williams, R.A. Dweik, F.B. Thunnissen, W. Calhoun, and S.C. Erzurum. 1997. Interferon gamma and interleukin 4 stimulate prolonged expression of inducible nitric oxide synthase in human airway epithelium through synthesis of soluble mediators. J. Clin. Invest. 100:829–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamill, O.P., A. Marty, E. Neher, B. Sakman, and F. Sigworth. 1981. Improved patch-clamp techniques for high-resolution current recording from cells and cell-free membrane patches. Pflugers Arch. 391:85–100. [DOI] [PubMed] [Google Scholar]
- Kakuta, Y., T. Kanno, H. Sasaki, and T. Takishima. 1985. Effect of Ca2+ on the ciliary beat frequency of skinned dog tracheal epithelium. Resp. Physiol. 60:9–19. [DOI] [PubMed] [Google Scholar]
- Korngreen, A., W. Ma, Z. Priel, and S.D. Silberberg. 1998. Extracellular ATP directly gates a cation-selective channel in rabbit airway ciliated epithelial cells. J. Physiol. 508:703–720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngreen, A., and Z. Priel. 1994. Simultaneous measurement of ciliary beating and intracellular calcium. Biophys. J. 67:377–380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korngreen, A., and Z. Priel. 1996. Purinergic stimulation of rabbit ciliated airway epithelia: control by multiple calcium sources. J. Physiol. 497:53–66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansley, A.B., M.J. Sanderson, and E.R. Dirksen. 1992. Control of the beat cycle of respiratory tract cilia by Ca2+ and cAMP. Am. J. Physiol. 263:L232–L242. [DOI] [PubMed] [Google Scholar]
- Levin, R., A. Braiman, and Z. Priel. 1997. Protein kinase C induces calcium influx and sustained enhancement of ciliary beating by extracellular ATP. Cell Calcium. 21:103–113. [DOI] [PubMed] [Google Scholar]
- Ma, W., A. Korngreen, N. Uzlaner, Z. Priel, and S.D. Silberberg. 1999. Extracellular sodium regulates airway ciliary motility by inhibiting a P2X receptor. Nature. 400:894–897. [DOI] [PubMed] [Google Scholar]
- Mao, H., and L.B. Wong. 1998. Fluorescence and laser photon counting measurements of epithelial [Ca2+]i or [Na+]i with ciliary beat frequency. Ann. Biomed. Eng. 26:666–678. [DOI] [PubMed] [Google Scholar]
- Marty, A., and E. Neher. 1995. Tight-Seal Whole-Cell Recording. Single-Channel Recording. B. Sakmann, and E. Neher, editors. Plenum Press, New York. 341–356.
- Paradiso, A.M., S.J. Mason, E.R. Lazarowski, and R.C. Boucher. 1995. Membrane-restricted regulation of Ca2+ release and influx in polarized epithelia. Nature. 377:643–646. [DOI] [PubMed] [Google Scholar]
- Porter, M.E., and W.S. Sale. 2000. The 9 + 2 axoneme anchors multiple inner arm dyneins and a network of kinases and phosphatases that control motility. J. Cell Biol. 151:F37–F42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rases, B.U., and P. Verdugo. 1982. Calcium-dependent reactivation of demembranated mammalian epitheliary ciliated cells: the effect of calmodulin. Mechanism and Control of Ciliary Movement. C.J. Brokaw and P. Verdugo, editors. Alan R. Liss, Inc. New York. 222 pp.
- Salathe, M., and R.J. Bookman. 1999. Mode of Ca2+ action on ciliary beat frequency in single ovine airway epithelial cells. J. Physiol. 520:851–865. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salathe, M., M.M. Pratt, and A. Wanner. 1993. Cyclic AMP-dependent phosphorylation of a 26kD axonemal protein in ovine cilia isolated from small tissue pieces. Am. J. Respir. Cell Mol. Biol. 9:306–314. [DOI] [PubMed] [Google Scholar]
- Sanderson, M.J., A.C. Charles, and E.R. Dirksen. 1990. Mechanical stimulation and intercellular communication increases intracellular Ca2+ in epithelial cells. Cell Reg. 1:585–596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamaoki, J., M. Kondo, and T. Takizawa. 1989. Effect of cAMP on ciliary function in rabbit tracheal epithelial cells. J. App. Physiol. 66:1035–1039. [DOI] [PubMed] [Google Scholar]
- Tamm, S. 1994. Ca2+ channels and signaling in cilia and flagella. Trends Cell Biol. 4:305–310. [DOI] [PubMed] [Google Scholar]
- Uzlaner, N., and Z. Priel. 1999. Interplay between the NO pathway and elevated [Ca2+]i enhances ciliary activity in rabbit trachea. J. Physiol. 516:179–190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdugo, P. 1980. Ca2+-dependent hormonal stimulation of ciliary activity. Nature. 283:764–765. [DOI] [PubMed] [Google Scholar]
- Villalon, M., T.R. Hinds, and P. Verdugo. 1989. Stimulus-response coupling in mammalian ciliated cells. Demonstration of two mechanism of control for cytosolic Ca2+. Biophys. J. 56:1255–1258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wanner, A., M. Salathe, and T.G. O'Riordan. 1996. Mucociliary clearance in the airways. Am. J. Respir. Crit. Care Med. 154:1868–1902. [DOI] [PubMed] [Google Scholar]
- Yang, B., R.Y. Schlosser, and T.V. McCaffrey. 1996. Dual signal transduction mechanism modulate ciliary beat frequency in upper airway epithelium. Am. J. Physiol. 270:L745–L751. [DOI] [PubMed] [Google Scholar]
- Yang, P., D.R. Diener, J.L. Rosenbaum, and W.S. Sale. 2001. Localization of calmodulin and dynein light chain LC8 in flagellar radial spokes. J. Cell Biol. 153:1315–1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zagoory, O., A. Braiman, and Z. Priel. 2001. Role of calcium and calmodulin in ciliary stimulation induced by acetylcholine. Am. J. Physiol. 280:C100–C109. [DOI] [PubMed] [Google Scholar]
- Zagoory, O., A. Braiman, and Z. Priel. 2002. The mechanism of ciliary stimulation by acetylcholine: roles of calcium, PKA and PKG. J. Gen. Physiol. 119:329–339. [DOI] [PMC free article] [PubMed] [Google Scholar]