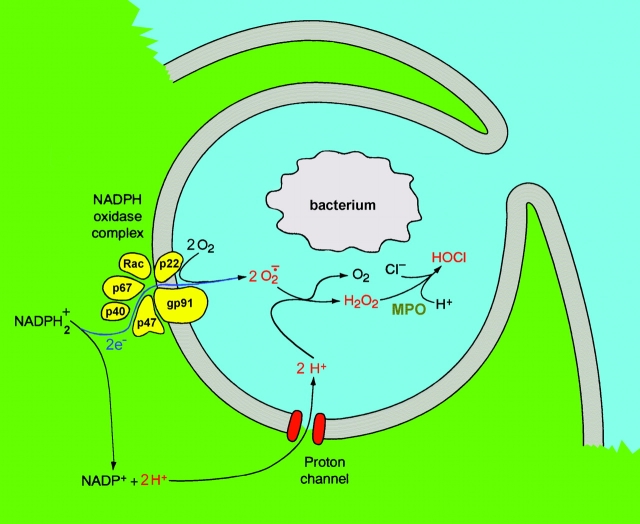
During the “respiratory burst” in phagocytes, NADPH oxidase helps kill microbes by producing superoxide anion, O2 −. As illustrated in the cartoon in Fig. 1 , the NADPH oxidase complex has several components. In unstimulated cells, four components (p67phox, p40phox, p47phox, and a G protein, Rac) are located in the cytosol, and gp91phox and p22phox are membrane bound. Upon stimulation by bacteria, PMA (phorbol ester), or chemotactic peptides, the complex assembles and begins to generate O2 −. This is accomplished by transporting electrons from NADPH inside the cell, across the cell membrane to reduce extracellular O2 to O2 −. Without charge compensation, the electron efflux in a human eosinophil would depolarize the membrane by ∼12 V/s, and this opposing voltage would shut down the oxidase within ∼20 ms. Henderson et al. (1987)(1988a,b) demonstrated that electrogenic H+ efflux in human neutrophils compensates for electrogenic electron translocation through the NADPH oxidase complex. The consensus is that the electron current is balanced by proton efflux mediated by voltage-gated proton channels. A recent suggestion that K+ efflux contributes ∼6% of the total charge compensation (Reeves et al., 2002), still leaves 94% of the job to proton channels. The molecular identity of these H+ channels is not known.
Figure 1.
Cartoon illustrating the electron and proton currents during the respiratory burst in phagocytes. The two membrane-bound components, gp91phox and p22phox, occur together in phagocytes in a complex called flavocytochrome b 558 that includes FAD and two heme groups, and comprises an electron pathway. The conversion of H2O2 to HOCl is catalyzed by myeloperoxidase (MPO), which causes pus to be green. We have added an extra proton to both sides of the reaction that usually is written: NADPH → NADP+ + H+. This was done because (a) the regeneration of NADPH produces one additional intracellular proton, (b) the dismutation of O2 − to H2O2 consumes two extracellular protons, and (c) balancing the two translocated electrons requires two protons. This figure was modified from a version originally published in DeCoursey and Grinstein (1999).
A variety of evidence has been interpreted to suggest that gp91phox can function as a proton channel, but critical examination of the evidence shows that this is not the case. The evidence on both sides falls into two general categories: heterologous expression studies and circumstantial evidence. The heterologous expression studies claiming proton channel function for gp91phox are unconvincing for two reasons. (a) In some cases, the putative H+ currents in gp91phox transfected cells simply do not have the properties of voltage-gated proton currents in native cells. (b) Those studies showing genuine H+ currents in gp91phox-transfected cells were done in an expression system that constitutively exhibits voltage-gated proton currents of amplitude similar to that reported in the transfected cells. When we expressed gp91phox in COS-7 cells that have no endogenous H+ channels, no H+ current could be detected, although expression was confirmed both by antibody staining and functional studies (Morgan et al., 2002). In addition to this evidence, there is substantial circumstantial evidence, which, by its nature is less conclusive. Because the circumstantial evidence exists historically, it will be discussed briefly.
Heterologous Expression of gp91phox Does Not Result in Voltage-gated Proton Currents
Heterologous expression of gp91phox and several gp91phox homologues, known as Nox proteins, has been reported to result in voltage-gated proton currents. Henderson et al. (1995) first reported that heterologous expression of gp91phox in CHO cells resulted in pH changes suggestive of conductive H+ flux. This result has been supported by several subsequent studies (Henderson et al., 1997; Henderson, 1998; Henderson and Meech, 1999; Mankelow and Henderson, 2001; Maturana et al., 2001). Bánfi et al. (2000) reported that the gp91phox homologue, Nox1 was a voltage-gated proton channel. This group subsequently reported that another homologue, Nox5 was a Ca2+-activated H+ channel (Bánfi et al., 2001).
The heterologous expression data are unconvincing and internally inconsistent. Of the three cell lines used for expression, two (CHO and HEK-293 cells) already express voltage-gated proton channels (Cherny et al., 1997; Bánfi et al., 2000; Eder and DeCoursey, 2001; Maturana et al., 2001). Expression of either Nox1 or gp91phox in HEK-293 cells results in H+ currents that are only ∼4-fold larger than in the background (Bánfi et al., 2000; Maturana et al., 2001), and of similar amplitude to H+ currents in control HEK-293 cells (Eder and DeCoursey, 2001). Transfection could induce up-regulation of the endogenous H+ channels. Up-regulation of endogenous ion channels by transfection with irrelevant proteins is a well-known phenomenon (Shimbo et al., 1995). In both Nox1 or gp91phox-transfected HEK-293 cells, H+ current densities were small, <4 pA/pF (at 60 mV at pHo 7.5, pHi 5.7). Under the same conditions (60 mV at pHo 7.5, pHi 5.7) this group observed native voltage-gated H+ currents >300 pA/pF in human eosinophils (Schrenzel et al., 1996). Putative H+ currents in gp91phox-transfected CHO cells were >10,000 pA (Henderson and Meech, 1999).
The currents reported in gp91phox-transfected CHO cells (Henderson and Meech, 1999) differ markedly from H+ currents in phagocytes. (a) They activate at least an order of magnitude more rapidly than phagocyte H+ currents. (b) Although the currents were extremely large, >10 nA in some cells, they did not exhibit the droop characteristically observed during large H+ currents (DeCoursey, 1991; Demaurex et al., 1993; Kapus et al., 1993; DeCoursey and Cherny, 1994; Gordienko et al., 1996; Schrenzel et al., 1996). Voltage-gated proton channels do not inactivate, but large H+ currents droop because H+ efflux increases pHi, which decreases the driving force (DeCoursey, 1991; Demaurex et al., 1993; Kapus et al., 1993; DeCoursey and Cherny, 1994). (c) Increasing pHi by only ∼0.6 U completely abolished the gargantuan putative H+ currents in CHO cells (Henderson and Meech, 1999). In stark contrast, in every study of voltage-gated proton channels in native cells, increasing pHi by 1 U reduced the limiting g H, g H,max by only ∼1/2 (DeCoursey, 1998). Although increasing pHi by 1 U shifts the g H-V relationship of all H+ channels by ∼40 mV (DeCoursey, 2003), to account for the mysterious disappearance of the putative H+ currents in CHO cells at 0.6 U higher pHi would require a shift of at least 140 mV; higher voltages were not reported. (d) Finally, 200 μM Zn2+ only partially inhibited the putative H+ current and did not appear to slow activation (Henderson and Meech, 1999). In phagocytes and other cells, 1 μM Zn2+ slows activation by ∼3–10-fold at pH ≥ 7 (Cherny and DeCoursey, 1999; DeCoursey et al., 2001a; Schilling et al., 2002).
Thus, CHO cells that heterologously express gp91phox may sometimes exhibit a conductance that superficially resembles voltage-gated proton currents, but the currents are fundamentally different.
The putative H+ currents in gp91phox-expressing COS-7 cells have not been characterized, and the data that exist indicate a nonselective conductance. The family of currents in Fig. 1 A of Maturana et al. (2001) was recorded at pHo 7.5, pHi 5.7, and thus E H was −105 mV. However, the large inward tail currents upon repolarization to −60 mV, as well as the activation of inward current during the pulse to 0 mV, both indicate that V rev > 0 mV. The conductance therefore is not H+ selective, because V rev deviates from E H by >100 mV. Astonishingly, in defiance of their own data, the authors insist that these are proton currents. In contrast, the currents in transfected HEK-293 cells in the same figure and recorded under identical conditions exhibit outward tail currents at −60 mV, consistent with H+ selectivity. Because voltage-gated proton channels are expressed by control HEK-293 cells (Bánfi et al., 2000; Eder and DeCoursey, 2001; Maturana et al., 2001; DeCoursey, 2003), their presence in transfected HEK-293 cells is not surprising. On the other hand, COS-7 cells do not express voltage-gated proton channels (Maturana et al., 2001; Morgan et al., 2002), and continue not to express H+ channels when transfected with gp91phox.
Direct Evidence That gp91phox Is Not a Proton Channel
H+ currents are normal in phagocytes from CGD patients who lack gp91phox.
Chronic granulomatous disease (CGD) results when a mutation in any one of the four main NADPH oxidase components prevents enzyme function. In 1994, Nanda et al. (1994b) demonstrated that peripheral blood monocytes from CGD patients lacking gp91phox had H+ currents with properties and amplitude identical to those in normal subjects. This voltage-clamp study (Nanda et al., 1994b) disproved their previous hypothesis (Nanda et al., 1993) that gp91phox might be a proton channel, and has been confirmed subsequently (Bánfi et al., 1999; DeCoursey et al., 2001b). Nanda et al. (1994a) also showed that in several rare forms of CGD, activation of H+ efflux was normal with mutations that permitted assembly of a dysfunctional NADPH oxidase complex, and that in patients with reduced gp91phox expression, activation of the g H was not reduced proportionately. The disparity between gp91phox levels and H+ current density also speaks against the idea that NADPH oxidase might contain the H+ channel. These studies showed convincingly that the dominant voltage-gated proton channel in unstimulated phagocytes is not gp91phox.
H+ currents are normal in gp91phox knockout cells, and are enhanced by PMA.
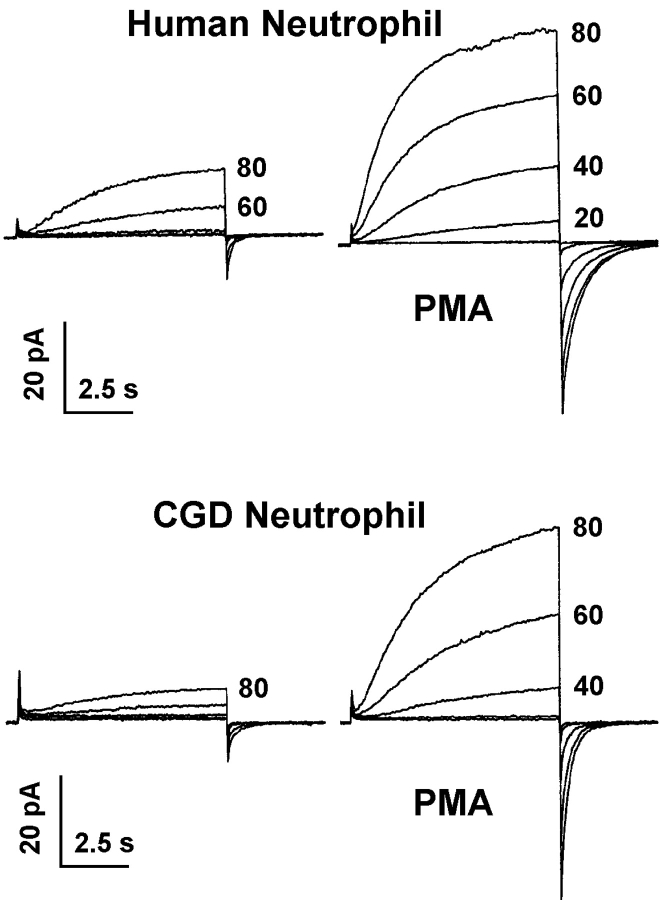
The CGD evidence was convincing, but not conclusive, because in the “dual channel hypothesis,” which was actually considered by Nanda et al. (1994b), the H+ channel in unstimulated cells might simply be a different molecule from the H+ channel in activated cells. This possibility was ruled out by our study of gp91phox knockout cells and CGD neutrophils lacking gp91phox (DeCoursey et al., 2001b). PLB-985 cells are of the myelocytic lineage and when induced by dimethylformamide become capable of producing superoxide via a fully functional NADPH oxidase system (Zhen et al., 1993). Identical large voltage-gated proton currents with completely normal properties were observed in wild-type PLB cells, gp91phox knockouts, and in knockouts rescued by retransfection with gp91phox. Studied in permeabilized-patch configuration, the H+ currents in gp91phox knockout cells also responded to PMA stimulation with increased g H,max and faster τact similar to controls. The slowing of τtail was not observed, consistent with this parameter being closely linked to NADPH oxidase activity (DeCoursey et al., 2000), which obviously was absent in the gp91phox knockout cells. As illustrated in Fig. 2 , similar results were obtained in CGD granulocytes from patients lacking gp91phox. Unstimulated neutrophils from CGD patients had normal H+ currents, and PMA stimulation increased the H+ current to the same extent as in normal neutrophils. Thus the increase in g H,max that occurs during the respiratory burst still occurs in the complete absence of gp91phox and cannot be mediated by gp91phox.
Figure 2.
Families of H+ currents recorded in neutrophils from a normal individual (top) and from a patient with CGD who lacked gp91phox expression (bottom). Voltage pulses were applied in 20-mV increments as labeled. Cells were studied in permeabilized patch configuration, with a symmetrical NH4 + gradient applied to clamp pHi to pHo, which was 7.0, as described (DeCoursey et al., 2001b). The currents on the left were recorded before stimulation, those on the right after stimulation with PMA. The average H+ current amplitudes are the same in CGD and normal cells (DeCoursey et al., 2001b). The constant small inward current at the holding potential in the normal cell after PMA treatment is due to electron current through the activated NADPH oxidase complex, and is absent in the CGD cell. The proton conductance in both resting and activated cells is the same in the presence or absence of gp91phox, which is inconsistent with the proposal that gp91phox is a proton channel. Figure from DeCoursey (2003).
COS-7 cells expressing gp91phox have no proton currents.
If gp91phox is not the H+ channel in resting phagocytes, nor responsible for the increased g H during the respiratory burst, then it could be a proton conductance only if it were too small to detect in the presence of the large g H of resting or activated phagocytes. Because COS-7 cells lack an intrinsic H+ conductance (Maturana et al., 2001; Morgan et al., 2002), they are a clean expression system. We expressed gp91phox in COS-7 cells together with the other three main NADPH oxidase components (Morgan et al., 2002). The expression of gp91phox was confirmed by antibody staining and by demonstrating that the cells generated O2 − upon stimulation with PMA. No proton currents were detected in whole-cell or permeabilized-patch studies, or after stimulation with PMA, under conditions shown previously to enhance H+ currents in neutrophils (DeCoursey et al., 2000), eosinophils (DeCoursey et al., 2001a; Cherny et al., 2001a), or PLB cells (DeCoursey et al., 2001b). The functional assays in this study showed not only that gp91phox was expressed, but also that it was oriented in the membrane in the same way as in phagocytes. Because COS-7 cells lack intrinsic H+ currents, we could have detected a few picoamperes of voltage-gated proton current in these cells, but detected none. This result precludes any significant proton channel function for gp91phox. It also rules out any proton channel function for the other components expressed in these cells, p67phox, p47phox, or p22phox.
In combination, the gp91phox knockout studies and the heterologous expression studies clearly prove that gp91phox is not a proton channel.
Circumstantial Evidence
Expression of voltage-gated proton channels is not correlated with NADPH oxidase activity.
It is possible to perceive a general correlation between NADPH oxidase activity and H+ channel expression. Cells with high levels of NADPH oxidase activity (eosinophils and neutrophils) have large proton currents. H+ channels are expressed roughly in proportion to the rate of O2 − production in phagocytes—the H+ currents are typically 1–2 orders of magnitude larger than needed to compensate maximal NADPH oxidase activity (Eder and DeCoursey, 2001). Promyelocytic HL-60 cells induced to differentiate along the granulocyte pathway acquire the ability to produce O2 − in parallel with an increase in H+ channel expression (Qu et al., 1994).
However, the correlation between NADPH oxidase activity and H+ current in other cells is weak or absent. Several cells express voltage-gated proton channels but have no detectable NADPH oxidase activity. Voltage-gated proton channels were discovered in snail neurons (Thomas and Meech, 1982), which may lack NADPH oxidase. Human basophils have very high H+ current density (Cherny et al., 2001b), but do not produce detectable O2 − (de Boer and Roos, 1986). Rat alveolar epithelial cells have large H+ currents (DeCoursey, 1991; Cherny et al., 1995), but are capable of at most a low level of O2 − production (van Klaveren et al., 1997). Human T lymphocytes express H+ channels (Schilling et al., 2002), but have no detectable cytochrome b 558 (the membrane-bound complex that includes gp91phox) (Káldi et al., 1996). Finally, there was no correlation between the amplitude of I e and I H activated by PMA in individual human neutrophils studied in the permeabilized patch configuration (DeCoursey et al., 2000). In conclusion, although cells may regulate the expression of H+ channels in accordance with their need for them, the many exceptions belie a strong correlation.
There are far more NADPH oxidase complexes (hence gp91phox molecules) per cell than there are H+ channels.
Based on the g H,max of ∼1.5 nS at pHi 6.5 (Gordienko et al., 1996) and unitary conductance ∼30 fS at pHi 6.5 (Cherny et al., 2002), there are ∼50,000 voltage-gated proton channels in a human eosinophil. Based on Ie (Schrenzel et al., 1998; DeCoursey et al., 2001a; Cherny et al., 2001b) and the turnover rate of NADPH oxidase (Koshkin et al., 1997; Cross et al., 1999), there are ∼830,000 active NADPH oxidase complexes per eosinophil. Therefore the number of gp91phox molecules in human eosinophils exceeds the number of H+ channels by more than an order of magnitude. The discrepancy increases if one considers that only a small fraction of the gp91phox molecules in the cell are in the plasma membrane simultaneously (Quinn et al., 1993). This argument could be challenged if gp91phox functioned as a proton channel only when the NADPH oxidase complex was active or at least assembled (Maturana et al., 2001). However, this idea is incompatible with studies showing putative H+ channel behavior of gp91phox expressed by itself in CHO (Henderson et al., 1995; Henderson, 1998; Henderson and Meech, 1999; Mankelow and Henderson, 2001) HEK-293, or COS-7 cells (Maturana et al., 2001). None of these cells has the biochemical machinery to assemble NADPH oxidase or to produce O2 − even when gp91phox is expressed.
The H+ channels active during the respiratory burst have properties markedly different from those in resting cells because H+ channel properties change during activation of NADPH oxidase; there is one channel with two gating modes.
Bánfi et al. (1999) made the important observation that under conditions that enable NADPH oxidase activity, the properties of voltage-gated proton channels are radically different than in cells studied under conventional whole-cell conditions, in which NADPH oxidase cannot function. They concluded that NADPH oxidase activity was associated with the activation of a distinct type of voltage-gated proton channel, not observed in unstimulated cells, and that this channel is formed by gp91phox.
Studies of phagocytes in permeabilized-patch configuration allow direct comparison of the properties of H+ currents in a single cell before, during, and after activation of NADPH oxidase with PMA, arachidonic acid, or other agonists (DeCoursey et al., 2000, 2001a,b; Cherny et al., 2001a). These studies confirm the observation of Bánfi et al. (1999) that the properties of voltage-gated proton channels are dramatically different when NADPH oxidase is active. However, they also provide strong evidence that respiratory burst agonists alter the properties of preexisting H+ channels, rather than inducing the appearance of a novel variety of channel.
(1) The PMA-enhanced g H,max in individual neutrophils correlated strongly with g H,max before stimulation, even though there was an order-of-magnitude range of resting g H,max (DeCoursey et al., 2000). No correlation is expected if there are two independent H+ channel types.
(2) In PMA-stimulated neutrophils, both tail current kinetics and the g H-V relationship shifted progressively from “classical” to “novel” behavior with no indication of two components (DeCoursey et al., 2000).
(3) When NADPH oxidase is inhibited by DPI (diphenylene iodinium), the H+ current amplitude remains large (Schrenzel et al., 1998; DeCoursey et al., 2000, 2001a; Cherny et al., 2001a).
(4) Inhibition of NADPH oxidase by DPI immediately reverses the effect on only one of the four H+ channel properties that are altered by PMA, τtail. Thus, the “novel” properties of the NADPH oxidase–associated H+ channel are separable from one another, and are not invariant properties of a distinct type of channel.
(5) Arachidonic acid activates I e in permeabilized patch studies and produces changes in H+ currents that for the most part are quantitatively identical to those produced by PMA (Cherny et al., 2001a). However, the profound slowing of τtail seen after PMA stimulation is not observed with arachidonic acid, again demonstrating that the properties of the putative novel channels are not invariant.
(6) The reported difference in Zn2+ sensitivity between the two putative types of H+ channels (Bánfi et al., 1999) was a predictable outcome of the experimental design in that study and is not observed when equivalent measurements are used (Appendix of DeCoursey et al., 2001a).
(7) Finally, the appearance of novel H+ currents is inconsistent with the cellular localization of gp91phox. In resting neutrophils, ∼75% of the cytochrome b 558 (which contains gp91phox) is located in intracellular granules and ∼25% is in the plasma membrane (Clark et al., 1987). If gp91phox were a proton channel, it should be detected in unstimulated cells, and its density in the surface membrane should increase upon stimulation. However, no component of H+ current with the novel properties of the NADPH oxidase–related H+ channel is detected in the plasma membrane of unstimulated phagocytes (DeCoursey and Cherny, 1993; Demaurex et al., 1993; Kapus et al., 1993, 1994; Gordienko et al., 1996; Schrenzel et al., 1996; DeCoursey et al., 2000, 2001a). Upon activation, all of the channels in the cell uniformly exhibit novel properties, with no residual component of H+ current with the classical properties seen in unstimulated cells (DeCoursey et al., 2000, 2001a,b).
Protons and electrons move independently of each other in phagocytes.
It has been proposed that electrons and protons permeate the same putative channel in NADPH oxidase (Maturana et al., 2001). However, the translocation of electrons through NADPH oxidase is unequivocally separable from the compensatory H+ flux through H+ channels.
(1) It is possible to measure continuous DPI-sensitive electron current in a cell that is voltage clamped at a potential at which H+ channels do not open (Schrenzel et al., 1998; Bánfi et al., 1999; DeCoursey et al., 2000, 2001a,b; Cherny et al., 2001a), or when the g H is inhibited by Zn2+ (Schrenzel et al., 1998). In these situations, the normal requirement for H+ channels to balance the electron flux is satisfied by the feedback amplifier of the patch-clamp circuit, which supplies the required compensatory current.
(2) Inhibition of NADPH oxidase by DPI neither prevents nor reverses the dramatic enhancement of g H in stimulated phagocytes (DeCoursey et al., 2000, 2001a; Cherny et al., 2001a).
(3) H+ flux is inhibited by lower concentrations of Zn2+ or Cd2+ than are required to reduce O2 − release in neutrophils (Kapus et al., 1992).
Summary
The preponderance of the evidence supports the idea that the voltage-gated proton channel is a separate entity from the NADPH oxidase complex. The idea that gp91phox is a proton channel should be abandoned. We have tested this hypothesis definitively (DeCoursey et al., 2001b; Morgan et al., 2002) and found no evidence to sustain it. Neither gp91phox nor any of its homologues has been demonstrated to function as a proton channel. On the contrary, expression of gp91phox does not result in H+ currents. Furthermore, all known components of H+ current in resting and activated phagocytes persist in cells lacking gp91phox. Because the molecular and genetic identity of the voltage-gated proton channel remains unknown, we still need to identify this molecule. Both NADPH oxidase and the H+ channel play important roles during the respiratory burst in phagocytes. Their interaction, regulation, modulation, expression, trafficking, and physiology are rich areas for future study.
Acknowledgments
The authors appreciate constructive criticism of the manuscript by Fred S. Cohen, Claudia Eder, and Larry L. Thomas.
This work was supported in part by the Heart, Lung, and Blood Institute of the National Institutes of Health (research grants HL52671 and HL61437 to T.E. DeCoursey).
References
- Bánfi, B., A. Maturana, S. Jaconi, S. Arnaudeau, T. Laforge, B. Sinha, E. Ligeti, N. Demaurex, and K.-H. Krause. 2000. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 287:138–142. [DOI] [PubMed] [Google Scholar]
- Bánfi, B., G. Molnár, A. Maturana, K. Steger, B. Hegeds, N. Demaurex, and K.-H. Krause. 2001. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 276:37594–37601. [DOI] [PubMed] [Google Scholar]
- Bánfi, B., J. Schrenzel, O. Nüsse, D.P. Lew, E. Ligeti, K.-H. Krause, and N. Demaurex. 1999. A novel H+ conductance in eosinophils: unique characteristics and absence in chronic granulomatous disease. J. Exp. Med. 190:183–194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny, V.V., and T.E. DeCoursey. 1999. pH-dependent inhibition of voltage-gated H+ currents in rat alveolar epithelial cells by Zn2+ and other divalent cations. J. Gen. Physiol. 114:819–838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny, V.V., L.M. Henderson, and T.E. DeCoursey. 1997. Proton and chloride currents in Chinese hamster ovary cells. Membr. Cell Biol. 11:337–347. [PubMed] [Google Scholar]
- Cherny, V.V., L.M. Henderson, W. Xu, L.L. Thomas, and T.E. DeCoursey. 2001. a. Activation of NADPH oxidase-related proton and electron currents in human eosinophils by arachidonic acid. J. Physiol. 535:783–794. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny, V.V., L.L. Thomas, and T.E. DeCoursey. 2001. b. Voltage-gated proton currents in human basophils. Membr. Cell Biol. In press. [Google Scholar]
- Cherny, V.V., V.S. Markin, and T.E. DeCoursey. 1995. The voltage-activated hydrogen ion conductance in rat alveolar epithelial cells is determined by the pH gradient. J. Gen. Physiol. 105:861–896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherny, V.V., R. Murphy, and T.E. DeCoursey. 2002. Single proton channel currents are really small. Biophys. J. 82:639a. [Google Scholar]
- Clark, R.A., K.G. Leidal, D.W. Pearson, and W.M. Nauseef. 1987. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J. Biol. Chem. 262:4065–4074. [PubMed] [Google Scholar]
- Cross, A.R., R.W. Erickson, B.A. Ellis, and J.T. Curnutte. 1999. Spontaneous activation of NADPH oxidase in a cell-free system: unexpected multiple effects of magnesium ion concentrations. Biochem. J. 338:229–233. [PMC free article] [PubMed] [Google Scholar]
- de Boer, M., and D. Roos. 1986. Metabolic comparison between basophils and other leukocytes from human blood. J. Immunol. 136:3447–3454. [PubMed] [Google Scholar]
- DeCoursey, T.E. 1991. Hydrogen ion currents in rat alveolar epithelial cells. Biophys. J. 60:1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E. 1998. Four varieties of voltage-gated proton channels. Front. Biosci. 3:d477–d482. [DOI] [PubMed] [Google Scholar]
- DeCoursey, T.E. 2003. Voltage-gated proton channels and other proton transfer pathways. Physiol. Rev. In press. [DOI] [PubMed] [Google Scholar]
- DeCoursey, T.E., and V.V. Cherny. 1993. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys. J. 65:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E., and V.V. Cherny. 1994. Voltage-activated hydrogen ion currents. J. Membr. Biol. 141:203–223. [DOI] [PubMed] [Google Scholar]
- DeCoursey, T.E., and S. Grinstein. 1999. Ion channels and carriers in leukocytes. Inflammation: Basic Principles and Clinical Correlates. Third edition. J.I. Gallin and R. Snyderman, editors. Lippincott Williams and Wilkins, Philadelphia, PA. 639–659.
- DeCoursey, T.E., V.V. Cherny, W. Zhou, and L.L. Thomas. 2000. Simultaneous activation of NADPH oxidase-related proton and electron currents in human neutrophils. Proc. Natl. Acad. Sci. USA. 97:6885–6889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E., V.V. Cherny, A.G. DeCoursey, W. Xu, and L.L. Thomas. 2001. a. Interactions between NADPH oxidase-related proton and electron currents in human eosinophils. J. Physiol. 535:767–781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E., V.V. Cherny, D. Morgan, B.Z. Katz, and M.C. Dinauer. 2001. b. The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps. J. Biol. Chem. 276:36063–36066. [DOI] [PubMed] [Google Scholar]
- Demaurex, N., S. Grinstein, M. Jaconi, W. Schlegel, D.P. Lew, and K.-H. Krause. 1993. Proton currents in human granulocytes: regulation by membrane potential and intracellular pH. J. Physiol. 466:329–344. [PMC free article] [PubMed] [Google Scholar]
- Eder, C., and T.E. DeCoursey. 2001. Voltage-gated proton channels in microglia. Prog. Neurobiol. 64:277–305. [DOI] [PubMed] [Google Scholar]
- Gordienko, D.V., M. Tare, S. Parveen, C.J. Fenech, C. Robinson, and T.B. Bolton. 1996. Voltage-activated proton current in eosinophils from human blood. J. Physiol. 496:299–316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M. 1998. Role of histidines identified by mutagenesis in the NADPH oxidase-associated H+ channel. J. Biol. Chem. 273:33216–33223. [DOI] [PubMed] [Google Scholar]
- Henderson, L.M., G. Banting, and J.B. Chappell. 1995. The arachidonate-activable (sic), NADPH oxidase-associated H+ channel: evidence that gp91-phox functions as an essential part of the channel. J. Biol. Chem. 270:5909–5916. [PubMed] [Google Scholar]
- Henderson, L.M., J.B. Chappell, and O.T.G. Jones. 1987. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., J.B. Chappell, and O.T.G. Jones. 1988. a. Internal pH changes associated with the activity of NADPH oxidase of human neutrophils: further evidence for the presence of an H+ conducting channel. Biochem. J. 251:563–567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., J.B. Chappell, and O.T.G. Jones. 1988. b. Superoxide generation by the electrogenic NADPH oxidase of human neutrophils is limited by the movement of a compensating charge. Biochem. J. 255:285–290. [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., and R.W. Meech. 1999. Evidence that the product of the human X-linked CGD gene, gp91-phox, is a voltage-gated H+ pathway. J. Gen. Physiol. 114:771–785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., S. Thomas, G. Banting, and J.B. Chappell. 1997. The arachidonate-activatable, NADPH oxidase-associated H+ channel is contained within the multi-membrane-spanning N-terminal region of gp91-phox. Biochem. J. 325:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Káldi, K., K. Szászi, G. Koncz, K. Suszták, and E. Ligeti. 1996. Arachidonic acid activatable electrogenic H+ transport in the absence of cytochrome b 558 in human T lymphocytes. FEBS Lett. 381:156–160. [DOI] [PubMed] [Google Scholar]
- Kapus, A., R. Romanek, and S. Grinstein. 1994. Arachidonic acid stimulates the plasma membrane H+ conductance of macrophages. J. Biol. Chem. 269:4736–4745. [PubMed] [Google Scholar]
- Kapus, A., R. Romanek, A.Y. Qu, O.D. Rotstein, and S. Grinstein. 1993. A pH-sensitive and voltage-dependent proton conductance in the plasma membrane of macrophages. J. Gen. Physiol. 102:729–760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kapus, A., K. Szászi, and E. Ligeti. 1992. Phorbol 12-myristate 13-acetate activates an electrogenic H+-conducting pathway in the membrane of neutrophils. Biochem. J. 281:697–701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koshkin, V., O. Lotan, and E. Pick. 1997. Electron transfer in the superoxide-generating NADPH oxidase complex reconstituted in vitro. Biochim. Biophys. Acta. 1319:139–146. [DOI] [PubMed] [Google Scholar]
- Mankelow, T.J., and L.M. Henderson. 2001. Inhibition of the neutrophil NADPH oxidase and associated H+ channel by diethyl pyrocarbonate (DEPC), a histidine-modifying agent: evidence for at least two target sites. Biochem. J. 358:315–324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maturana, A., S. Arnaudeau, S. Ryser, B. Bánfi, J.P. Hossle, W. Schlegel, K.-H. Krause, and N. Demaurex. 2001. Heme histidine ligands within gp91phox modulate proton conduction by the phagocyte NADPH oxidase. J. Biol. Chem. 276:30277–30284. [DOI] [PubMed] [Google Scholar]
- Morgan, D., V.V. Cherny, M.O. Price, M.C. Dinauer, and T.E. DeCoursey. 2002. Absence of proton channels in COS-7 cells expressing functional NADPH oxidase components. J. Gen. Physiol. 119:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda, A., S. Grinstein, and J.T. Curnutte. 1993. Abnormal activation of H+ conductance in NADPH oxidase-defective neutrophils. Proc. Natl. Acad. Sci. USA. 90:760–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda, A., J.T. Curnutte, and S. Grinstein. 1994. a. Activation of H+ conductance in neutrophils requires assembly of components of the respiratory burst oxidase but not its redox function. J. Clin. Invest. 93:1770–1775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nanda, A., R. Romanek, J.T. Curnutte, and S. Grinstein. 1994. b. Assessment of the contribution of the cytochrome b moiety of the NADPH oxidase to the transmembrane H+ conductance of leukocytes. J. Biol. Chem. 269:27280–27285. [PubMed] [Google Scholar]
- Qu, A.Y., A. Nanda, J.T. Curnutte, and S. Grinstein. 1994. Development of a H+-selective conductance during granulocytic differentiation of HL-60 cells. Am. J. Physiol. 266:C1263–C1270. [DOI] [PubMed] [Google Scholar]
- Quinn, M.T., T. Evans, L.R. Loetterle, A.J. Jesaitis, and G.M. Bokoch. 1993. Translocation of Rac correlates with NADPH oxidase activation: evidence for equimolar translocation of oxidase components. J. Biol. Chem. 268:20983–20987. [PubMed] [Google Scholar]
- Reeves, E.P., H. Lu, H.L. Jacobs, C.G.M. Messina, S. Bolsover, G. Gabella, E.O. Potma, A. Warley, J. Roes, and A.W. Segal. 2002. Killing activity of neutrophils is mediated through activation of proteases by K+ flux. Nature. 416:291–297. [DOI] [PubMed] [Google Scholar]
- Schilling, T., A. Gratopp, T.E. DeCoursey, and C. Eder. 2002. Voltage-activated proton currents in human lymphocytes. J. Physiol. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenzel, J., D.P. Lew, and K.-H. Krause. 1996. Proton currents in human eosinophils. Am. J. Physiol. 271:C1861–C1871. [DOI] [PubMed] [Google Scholar]
- Schrenzel, J., L. Serrander, B. Bánfi, O. Nüsse, R. Fouyouzi, D.P. Lew, N. Demaurex, and K.-H. Krause. 1998. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 392:734–737. [DOI] [PubMed] [Google Scholar]
- Shimbo, K., D.L. Brassard, R.A. Lamb, and L.H. Pinto. 1995. Viral and cellular small integral membrane proteins can modify ion channels endogenous to Xenopus oocytes. Biophys. J. 69:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, R.C., and R.W. Meech. 1982. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurones. Nature. 299:826–828. [DOI] [PubMed] [Google Scholar]
- van Klaveren, R.J., C. Roelant, M. Boogaerts, M. Demedts, and B. Nemery. 1997. Involvement of an NAD(P)H oxidase-like enzyme in superoxide anion and hydrogen peroxide generation by rat type II cells. Thorax. 52:465–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhen, L., A.A. King, Y. Xiao, S.J. Chanock, S.H. Orkin, and M.C. Dinauer. 1993. Gene targeting of X chromosome-linked chronic granulomatous disease locus in a human myeloid leukemia cell line and rescue by expression of recombinant gp91phox. Proc. Natl. Acad. Sci. USA. 90:9832–9836. [DOI] [PMC free article] [PubMed] [Google Scholar]