Introduction
Previous Perspectives in the Journal of General Physiology have considered (a) the different advantages of two models of ion channel permeation and (b) whether ryanodine receptor adaptation might arise from flash photolysis. Each argument, over the choice of model or influence of technique, is an example of a classic scientific dispute. Here we turn to another hoary old problem—the identification of a “prime mover.”
The specific problem we are dealing with here concerns the structure and function of the voltage-gated proton channel in mammalian neutrophils. We have presented evidence recently that a component of the NADPH oxidase, gp91phox, functions as a proton channel and as such plays an essential role in neutrophil action (Henderson and Meech, 1999). The proposal has proved controversial because the evidence we provided was necessarily circumstantial.
Each time this kind of controversy arises standard pieces of circumstantial evidence are assembled. The following is a list of the kinds of evidence that would generally be considered relevant:
(a) Deletion: destruction of gp91phox abolishes function.
(b) Addition: recovery of function when gp91phox is restored.
(c) Modification: altered gp91phox structure leads to altered function.
(d) Location: gp91phox is located at a site appropriate to its proposed function.
(e) Quantity: gp91phox is present in sufficient quantity to perform the function.
(f) Conditions: gp91phox is active under conditions appropriate to its proposed function.
The key question is whether gp91phox is a “prime mover,” i.e., a proton channel, or whether it simply acts as some kind of cofactor. It has been suggested that gp91phox is not the proton channel itself but the modulator of a proton channel formed by a different, as yet unidentified protein (DeCoursey et al., 2001). The experimental basis for this suggestion comes from Xenopus oocytes where high levels of heterologous expression of many different membrane proteins induce the appearance of an endogenous chloride current (Shimbo et al., 1995; Tzounopoulos et al., 1995). The question is an important one because the proton channel poses an interesting problem in membrane permeation; if gp91phox is the channel, further analysis of proton permeation becomes possible.
Before we start to evaluate the evidence we have tried to summarize the key findings in this area over the past 20 yr. So new readers start here!
Background
(1) In 1980, one of us (Robert Meech) found that depolarized neurons of the land snail, Helix aspersa, were highly proton permeable. In fact, depolarization increased the proton permeability so much that the intracellular pH (pHi) was set by the trans-membrane potential. Subsequent experiments in Roger Thomas's laboratory showed that injection of protons at depolarized membrane potentials produced a large outward current which declined in an exponential fashion once the injection had stopped (Thomas and Meech, 1982; Meech and Thomas, 1987). The acidified cell cytoplasm returned to its resting pH with a similar time course; both pHi recovery and outward current were blocked reversibly by 2 mM Cd2+. After proton injection at the resting potential, pHi remained acidified and there was no obvious outward current. Thus, the current appeared when protons moved through a voltage-gated pathway. It always seemed simpler to think of this pathway as a Cd2+-sensitive “channel.”
(2) Further characterization of this voltage-gated proton channel took place in Lou Byerly's laboratory in 1982 and 1983. Byerly and Hagiwara (1982) had identified a “residual current” present in the perfused neurons of the pond snail, Lymnaea stagnalis, when the major ions, both internal and external, had been replaced by large impermeable substitutes. Armed with the knowledge that snail neurons contained a voltage-gated proton pathway, our key experiment was to show that “residual” tail current reversed at EH, the H+ equilibrium potential (Byerly et al., 1984). The protocol used to control pHi in the perfused neurons was that developed by Byerly and Moody and later published by them (Byerly and Moody, 1986); variants on this protocol have been used in all subsequent experiments on voltage-gated proton channels.
(3) The perfused neuron experiments not only established that the “residual” conductance was proton selective, they also showed that its voltage dependence was affected by both internal and external pH. Acidification of pHi shifted activation toward less positive potentials; acidification of the bathing medium shifted activation in the opposite direction. As a result, activation seemed to occur when the main driving force for protons was outwards. It was as if the channel's primary role was to promote recovery from acidification. Protons could move inwards through the channel—during tail current experiments for example—but these movements were short lived. A second important finding was that Cd2+ inhibited the channel in two ways; it not only shifted activation toward positive membrane potentials but also reduced the maximum available conductance. Cd2+ increased the time to half steady-state current, but this effect was reduced or absent at positive membrane potentials.
(4) Patch clamp experiments by Byerly and Suen (1989) established that proton channels were not distributed evenly over the cell surface, that the proton current had a Q10 of 2.1 and that extracellular divalent cations blocked the proton current in the sequence: Cu2+ = Zn2+ > Ni2+ > Cd2+ > Co2+ > Mn2+ >Mg2+ = Ca2+ = Ba2+. Mahaut-Smith (1989) showed that in land snails Zn2+ block at 45 mV had the form of a monomolecular reaction with a Kd of 1.48 × 10−5M; 1 mM Zn2+ produced 100% block.
(5) Byerly and Suen (1989) examined the current fluctuations at different membrane potentials and found that activation of the proton conductance produced no significant increase in the current variance. They estimated the unitary proton current to be <0.004 pA with a driving force of ∼100 mV. Thus, the unitary conductance was <0.04 pS.
(6) In human neutrophils, a Cd2+-sensitive proton–permeable pathway becomes evident once the enzyme NADPH oxidase is made active. NADPH oxidase is found in the plasma and granular membranes of the white blood cells (basophils, neutrophils, macrophages, and eosinophils) that surround and kill invading infectious microorganisms. The enzyme uses electrons derived from the oxidation of NADPH to reduce O2 to the reactive radical, superoxide O2 −. It is not continuously active but is rapidly (0.5–2 min) activated by a range of compounds and physiological stimuli. Activation results in a rapid depolarization of the plasma membrane to a level that depends on the transmembrane pH gradient; the larger the pH gradient the smaller the depolarization. Activation can be inhibited by diphenelyene iodonium (DPI) (Henderson et al., 1987).
(7) Activation of the NADPH oxidase leads to the synthesis of O2 − and an associated efflux of electrons recorded as an electric current by Schrenzel et al. (1998). The electron efflux is sufficient to depolarize the cell membrane by several volts and the remaining protons are sufficient to acidify the cell cytoplasm by many pH units. Henderson (1988) proposed that a proton channel associated with the NADPH oxidase would account for the observed high proton permeability in activated neutrophils. Their proposal was that the channel provided a path for proton movement to compensate for both the intracellular acidosis and the charge difference set up by the efflux of electrons. A reduced compensatory movement of protons explained the increased acidosis of pHi in the presence of the K+ ionophore valinomycin, or 1 mM Cd2+.
(8) A number of compounds activate the NADPH oxidase. These include PMA, phorbol ester, and unsaturated fatty acids such as arachidonic and oleic acids. If there is an electrochemical gradient for protons, concentrations of arachidonic acid that activate the NADPH oxidase initiate a rapid change in pHi when studied in cell suspensions. Henderson and Chappell (1992) concluded that arachidonate induces a selective increase in the permeability of the membrane to protons and suggested that it acted on the NADPH oxidase associated proton channel.
(9) The NADPH oxidase is a multisubunit enzyme (phagocyte oxidase), consisting of two membrane proteins, gp91phox and p22phox, and two cytosolic proteins, p47phox and p67phox. Upon activation, the two cytostolic subunits move to the membrane and bind to the membrane subunits. The gene for gp91phox is X-linked and a mutation in this or any of the genes encoding one of the other subunits results in chronic granulomatous disease (CGD). Patients with CGD lack a functional NADPH oxidase and are highly susceptible to infection.
(10) In cell lines established from CGD patients, the absence of an arachidonic acid–activated proton channel correlates with the absence of gp91phox rather than the absence of p47phox or p67phox (Henderson et al., 1995). Furthermore, suppressing the expression of p22phox in differentiating HL60 cells had little effect on the arachidonic acid–activated proton flux associated with superoxide production. Consequently, gp91phox seemed likely to be the protein that forms the proton channel.
(11) Soon after the description of voltage-gated proton currents in Helix and Lymnaea neurons, Barish and Baud (1984) identified similar currents in Ambystoma oocytes. Proton currents were also found in rat alveolar epithelial cells (DeCoursey, 1991), microglial cells (Morihata et al., 2000), and a range of myeloid or myeloid derived cells, such as human neutrophils (DeCoursey and Cherny, 1993) and human granulocytes (Demaurex et al., 1993). In addition, the selective proton permeability of the influenza virus M2 channel was established (Chizhmakov et al., 1996; Shimbo et al., 1996)
(12) Arachidonic acid increases the amplitude of the voltage-gated proton current in human neutrophils (DeCoursey and Cherny, 1993). This important finding indicated that the proton channel associated with the NADPH oxidase might correspond to the voltage-gated proton channel. This idea is supported by whole-cell clamp experiments on gp91phox-transfected Chinese hamster ovary (CHO) cells. CHO cells, studied in suspension, show no evidence of having an arachidonate-activated proton pathway until they are transfected with cDNA encoding for gp91phox (Henderson et al., 1995). What is more, studied under whole cell patch-clamp, these transfected cells generate large outward proton currents even during short (150–750 ms) depolarizing command pulses (Henderson and Meech, 1999). These currents, absent in control cells, have all the classical characteristics of the proton channels described by Byerly et al. (1984): their tail currents reverse at EH and the reversal potential shifts 56 mV for 1 U change in pHo. They are also rapidly and reversibly inhibited by 200 μM Zn2+ which produces 60% inhibition during activation at 100 mV (see section 3 above). In addition, their amplitude is reversibly increased by arachidonic acid.
(13) The actual membrane topology of gp91phox is currently unknown. The hydropathy plot suggests that it consists of either four or six transmembrane domains, followed by a large hydrophobic domain, which contains the predicted binding sites for the NADPH oxidase cofactor FAD and the substrate NADPH. CHO cells expressing only the putative transmembrane domain (amino acids 1–230) of gp91phox (CHO-NT) exhibit all the properties of a voltage-gated proton pathway (Henderson et al., 1997; Henderson and Meech, 1999). The third transmembrane domain of gp91phox contains four histidine residues, of which 111, 115, and 119 are predicted to align one above another in an α-helix. If the histidine at position 115 is mutated to either leucine or lysine, the expressed protein appears at the cell membrane, but neither voltage-gated proton currents nor an arachidonic activated proton flux can be elicited. However, some function is retained if the histidine is mutated to aspartic acid (Henderson, 1998; Henderson and Meech, 1999). Aspartic acid and histidine each have a similar pKa, suggesting that proton permeation may require the amino acid side chain at position 115 to alternate between accepting and donating protons at physiological pH.
(14) In recent years it has become evident that the human genome contains more than one gene encoding a gp91phox-like protein. At present, four homologues have been identified, each with a similar molecular weight to gp91phox. They have been given the names Nox1 (from NADPH Oxidase), Nox2 (the renamed gp91phox), Nox3, and Nox4 (Lambeth et al., 2000). In addition, there is a higher molecular weight homologue, Nox5, with an NH2-terminal extension containing three EF hands (Banfi et al., 2001) and two Duox proteins, expressed in the thyroid, which consist of a fusion of NH2-terminal peroxidase and COOH-terminal gp91phox domains (Dupuy et al., 1999). Splice variants of Nox1 (Nox1S) (Banfi et al., 2000) and Nox5 (Banfi et al., 2001) have been reported. Voltage-dependent proton currents have been recorded from HEK-293 cells transfected with either Nox1S (Banfi et al., 2000) or Nox5 (Banfi et al., 2001). However, untransfected HEK-293 cells express low levels of Nox4 (Shiose et al., 2001) that could account for the proton currents observed in these wild type cells.
(15) The predicted membrane topology for the different Nox homologues is of four to six transmembrane domains with a large cytosolic domain at the COOH-terminal, much as described for gp91phox (Nox2). The histidines in position 115 and 119 in Nox2 are conserved in all the human homologues, in keeping with the proposed mechanism for proton permeation.
Is gp91phox (Nox2) a Voltage-gated Proton Channel? A Review of the Evidence
The question presented for discussion contains a number of tangled threads so that it would be as well to tease apart and identify the different components, as we understand them. First, is gp91phox a voltage-gated proton channel? Second, is such a channel required for superoxide generation in neutrophils? Third, is gp91phox the voltage-gated proton channel in neutrophils?
We have attempted to answer the first question by showing that CHO cells expressing gp91phox have large time and voltage-dependent proton currents (Henderson and Meech, 1999). We cannot exclude the possibility that gp91phox expression simply up-regulates a channel already present in the wild-type CHO cells (DeCoursey et al., 2001) but we feel that some observations are difficult to reconcile with a hypothesis that requires a specific protein–protein interaction of this kind. A key finding is that histidine 115 is essential for function. This supreme specificity is very different to the situation in Xenopus oocytes where the expression of a wide variety of cellular and synthetic proteins, such as phospholemman and the NB protein of influenza B virus, can activate an endogenous chloride conductance (Shimbo et al., 1995).
As to whether a voltage-gated proton channel is essential for superoxide generation in neutrophils, this appears to be well established so there is little need for further discussion here. The final question is more difficult to answer. There is little direct evidence but much indirect inference based on neutrophil surrogates. Our discussion reviews the evidence from a number of different cell types.
(a). Deletion: Does Destruction of gp91phox Abolish Function?
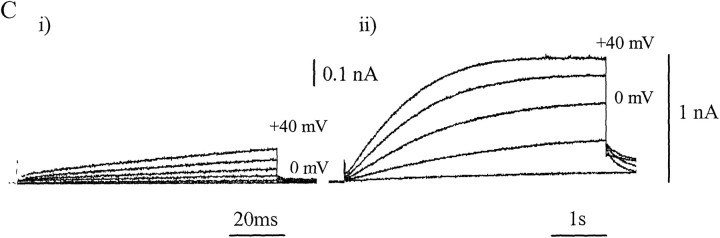
Finding voltage-gated proton currents in the absence of membrane-bound gp91phox would exclude gp91phox from consideration as the only voltage-gated proton channel in neutrophils. We know of no unequivocal evidence of this kind from neutrophils but exon 3 of the gene encoding gp91phox has been knocked out in a PLB-985 cell line. The resulting PLBKO cells appear not to express gp91phox, but nevertheless exhibit large outward proton currents (DeCoursey et al., 2001). In Fig. 1 C we reproduce typical proton currents recorded from the PLBKO cells given to us by Dr. Mary C. Dinauer. The figure also shows that both PLB-985 and PLBKO cells immunostained with an antibody to gp91phox have high levels of stain in the region of the cell membrane. As might be expected, differentiation of PLB-985 cells results in an increase in the expression of this gp91phox-like protein (Fig. 1 A) together with an increase in PMA-activated superoxide generation (not depicted). However, undifferentiated PLBKO cells also show a high level of expression of a gp91phox-like protein, and once again expression increases after differentiation (Fig. 1 B). The COOH-terminal regions of other Nox proteins have such a high degree of sequence homology that we would expect the anti-gp91phox antibody would detect any of them. We have confirmed the presence of gp91phox-like protein in PLBKO cells with a second anti-gp91phox antibody supplied by M.C. Dagher (Grenoble, France).
Figure 1.
Expression of NADPH Oxidase (Nox) protein in PLB and PLBKO cells. (A) Nox protein immunodetected in undifferentiated (i) and differentiated (ii) PLB-298 cells after incubation with an anti–COOH-terminal gp91phox polyclonal antibody and FITC-labeled anti–rabbit antibody. The polyclonal anti-gp91phox was raised to the COOH-terminal 14-amino acid peptide coupled to keyhole limpet hemocyanin. It was verified by Western blot against detergent-solubilized membrane proteins from human neutrophils (Henderson et al., 1995). The images, which show an optical slice close to the cell center, were obtained using a BioRad MRC 600 inverted confocal microscope. A pseudocolor scale in which the highest intensity of emitted fluorescence is denoted by red through orange, yellow and green to blue, low fluorescence, indicates the presence of a Nox protein. Differentiated PLB-985 cells treated in the absence of the anti-gp91phox antibody are shown in iii. (B) Immunodetection of Nox protein in PLBKO cells using the same technique and antibody as described for A. As before, undifferentiated (i), differentiated (ii), and control cells in the absence of anti-gp91phox antibody (iii) are shown. (C) Whole-cell currents recorded from a single differentiated PLB-985 cell under conditions designed to maximize the amplitude of H+ current (see Henderson and Meech, 1999). The patch pipette–filling solution, which was adjusted to pH 6.5, contained 119 mM TMA hydroxide, to block potassium currents, a small quantity of calcium buffer (3.7 mM EGTA, 0.74 mM CaCl2) and ∼120 mM MES pH buffer. The bathing solution contained 110 mM TMA methane-sulphonate, 2 mM Ca(OH)2, 2 mM Mg(OH)2, 5 mM glucose, and 100 mM EPPS buffer adjusted to pH 8.0. Low amplitude prepulses in the linear current–voltage range (−60 to −100 mV) were scaled by PCLAMP 6 software (Axon Instruments, Inc.) and used for online subtraction of linear capacitive and leakage currents (Armstrong and Bezanilla, 1974). The superimposed currents shown were recorded in response to a standard stepped-voltage protocol; commands were in the range -40 to 40 mV (20-mV increments). The command duration was 100 ms (i) or 5 s (ii). The immunostaining of PLB-985 and PLBKO cells and recording of whole cell currents are the work of X. Wen Hu. Mary C. Dinauer provided the PLBKO cells. EPPS is N-[2-hydroxyethyl]-piperazine-N'-[3-propane-sulphonic acid]; TMA, tetramethylammonium.
The presence of a gp91phox-like protein in the gp91phox knockout cell line may be a consequence of an enhanced expression of other Nox homologues. Compensation of this kind is seen in osteoclasts isolated from the gp91phox knockout mouse; these osteoclasts express Nox4 at a higher level than osteoclasts from wild-type mice (Yang et al., 2001). Compensatory expression of one or more of the homologues of gp91phox in the PLBKO cells would account for the presence of the voltage-dependent proton current reported by DeCoursey et al., (2001). The identity of the gp91phox homologue expressed in these cells is currently under investigation.
(b). Addition: Does Function Recover if gp91phox Is Restored?
Once again we know of no direct evidence of this kind. However, gp91phox has been inserted into both CHO cells (Henderson and Meech, 1999; see section 12 above) and Green monkey kidney (COS-7) cells (Maturana et al., 2001), and time- and voltage-dependent currents were recorded in each case. Now the question arises: do the characteristics of the expressed gp91phox channels correspond to those of the voltage-gated proton conductance in neutrophils?
A puzzling aspect of the voltage-gated proton channels recorded from many mammalian cells is that their kinetics are so slow. Fig. 1 C shows whole cell currents from a single differentiated PLB-985 cell on two different time scales. In Fig. 1 C, ii, the currents take several seconds to reach a steady-state, even at 40 mV, and at −20 mV the currents fail to reach a steady-state after 5 s. However, although they take many seconds to reach steady-state, the proton currents do develop without a delay (see Figure C i). The currents recorded from gp91phox-expressing CHO cells are similar. In most of our studies on CHO cells we have used short command pulses to demonstrate the early stages of the development of the proton current and to avoid changes in the proton gradient with time. As a practical consideration, these slow kinetics mean that a true steady-state activation curve is hard to achieve and the position of the “activation curve” on the voltage axis will depend on the duration of the test pulse. As always, it is essential to compare like with like.
Whether CHO cells have detectable endogenous proton currents is in dispute but it is generally accepted that they are absent from COS-7 cells (Maturana et al., 2001; Morgan et al., 2002). However, the large volume of these cells (cell diameter as much as 50 μm) makes it hard (perhaps impossible) to control pHi satisfactorily using a standard patch pipette with a 1 μm tip. Cell cytoplasm contains highly effective mobile and fixed pH buffers that dictate pHi. Byerly and Moody (1986) found that it was not possible to control pHi in dialyzed snail neurons (100 μm diameter) if the pipette diameter was less than one third of the diameter of the cell, even if the dialysis solution contained 120 mM pH buffer. The molecular size of the buffer, the volume of the cell and the diameter of the pipette tip govern the time required for full dialysis. In CHO cells (diameter 25 μm), it takes at least 10 min for a fluorescent dye (with a comparable molecular weight to pH buffer) to perfuse from a 3-μm pipette tip throughout the entire cell (Henderson and Meech, 1999). Even so pHi dropped to pH 6.8 only after 20 min dialysis with a pH 5.5 pipette solution. Problems associated with controlling pHi when recording voltage-gated proton currents have also been reported by Morihata et al. (2000) in experiments on microglial cells.
Characterization of the voltage-dependent currents that Maturana et al. (2001) recorded from their gp91phox-transfected COS-7 cells remains uncertain because the presumed proton tail currents do not reverse at EH as calculated from the pH of the pipette solution. However, our experience with CHO cells would lead us to suggest that pHi was in reality close to normal and not the pH of the pipette solution. Whether or not the time-dependent currents observed were proton currents they stand in contrast to the findings of Morgan et al. (2002), who were unable to see comparable currents even after 24 min dialysis. The COS-7 cells used were transfected with all four of the main subunits of NADPH oxidase (although direct evidence for gp91phox in the cell membrane is absent). The cells were smaller than normal COS-7 cells (∼15–25 instead of 20–50 μm) and they regularly developed a high leakage current before any proton current became evident. We can confirm that even in CHO cells this is an ongoing problem that we attribute to cell swelling. In the smaller CHO cells, proton currents may be evident because pHi becomes acidified before irreversible damage is done to the cell membrane and the cell contents. In conclusion: COS-7 cells transfected with gp91phox may generate time-dependent currents but there appears to be considerable variability in the response.
(c). Modification: Does Altered gp91phox Structure Lead To Altered Function?
“Deletion” and “addition” experiments of the kind considered above do not exclude the possibility that the structure concerned is an intermediate agent rather than a “prime mover.” More direct evidence is obtained by modifying the gp91phox product by mutagenesis (Henderson and Meech, 1999). Conserve the positive charge at position 115 (i.e., by changing the histidine residue to lysine), and the expressed product fails to conduct protons. But change the positive charge at position 115 to negative (i.e., by changing the histidine to aspartic acid), and the expressed product retains the capacity to conduct protons, at least partially. Such observations are difficult to reconcile with a hypothesis that requires a specific protein–protein interaction between gp91phox and a second proton channel–forming protein. However, if gp91phox is the proton channel these findings fall into place with a simpler, more general hypothesis, that the histidine residue, with its capacity to undergo cycles of protonation and deprotonation, is the key element of the proton conduction pathway.
This proposal finds support not only in work on the influenza virus M2 proton channel, where histidine 37 is essential for both ion selectivity and activation (Wang et al., 1995), but also in experiments on the S4 region of the Shaker K+ channel (Starace and Bezanilla, 2001). S4 is thought to act as a voltage sensor because every third amino acid residue has a positive charge (arginine or lysine). When the arginine in positions 365, 368, and 371 is changed to histidine the resulting channels are capable of conducting protons down their electrochemical gradient. Starace and Bezanilla conclude that because the three histidine residues are accessible to the cytosol and to the external environment they are able to conduct protons across the cell membrane.
(d). Location: Is gp91phox Located at a Site Appropriate to its Proposed Function?
A variety of cell fractionation experiments show that there are large quantities of gp91phox in the neutrophil cell membrane.
(e). Quantity: Is gp91phox Present in Neutrophils in Sufficient Quantity?
If we were to measure the amount of gp91phox present in neutrophils and show that the concentration was insufficient to generate the measured proton conductance the discussion would be over. Instead we can show by calculation that there is sufficient gp91phox present in the neutrophil surface membrane to account for the proton currents observed. The concentration of cytochrome b558 present in human neutrophils corresponds to ∼1.66 × 106 molecules of gp91phox (Henderson, 1988). Of this, ∼25% of the gp91phox molecules are in the surface membrane (Clark et al., 1987). If we take the Shaker K+ channel as our model and assume that there are four subunits of gp91phox/proton channel, we end up with ∼1 × 105 channels/neutrophil. If each proton channel had a unitary conductance of no more than 0.04 pS (Byerly and Suen, 1989), the neutrophil would have a total proton conductance of no more than 4.2 nS. This value, which is ∼20 times higher than that actually found, suggests that there would be sufficient gp91phox even if the individual proton channels had an unusually low open probability or if we have overestimated the unitary conductance.
(f). Conditions: Is gp91phox Active under Appropriate Conditions?
Direct activation of neutrophils by agents such as arachidonic acid leads to production of superoxide, depolarization of the cell membrane and acidification of the cell contents. In gp91phox-expressing CHO cells, cell acidification and arachidonic acid both shift the voltage dependence of the proton conductance toward the resting potential. Thus, the characteristics of the proton conductance are exactly those required to fulfill its function.
Indirect activation by PMA is thought to be via a cytosolic phospholipase A2 (cPLA2) that releases arachidonic acid. Experiments using a PLB-985 cell line deficient in cPLA2 have established that the enzyme is essential for the PMA activation of proton efflux (Lowenthal and Levy, 1999). This is presumably because arachidonic acid shifts the voltage dependence of the proton conductance toward the resting potential. The generation of superoxide by PMA is also dependent on the expression of cPLA2 (Dana et al., 1998). It follows that PMA should shift the activation curve of the proton conductance in PLB-985 cells but not that in a cPLA2-deficient cell line.
Future
At one time gp91phox expression was believed to be restricted to the phagocytes and myeloid-derived cells. Now, however, homologues of gp91phox have been found in the genomes of a wide range of organisms such as Drosophila, Dictystelium, C. elegans, Arabidopsis, tomato, rice, and some bacteria. Function has yet to be assigned to gp91phox-like proteins in these species, although superoxide generation is assumed. Histidines 115 and 119 are conserved in each of the gp91phox homologues so far identified, and so they too may function as proton channels. That homologues have been shown to be expressed in such a wide range of tissues and species forces us to reevaluate the assumption that cell lines and tissues are devoid of gp91phox unless shown otherwise. It may be safer to assume that gp91phox has a widespread distribution and that it performs an essential function as a proton permeable pathway in all cells.
Acknowledgments
We thank Dr. Mary C. Dinauer (Indiana University Medical Center) for providing the PLBKO cells.
The work reviewed in this perspective was supported in part by grant no. HO522 and H0604 from the Arthritis Research Campaign, UK.
References
- Armstrong, C.M., and F. Bezanilla. 1974. Charge movement associated with the opening and closing of the activation gates of the Na channels. J. Gen. Physiol. 63:533–552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Banfi, B., A. Maturana, S. Jaconi, S. Arnaudeau, T. Laforge, B. Sinha, E. Ligeti, N. Demaurex, and K.H. Krause. 2000. A mammalian H+ channel generated through alternative splicing of the NADPH oxidase homolog NOH-1. Science. 287:138–142. [DOI] [PubMed] [Google Scholar]
- Banfi, B., G. Molnar, A. Maturana, K. Steger, B. Hegedus, N. Demaurex, and K.H. Krause. 2001. A Ca2+-activated NADPH oxidase in testis, spleen, and lymph nodes. J. Biol. Chem. 276:37594–37601. [DOI] [PubMed] [Google Scholar]
- Barish, M.E., and C. Baud. 1984. A voltage-gated hydrogen ion current in the oocyte membrane of the axolotl, Ambystoma. J. Physiol. 352:243–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly, L., and S. Hagiwara. 1982. Calcium currents in internally perfused nerve cell bodies of Limnea stagnalis. J. Physiol. 322:503–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly, L., and W.J. Moody. 1986. Membrane currents of internally perfused neurones of the snail, Lymnaea stagnalis, at low intracellular pH. J. Physiol. 376:477–491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly, L., and Y. Suen. 1989. Characterization of proton currents in neurones of the snail, Lymnaea stagnalis. J. Physiol. 413:75–89. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byerly, L., R. Meech, and W. Moody, Jr. 1984. Rapidly activating hydrogen ion currents in perfused neurones of the snail, Lymnaea stagnalis. J. Physiol. 351:199–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chizhmakov, I.V., F.M. Geraghty, D.C. Ogden, A. Hayhurst, M. Antoniou, and A.J. Hay. 1996. Selective proton permeability and pH regulation of the influenza virus M2 channel expressed in mouse erythroleukaemia cells. J. Physiol. 494:329–336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark, R.A., K.G. Leidal, D.W. Pearson, and W.M. Nauseef. 1987. NADPH oxidase of human neutrophils. Subcellular localization and characterization of an arachidonate-activatable superoxide-generating system. J. Biol. Chem. 262:4065–4074. [PubMed] [Google Scholar]
- Dana, R., T.L. Leto, H.L. Malech, and R. Levy. 1998. Essential requirement of cytosolic phospholipase A2 for activation of the phagocyte NADPH oxidase. J. Biol. Chem. 273:441–445. [DOI] [PubMed] [Google Scholar]
- DeCoursey, T.E. 1991. Hydrogen ion currents in rat alveolar epithelial cells. Biophys. J. 60:1243–1253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E., and V.V. Cherny. 1993. Potential, pH, and arachidonate gate hydrogen ion currents in human neutrophils. Biophys. J. 65:1590–1598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeCoursey, T.E., V.V. Cherny, D. Morgan, B.Z. Katz, and M.C. Dinauer. 2001. The gp91phox component of NADPH oxidase is not the voltage-gated proton channel in phagocytes, but it helps. J. Biol. Chem. 276:36063–36066. [DOI] [PubMed] [Google Scholar]
- Demaurex, N., S. Grinstein, M. Jaconi, W. Schlegel, D.P. Lew, and K.-H. Krause. 1993. Proton channels in human granulocytes: regulation by membrane potential and intracellular pH. J. Physiol. 466:329–344. [PMC free article] [PubMed] [Google Scholar]
- Dupuy, C., R. Ohayon, A. Valent, M.S. Noel-Hudson, D. Deme, and A. Virion. 1999. Purification of a novel flavoprotein involved in the thyroid NADPH oxidase. Cloning of the porcine and human cdnas. J. Biol. Chem. 274:37265–37269. [DOI] [PubMed] [Google Scholar]
- Henderson, L.M. 1988. The electrogenic NADPH oxidase of human neutrophils derived cytoplasts. Ph.D. Thesis. University of Bristol, UK.
- Henderson, L.M. 1998. Role of histidines identified by mutagenesis in the NADPH oxidase-associated H+ channel. J. Biol. Chem. 273:33216–33223. [DOI] [PubMed] [Google Scholar]
- Henderson, L.M., G. Banting, and J.B. Chappell. 1995. The arachidonate-activable, NADPH oxidase-associated H+ channel. Evidence that gp91-phox functions as an essential part of the channel. J. Biol. Chem. 270:5909–5916. [PubMed] [Google Scholar]
- Henderson, L.M., and J.B. Chappell. 1992. The NADPH-oxidase-associated H+ channel is opened by arachidonate. Biochem. J. 283:171–175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., J.B. Chappell, and O.T. Jones. 1987. The superoxide-generating NADPH oxidase of human neutrophils is electrogenic and associated with an H+ channel. Biochem. J. 246:325–329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., and R.W. Meech. 1999. Evidence that the product of the human X-linked CGD gene, gp91-phox, is a voltage-gated H+ pathway. J. Gen. Physiol. 114:771–786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson, L.M., S. Thomas, G. Banting, and J.B. Chappell. 1997. The arachidonate-activatable, NADPH oxidase-associated H+ channel is contained within the multi-membrane-spanning N-terminal region of gp91-phox. Biochem. J. 325:701–705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lambeth, J.D., G. Cheng, R.S. Arnold, and W.A. Edens. 2000. Novel homologs of gp91phox. Trends Biochem. Sci. 25:459–461. [DOI] [PubMed] [Google Scholar]
- Lowenthal, A., and R. Levy. 1999. Essential requirement of cytosolic phospholipase A2 for activation of the H+ channel in phagocyte-like cells. J. Biol. Chem. 274:21603–21608. [DOI] [PubMed] [Google Scholar]
- Mahaut-Smith, M.P. 1989. The effect of zinc on calcium and hydrogen ion currents in intact snail neurons. J. Exp. Biol. 145:455–464. [DOI] [PubMed] [Google Scholar]
- Maturana, A., S. Arnaudeau, S. Ryser, B. Banfi, J.P. Hossle, W. Schlegel, K.H. Krause, and N. Demaurex. 2001. Heme histidine ligands within gp91(phox) modulate proton conduction by the phagocyte NADPH oxidase. J. Biol. Chem. 276:30277–30284. [DOI] [PubMed] [Google Scholar]
- Meech, R.W., and R.C. Thomas. 1987. Voltage-dependent intracellular pH in Helix aspersa neurones. J. Physiol. 390:433–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgan, D., V.V. Cherny, M.O. Price, M.C. Dinauer, and T.E. DeCoursey. 2002. Absence of proton channels in COS-7 cells expressing functional NADPH oxidase components. J. Gen. Physiol. 119:571–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morihata, H., F. Nakamura, T. Tsutada, and M. Kuno. 2000. Potentiation of a voltage-gated proton current in acidosis-induced swelling of rat microglia. J. Neurosci. 20:7220–7227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schrenzel, J., L. Serrander, B. Banfi, O. Nusse, R. Fouyouzi, D.P. Lew, N. Demaurex, and K. Krause. 1998. Electron currents generated by the human phagocyte NADPH oxidase. Nature. 392:734–737. [DOI] [PubMed] [Google Scholar]
- Shimbo, K., D.L. Brassard, R.A. Lamb, and L.H. Pinto. 1995. Viral and cellular small integral membrane proteins can modify ion channels endogenous to Xenopus oocytes. Biophys. J. 69:1819–1829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimbo, K., D.L. Brassard, R.A. Lamb, and L.H. Pinto. 1996. Ion selectivity and activation of the M2 ion channel of influenza virus. Biophys. J. 70:1335–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiose, A., J. Kuroda, K. Tsuruya, M. Hirai, H. Hirakata, S. Naito, M. Hattori, Y. Sakaki, and H. Sumimoto. 2001. A novel superoxide-producing NAD(P)H oxidase in kidney. J. Biol. Chem. 276:1417–1423. [DOI] [PubMed] [Google Scholar]
- Starace, D.M., and F. Bezanilla. 2001. Histidine scanning mutagenesis of basic residues of the s4 segment of the Shaker K+ channel. J. Gen. Physiol. 117:469–490. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas, R.C., and R.W. Meech. 1982. Hydrogen ion currents and intracellular pH in depolarized voltage-clamped snail neurons. Nature. 299:826–828. [DOI] [PubMed] [Google Scholar]
- Tzounopoulos, T., J. Maylie, and J.P. Adelman. 1995. Induction of endogenous channels by high levels of heterologous membrane proteins in Xenopus oocytes. Biophys. J. 69:904–908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang, C., R.A. Lamb, and L.H. Pinto. 1995. Activation of the M2 ion channel of influenza virus: a role for the transmembrane domain histidine residue. Biophys. J. 69:1363–1371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang, S., P. Madyastha, S. Bingel, W. Ries, and L. Key. 2001. A new superoxide-generating oxidase in murine osteoclasts. J. Biol. Chem. 276:5452–5458. [DOI] [PubMed] [Google Scholar]