Abstract
The α subunit of voltage-gated Na+ channels of brain, skeletal muscle, and cardiomyocytes is functionally modulated by the accessory β1, but not the β2 subunit. In the present study, we used β1/β2 chimeras to identify molecular regions within the β1 subunit that are responsible for both the increase of the current density and the acceleration of recovery from inactivation of the human heart Na+ channel (hH1). The channels were expressed in Xenopus oocytes. As a control, we coexpressed the β1/β2 chimeras with rat brain IIA channels. In agreement with previous studies, the β1 extracellular domain sufficed to modulate IIA channel function. In contrast to this, the extracellular domain of the β1 subunit alone was ineffective to modulate hH1. Instead, the putative membrane anchor plus either the intracellular or the extracellular domain of the β1 subunit was required. An exchange of the β1 membrane anchor by the corresponding β2 subunit region almost completely abolished the effects of the β1 subunit on hH1, suggesting that the β1 membrane anchor plays a crucial role for the modulation of the cardiac Na+ channel isoform. It is concluded that the β1 subunit modulates the cardiac and the neuronal channel isoforms by different molecular interactions: hH1 channels via the membrane anchor plus additional intracellular or extracellular regions, and IIA channels via the extracellular region only.
Keywords: β2 subunit, cardiac electrophysiology, Nav1.2, Nav1.5, subunit interaction
INTRODUCTION
Voltage-gated sodium (Na+) channels are responsible for the initiation and propagation of action potentials in electrically excitable cells (Catterall, 1992). These channels are heteromultimeric proteins of the plasma membrane consisting of a pore-forming α subunit and accessory β subunits. Screening for cDNAs encoding Na+ channel subunits revealed the existence of 10 α and 3 β subunit isoforms in mammalian cells (Goldin, 2001).
As demonstrated by heterologous expression experiments, the α subunit determines the main electrophysiological and pharmacological properties of a given Na+ channel complex (Catterall, 1992), while two of the three β subunits (β1 and β3) modulate the function of the α subunits (Patton et al., 1994; Morgan et al., 2000). When expressed in Xenopus oocytes, the β1 subunit increases the current amplitude and accelerates the recovery from inactivation in currents generated by cardiac (Nuss et al., 1995; Qu et al., 1995), skeletal muscle (Wallner et al., 1993; Yang et al., 1993; Makita et al., 1994) and neuronal Na+ channels (Isom et al., 1992; Smith and Goldin, 1998; Vijayaragavan et al., 2001). In addition to this, neuronal and skeletal muscle Na+ channels require the β1 subunit for fast inactivation (Isom et al., 1992; Wallner et al., 1993; Yang et al., 1993; Makita et al., 1994; Patton et al., 1994; Nuss et al., 1995; Smith and Goldin, 1998; Vijayaragavan et al., 2001).
The molecular mechanisms leading to the increased current densities and to the accelerated recovery from inactivation have not been elucidated. Recent data indicate that the human heart Na+ channel (hH1; Gellens et al., 1992) assembles with the β1 subunit already within the endoplasmic reticulum (Zimmer et al., 2002). This may result in an improved trafficking of the channel complex to the plasma membrane, similarly as reported for ATP-sensitive K+ channels (Zerangue et al., 1999). Single-channel experiments with hH1 indicated that the larger current amplitude upon β1 coexpression is not due to a change of the channel open probability (Nuss et al., 1995). Together, these data suggest that increased current amplitudes are due to an increase of the number of functional channels in the plasma membrane. In this context, it is interesting to note that Na+ channel β subunits are highly homologous to cell adhesion molecules (CAM) of the Ig superfamiliy (Isom et al., 1995; Isom, 2001). Their extracellular domains bind to extracellular matrix molecules (Srinivasan et al., 1998; Xiao et al., 1999), strongly suggesting a function of Na+ channel β subunits in promoting cell–cell contacts and in modulating localization and cell-surface density of α subunits.
Molecular regions of the β1 subunit that are responsible for the modulation of the electrophysiological properties of IIA and human skeletal muscle (hSKM1) Na+ channels are located within the extracellular domain (Chen and Cannon, 1995; McCormick et al., 1998, 1999). In these studies it was shown that neither the putative β1 membrane anchor nor the β1 intracellular domain is required for the β1-like modulation of sodium channel gating. This result was further substantiated by the finding that the corresponding β1 subunit response element in IIA and hSKM1 channels is localized within an extracellular loop (domain IV, loop S5/S6; Makita et al., 1996; Qu et al., 1999).
In the present study we used chimeras and deletion variants of β1 and β2 subunits to identify β1 molecular regions involved in the modulation of hH1. We show that—in contrast to the result with IIA channels—the β1 extracellular domain is neither sufficient nor necessary for the β1 effect on the recovery kinetics and current density of hH1 channels expressed in Xenopus oocytes. Instead, the putative membrane anchor plus either the extracellular or the intracellular domain of the β1 subunit are required to modulate hH1. We conclude that hH1 and IIA channels interact specifically with different molecular regions of the β1 subunit.
MATERIALS AND METHODS
cDNAs of Na+ Channel Subunits
Plasmids pSP64T-hH1, pNa200, and pSPNaβ coding for hH1 (EMBL/GenBank/DDBJ accession no. M77235; Gellens et al., 1992), for the rat brain IIA Na+ channel (EMBL/GenBank/DDBJ accession no. X61149; Auld et al., 1988) and for the rat β1 subunit (EMBL/GenBank/DDBJ accession no. M91808; Isom et al., 1992) were provided by A.L. George (Vanderbilt University), A.L. Goldin (University of California) and W. Stühmer (Max Planck Institute, Göttingen), respectively. The β2 subunit (EMBL/GenBank/DDBJ accession no. U37026, Isom et al., 1995) was isolated by RT-PCR from the human brain astrocytoma cell line 1321N1, as already described (Zimmer et al., 2002).
Recombinant DNA Procedures
To obtain a comparable translation efficiency of the different β subunit variants in Xenopus oocytes, we subcloned each of the β subunit constructs into the same in vitro transcription vector (pGEMHEnew; Liman et al., 1992). This vector contains the T7 promoter, a 5′-untranslated region (UTR)* of the Xenopus β-globin gene, a multicloning site (mcs) used to insert the β subunit variants, and a 3′-UTR of the Xenopus β-globin gene. Since this 3′-UTR is not present in the hH1-containing vector pSP64T-hH1, we linearized all β subunit plasmids for in vitro transcription using a restriction site within the mcs downstream to the β subunit sequence so that also the resulting cRNAs did not contain the β-globin 3′-UTR. Thus, the hH1 and each of the β subunit cRNAs were composed of the β-globin 5′-UTR and the hH1 or the respective β subunit sequences.
The β1 cDNA was isolated from pSPNaβ and subcloned into pGEMHEnew using the HindIII-XbaI and EcoRI-XbaI sites, respectively, resulting in pGEM-β1. The HindIII and EcoRI sites were treated with Klenow enzyme to allow for blunt end ligation. The β2 cDNA was inserted into the BamHI-HindIII site of pGEMHEnew, resulting in pGEM-β2, as described previously (Zimmer et al., 2002).
The β subunit chimeras constructed are shown in Fig. 2. To create the constructs β122, β211, β122a, β211a, and β221, the desired β1 and β2 subunit regions were first separately amplified by PCR and then linked by a recombinant PCR step (Higuchi, 1989) using the following internal primer pairs: 5′-CACCCACAATCTCTGACACGATGGATGCCAT-3′ and 5′-CGTGTCAGAGATTGTGGGTGCCTCCGTCGG-3′ for the construction of β122, 5′-GGTGGCCGTGATCATGATGTACGTGCTCAT-3′ and 5′-ACATCATGATCACGGCCACCGTGAAGTCCC-3′ for the construction of β211, 5′-CCTGCAGATGGATCTTCTTGACGACGCTGG-3′ and 5′-CAAGAAGATCCATCTGCAGGTCCTCATGGA-3′ for the construction of β122a, 5′-TGGCAAGATCCACCTGGAGGTGGTGGACAA-3′ and 5′-CCTCCAGGTGGATCTTGCCATGGCCACGGT-3′ for the construction of β211a, and 5′-GGTGCTGATGGTGTACTGCTACAAGAAGAT-3′ and 5′-AGCAGTACACCATCAGCACCAAGATGACCA-3′ for the construction of β221. Recombinant fragments were subcloned into the BamHI-HindIII (β122, β122a) or Asp718-EcoRI sites (β211, β211a, β221) of pGEMHEnew, resulting in pGEM-β122, pGEM-β122a, and in pGEM-β211, pGEM-β211a, pGEM-β221, respectively. Chimeras β121 and β212 were created using the β122 and β211 constructs as initial templates for PCR and the following internal primer pairs: 5′-GGTGCTGATGGTGTACTGCTACAAGAAGAT-3′ and 5′-AGCAGTACACCATCAGCACCAAGATGACCA-3′ for the construction of β121, and 5′-ACTTGACCACCATCTCCGCCACGAGCCATA-3′ and 5′-GGCGGAGATGGTGGTCAAGTGTGTGAGGAG-3′ for the construction of β212. Recombinant PCR fragments were subcloned into the Asp718-Bsp1407 (β121) and Asp718-Bpu1102 sites (β212) of pGEM-β1 and pGEM-β2, resulting in pGEM-β121 and pGEM-β212, respectively. The deletion variants β11Δ and β21Δ were constructed by PCR. For the introduction of a stop codon at the desired position (underlined in the primer sequence) and an XbaI site for the subsequent cloning step (indicated in italics in the primer sequence), we used oligonucleotide 5′-AAATCTAGA CTAAATCTTCTTGTAGCAGTACAC-3′ as one of the flanking primers. The sequence of the T7 promoter in pGEM-β1 and pGEM-β211 served as the second primer site. The β11Δ and β21Δ PCR fragments were subcloned into the Asp718-XbaI site of pGEMHEnew, resulting in pGEM-β11Δ and pGEM-β21Δ, respectively. Construct β12Δ was also obtained by PCR. We used oligonucleotide 5′-AAAAAGCTTCAACCTGCTCTACCTCCTCACACACTTGACCAC-3′ (underlined: stop codon; italics: HindIII site) and the T7 promoter sequence to amplify the shortened chimera from plasmid pGEM-β122. The product was subcloned into the Asp718-HindIII site of pGEMHEnew, resulting in pGEM-β12Δ.
Figure 2.
Structure of the chimeras between the Na+ channel β1 and β2 subunits used in this study. The corresponding terminal amino acids of the β1 (white boxes) and β2 (gray boxes) subunit regions are indicated. The assumed topology of both subunits in the plasma membrane is shown below the cartoons of the chimeras.
PfuTurbo DNA Polymerase (Stratagene) was used for all PCR reactions to minimize PCR-mediated nucleotide exchanges. The correctness of the DNA constructs was checked by the dideoxy DNA sequencing method. Preparation, digestion, and ligation of DNA were performed according to standard procedures (Sambrook et al., 1989).
Heterologous Expression in Xenopus Oocytes
Capped cRNAs of hH1 and of IIA were prepared by SpeI and NotI digestion of plasmids pSP64T-hH1 and pNa200, respectively, followed by in vitro transcription reaction with SP6 (hH1) and T7 (IIA) RNA polymerase (Roche Diagnostics GmbH). Vectors pGEM-β2, pGEM-β122, pGEM-β122a, pGEM-β211, pGEM-β211a, pGEM-β221, pGEM-β121, pGEM-β212, and pGEM-β21Δ were linearized by HindIII digestion, and vectors pGEM-β1 and pGEM-β11Δ were linearized by XbaI digestion. The in vitro transcription reaction was performed using T7 RNA polymerase.
Oocytes from Xenopus laevis were obtained as described previously (Zimmer et al., 2002). Glass micropipettes were used to inject a cRNA volume per oocyte of 40–60 nl. Concentrations of the different cRNA preparations were assessed by agarose gel electrophoresis using the 0.24–9.5 kb RNA ladder from GIBCO BRL. The hH1 and IIA cRNA preparations were injected at a final concentration of ∼0.1 μg/μl and 0.05 μg/μl, respectively. The different cRNAs encoding the β subunit variants were at a concentration of ∼0.2 μg/μl. Thus, the final molar ratio of hH1 to β subunit variant was ∼1:20 at the cRNA level. Injected oocytes were incubated for 3 d at 18°C in Barth medium. In control experiments, we tested the influence of the hH1/β1 cRNA ratio on current density and recovery from inactivation. Significant modulation of hH1 currents was already observed at a 1:1 ratio. The effects saturated at a ratio of 1:5 to 1:10, and were obtained also at higher β1 cRNA concentrations (1:40). However, only about one fifth of the β1 cRNA was required to modulate hH1 channels when incorporating the 3′-UTR of the β-globin sequence into the β1 cRNA (NotI digestion of pGEM-β1). Current amplitudes did not increase when coinjecting undiluted β1 cRNA containing this β-globin sequence, although the recovery from inactivation of hH1 was clearly accelerated (unpublished data). A 3- to 10-fold dilution of this β1 cRNA containing both the 5′- and 3′-UTR of the Xenopus β-globin gene resulted in two- to fourfold higher peak current amplitudes accompanied by the described effect on the recovery kinetics. We think that this 3′-UTR enhances the translation efficiency of the β1 subunit. Thus, expression of hH1 whose cRNA does not contain this sequence is probably suppressed relative to the expression of β1.
Electrophysiology
Whole-cell Na+ currents were recorded with the two-microelectrode voltage clamp technique using a commercial amplifier (OC725C; Warner Instruments Corp.). Glass microelectrodes were filled with 3 M KCl solution. The microelectrode resistance was between 0.2 and 0.5 MΩ. The bath solution contained (in mM): 20 NaCl, 97.5 KCl, 1.8 CaCl2, 10 HEPES/KOH, pH 7.2. The currents were elicited by test potentials from −80 to 40 mV in 5-mV increments from a holding potential of −120 mV. The pulsing frequency was 0.2 Hz. Recovery from inactivation was determined with a standard protocol (Fig. 1 C, inset) at a frequency of 0.2 Hz. The amplitude of I Na, measured 3 d after injection at the test potential of −25 mV (hH1) and −10 mV (IIA) was between 0.5 to 5.0 μA. The recovery from inactivation was determined from Na+ currents with an amplitude between 1.5 to 3 μA. Recording and analysis of the data were performed on a PC with the ISO2 software (MFK). The sampling rate was 20 kHz.
Figure 1.
Modulation of hH1 and IIA Na+ channels by the β1 subunit. (A) Representative current traces for hH1 and hH1/β1 channels at the test potentials −10 and −30 mV. The τh values of hH1 versus hH1/β1 channels were statistically indistinguishable (−30 mV: τh = 2.21 ± 0.18 for hH1, τh = 2.01 ± 0.43 for hH1/β1 [P = 0.64]; −10 mV: τh = 1.25 ± 0.07 for hH1, τh = 1.12 ± 0.08 for hH1/β1 [P = 0.23]). Number of experiments: n = 11 for hH1, n = 6 for hH1/β1. Calibration bars = 4 ms, at −30 mV: 0.25 μA for hH1, 0.62 μA for hH1/β1; at −10 mV: 0.45 μA for hH1, 1.06 μA for hH1/β1. (B) Effect of the β1 subunit on the hH1 peak current amplitude in Xenopus oocytes (*P < 0.001). Currents were measured 3 d after injection at the test potential of −25 mV. Measurements were performed in seven different batches of oocytes. Data from a single batch of oocytes were normalized with respect to the mean current of hH1-injected oocytes (n = 63 for hH1, n = 50 for hH1/β1, and n = 52 for hH1/β2). (C) Time course of recovery from inactivation of hH1 channels. The respective voltage protocol is shown in the inset (n = 32 for hH1, n = 38 for hH1/β1, and n = 21 for hH1/β2). (D) Effect of the β1 subunit on the inactivation time course of rat brain IIA Na+ currents. The Na+ currents were elicited by a test pulse to −10 mV, and normalized with respect to the peak current. Calibration bars = 5 ms, 0.9 μA for IIA, 0.8 μA for IIA/β1, and 0.7 μA for IIA/β2. Statistically, the inactivation time constant τh was not different for IIA and IIA/β2 channels at the applied test pulses (unpublished data). (E) Effect of the β1 subunit on the IIA peak current amplitude (*P < 0.001). Currents were measured 3 d after injection at the test potential of −10 mV (n = 13 for IIA, n = 13 for IIA/β1 and n = 13 for IIA/β2). (F) Time course of recovery from inactivation of IIA channels. Currents were elicited by the same voltage protocol as indicated in C, except that a test pulse to −10 mV was used (n = 7 for IIA, n = 7 for IIA/β1 and n = 6 for IIA/β2). Bars indicate SEM.
Statistics
Student's t test was applied to test for statistical significance using the Microcal Origin 5.0 software (Microcal Software, Inc.). Statistical significance was assumed for P < 0.05.
RESULTS
The β1 Subunit Modulates both hH1 and IIA Channels
We first analyzed the effects of the β1 and β2 subunit on the current density, the recovery from inactivation, and the time course of inactivation in both hH1 and IIA currents. We found that the β2 subunit neither modulated hH1 nor IIA channels (Fig. 1) . In contrast, the β1 subunit produced significantly larger whole-cell currents (Fig. 1, B and E) and accelerated recovery from inactivation of both hH1 and IIA channels (Fig. 1, C and F). In addition to this, we observed rapidly inactivating Na+ currents in IIA/β1 channels that were not seen when expressing IIA channels alone (Fig. 1 D). A statistically significant effect of the β1 subunit on hH1 inactivation was not observed (Fig. 1 A).
The similarity of the β1 subunit effects on current density and recovery from inactivation of the cardiac and brain Na+ channels suggests a similar mechanism for the α/β1 subunit interaction. We tested this hypothesis by coexpressing various β1/β2 subunit chimeras (Fig. 2) with hH1 and IIA channels in the oocyte system. Although both β subunits share little contiguous primary sequence similarity (∼14% identity throughout the sequences), their conformation and topology are presumably very similar (Isom et al., 1992; Isom et al., 1995). Both subunits are predicted to be membrane anchored, exposing the larger NH2-terminal domain to the extracellular side and the smaller COOH-terminal region into the cytosol (Fig. 2).
hH1 and IIA Channels Are Modulated by Different β1 Subunit Regions
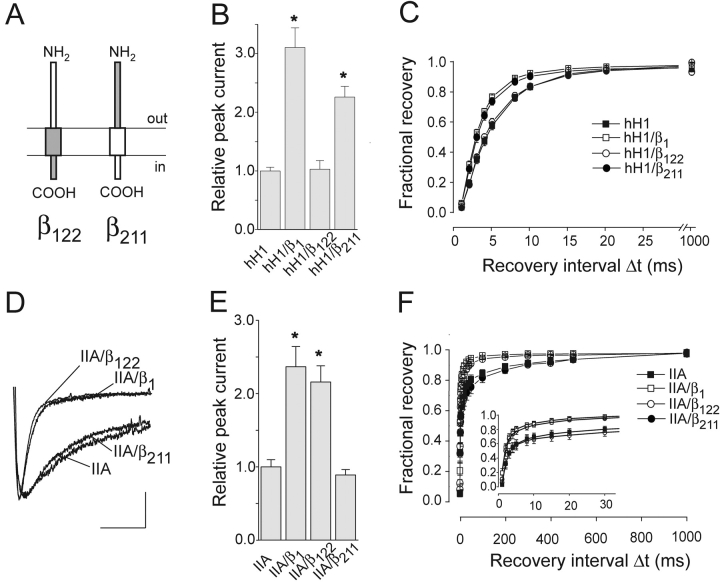
Coexpression of chimera β122 that consisted of the β1 extracellular domain (ED), the β2 membrane anchor (MA) and the β2 intracellular domain (ID; see Figs. 2 and 3 A) did neither enhance the current density nor accelerate the recovery from inactivation of hH1 channels (Fig. 3, B and C). In contrast, β1-like effects on hH1 currents were observed when coexpressing the opposite chimera β211, indicating that the MA plus the ID of the β1 subunit are required to modulate hH1 channels (Fig. 3, B and C, Table I) . In control experiments, we tested the effect of both chimeras on IIA channels and observed that only β122, but not β211, modulated the inactivation time course, current density, and recovery from inactivation (Fig. 3, D–F). This indicates that the β1 ED is necessary and sufficient to modulate IIA channels, similarly as reported previously (McCormick et al., 1999). Coexpression of β211, however, that was sufficient to modulate hH1, had no effect on IIA channels (Fig. 3, B and C). The same results were obtained when using a structurally similar pair of β subunit chimeras (β122a and β211a in Fig. 2; Table I). In conclusion, the cardiac Na+ channel isoform hH1 is modulated by the β1 MA plus the ID, whereas the β1 ED was sufficient to modulate the neuronal isoform IIA.
Figure 3.
Modulatory effect of chimeras β122 and β211 on hH1 and IIA channels. (A) Schematic representation of the domain structures of both chimeras. (B) Relative current amplitudes (test pulse to −25 mV; *P < 0.001; n = 55 for hH1, n = 37 for hH1/β1, n = 38 for hH1/β122, and n = 50 for hH1/β211). (C) Time course of recovery from inactivation of hH1 channels (n = 7 for hH1, n = 6 for hH1/β1, n = 5 for hH1/β122, and n = 6 for hH1/β211). (D) Effect of β122 on the inactivation time course of rat brain IIA Na+ currents (test pulse: −10 mV). Calibration bars = 5 ms, 0.25 μA for IIA, 0.52 μA for IIA/β1, 0.27 μA for IIA/β122, and 0.21 μA for IIA/β211. (E) Effect of β122 on the IIA peak current amplitude (test pulse to −10 mV; *P < 0.001; n = 19 for IIA, n = 13 for IIA/β1, n = 19 for IIA/β122, and n = 17 for IIA/β211). (F) Time course of recovery from inactivation of IIA channels. For voltage protocol, see legend to Fig. 1 (n = 13 for IIA, n = 12 for IIA/β1 and n = 12 for IIA/β122, and n = 12 for IIA/β211). Bars indicate SEM.
TABLE I.
Effect of the Different β Subunit Constructs on Current Densityand Recovery from Inactivation of hH1 Channels
| Channels | Imax a | τrec b |
|---|---|---|
| ms | ||
| hH1 | 1 | 5.87 ± 0.18 |
| hH1/β1 | 3.47 ± 0.32c | 3.20 ± 0.13c |
| hH1/β2 | 0.86 ± 0.50 | 5.79 ± 0.47 |
| hH1/β122 | 1.03 ± 0.15 | 5.24 ± 0.27 |
| hH1/β211 | 2.26 ± 0.18c | 3.74 ± 0.23c |
| hH1/β122a | 1.22 ± 0.30 | 5.30 ± 0.17 |
| hH1/β211a | 2.61 ± 0.48c | 3.33 ± 0.04c |
| hH1/β121 | 1.16 ± 0.11 | 5.01 ± 0.37c |
| hH1/β212 | 0.88 ± 0.07 | 5.31 ± 0.23 |
| hH1/β221 | 1.19 ± 0.11 | 4.79 ± 0.45c |
| hH1/β11Δ | 1.33 ± 0.15c | 3.95 ± 0.29c |
| hH1/β12Δ | 0.81 ± 0.21 | 5.58 ± 0.30 |
| hH1/β21Δ | 0.78 ± 0.06 | 5.70 ± 1.10 |
Peak current amplitudes relative to hH1.
Recovery time constants τrec determined with monoexponential fits.
Significantly different compared to hH1 channels (P < 0.05).
The β1 Intracellular Domain Requires its Own Membrane Anchor for Full Effect on hH1
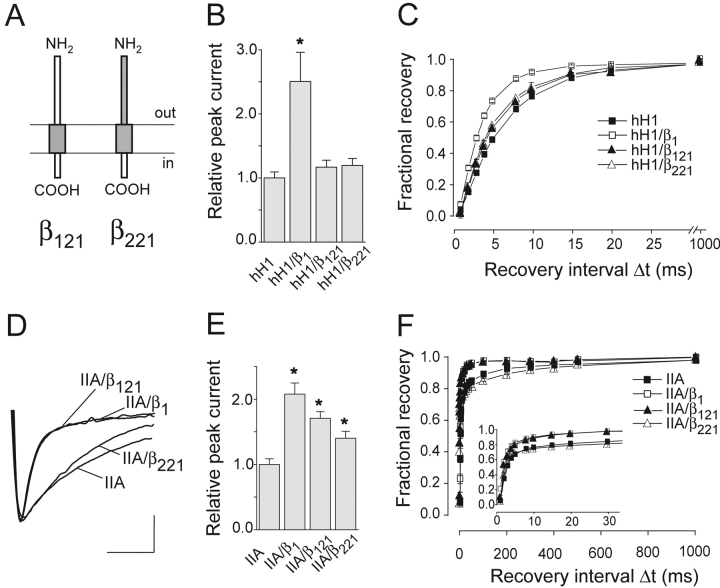
To test whether or not the β1 ID is sufficient to modulate hH1 currents, two chimeras were constructed that contained the β1 ID, the β2 MA, and either the β1 or the β2 ED (β121 and β221; Fig. 4 A). As result, none of the chimeras increased the hH1 current density (Fig. 4 B). We observed a moderate but significant acceleration of recovery from inactivation that was, however, significantly less pronounced compared with the effect of the wild-type β1 subunit (Fig. 4 C, Table I). In parallel experiments with IIA channels, β121 induced the full β1 effect on IIA inactivation, current density, and recovery from inactivation (Fig. 4, D–F). These results indicate that the β1 ED in the β121 chimera was functionally active to modulate IIA channels. As expected, chimera β221 had no effect on the inactivation time course (Fig. 4 D) and the recovery from inactivation (Fig. 4 F) of IIA currents. Interestingly, we found a small but significant increase of the IIA current density (Fig. 4 E), suggesting that also the β1 ID modulates the current density of IIA channels.
Figure 4.
Coexpression of chimeras β121 and β221 with hH1 and IIA channels. (A) Schematic representation of the domain structures of both chimeras. (B) Relative current amplitudes (test pulse to −25 mV; *P < 0.001; n = 29 for hH1, n = 17 for hH1/β1, n = 28 for hH1/β121, and n = 21 for hH1/β221). (C) Time course of recovery from inactivation of hH1 channels (n = 21 for hH1, n = 17 for hH1/β1, n = 12 for hH1/β121, and n = 19 for hH1/β221). (D) Effect of β121 on the time course of inactivation of rat brain IIA Na+ currents (test pulse to −10 mV). Calibration bars = 5 ms, 0.93 μA for IIA, 0.90 μA for IIA/β1, 0.62 μA for IIA/β121, and 0.90 μA for IIA/β221. (E) Effect of β121 and β221 on the IIA peak current amplitude (test pulse to −10 mV; *P < 0.001; n = 15 for IIA, n = 9 for IIA/β1, n = 12 for IIA/β121, and n = 19 for IIA/β221). (F) Time course of recovery from inactivation of IIA channels (n = 13 for IIA, n = 9 for IIA/β1 and n = 5 for IIA/β121, and n = 13 for IIA/β221). Bars indicate SEM.
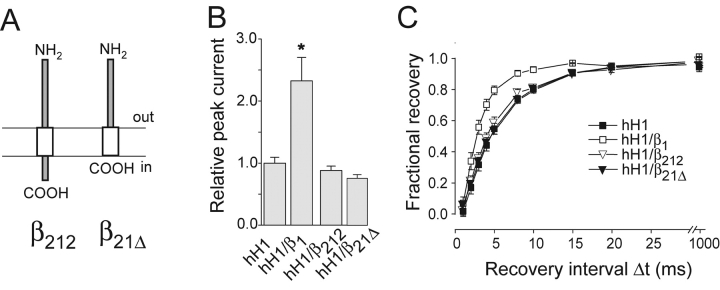
Considering the results with hH1, the exchange of the β1 MA by the corresponding β2 MA disturbed the β1-like effects (β1 versus β121, Fig. 4; β211 versus β221, Figs. 3 and 4, Table I). Hence, the β1 MA plays a crucial role in the interaction of the β1 subunit with hH1. However, when fused to the ED and the ID of the β2 subunit, the β1 MA alone did neither enhance current density nor accelerate recovery from inactivation of hH1 currents (β212, Fig. 5 , Table I). Similar results were obtained with a deletion mutant consisting of the β2 ED plus the β1 MA (β21Δ, Fig. 5, Table I), confirming that the β1 MA is not sufficient to modulate hH1 currents. In addition to this membrane-spanning region, the β1 ID is required for an efficient modulation of hH1 channels (β21Δ vs. β211, Figs. 3 and 5, Table I).
Figure 5.
Coexpression of chimera β212 and β21Δ with hH1 channels. (A) Schematic representation of the domain structure of β212 and β21Δ. (B) Relative current amplitudes (test pulse to −25 mV; *P < 0.001; n = 17 for hH1, n = 10 for hH1/β1, n = 21 for hH1/β212, and n = 11 for β21Δ). (C) Time course of recovery from inactivation of hH1 channels (n = 10 for hH1, n = 4 for hH1/β1, n = 11 for hH1/β212, and n = 7 for β21Δ). Bars indicate SEM.
The β1 Extracellular Domain Plus the β1 Membrane Anchor has Partially β1-like Effects on hH1
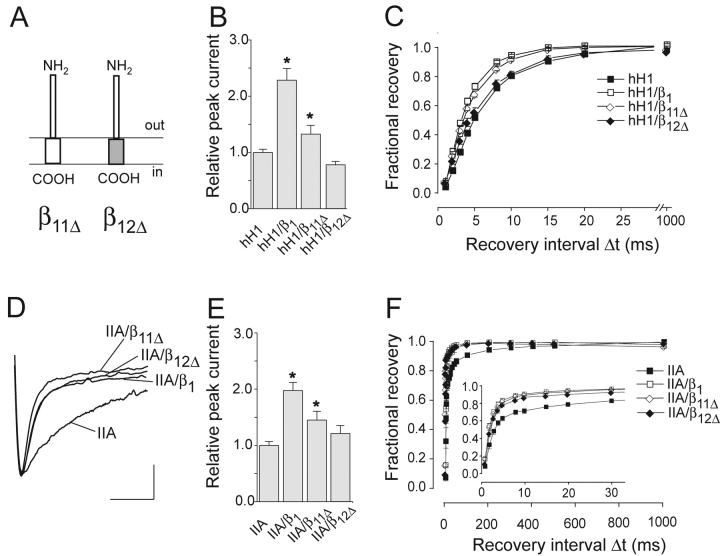
Because the β1 ID efficiently modulates hH1 channels only when linked to the β1 MA, we tested whether also the β1 ED requires the fusion to the β1 MA in order to functionally interact with hH1. To address this question we fused the β1 ED either to the β1 or to the β2 MA (resulting in β11Δ or β12Δ, respectively), and expressed these deletion variants lacking the β1 ID with hH1 or IIA channels (Fig. 6 A).
Figure 6.
Coexpression of β11Δ and β12Δ with hH1 and IIA channels. (A) Schematic representation of the domain structures of both deletion variants. (B) Relative current amplitudes (test pulse to −25 mV; *P < 0.001; n = 38 for hH1, n = 38 for hH1/β1, n = 30 for hH1/β11Δ, and n = 23 for hH1/β12Δ). (C) Time course of recovery from inactivation of hH1 channels (n = 23 for hH1, n = 21 for hH1/β1, n = 19 for hH1/β11Δ, and n = 20 for hH1/β12Δ). (D) Effect of β11Δ and β12Δ on the inactivation time course of rat brain IIA Na+ currents (test pulse to −10 mV). Calibration bars = 5 ms, 0.92 μA for IIA, 0.76 μA for IIA/β1, 0.80 μA for IIA/β11Δ, and 0.28 μA for IIA/β12Δ. (E) Effect of β11Δ and β12Δ on the IIA peak current amplitude (test pulse to −10 mV; *P < 0.001; n = 16 for IIA, n = 14 for IIA/β1, n = 21 for IIA/β11Δ, and n = 9 for IIA/β12Δ). (F) Time course of recovery from inactivation of IIA channels (n = 13 for IIA, n = 13 for IIA/β1, n = 16 for IIA/β11Δ, and n = 10 for IIA/β12Δ). Bars indicate SEM.
As a result, β11Δ accelerated the recovery from inactivation of hH1 currents (Fig. 6, B and C, Table I). In contrast to this, coexpression of β12Δ did not produce β1-like effects on hH1. This result shows that the β1 MA is required in β11Δ to modulate hH1 channels. In β11Δ, a few amino acids are probably exposed to the intracellular side, thus belonging to the ID. These residues should, however, not be responsible for the modulation of hH1, because the same amino acids are present in β21Δ which had no effect on hH1 (Fig. 5).
In case of IIA channels, both β11Δ and β12Δ accelerated the inactivation time course and the recovery process from inactivation (Fig. 6, D–F), again confirming that the β1 ED suffices to modulate IIA channels.
Although the hH1 and IIA current amplitudes increased significantly when coexpressing β11Δ, respective values were clearly smaller compared with the data obtained with hH1/β1 or IIA/β1 channels (Fig. 6, B and E). Thus, the absence of the β1 ID caused a partial loss of function, suggesting an α/β1 subunit interaction on the cytoplasmic side.
DISCUSSION
In the present study we took advantage of the nonmodulating β2 subunit to explore molecular regions of the β1 subunit that functionally interact with hH1 to enhance current density and to accelerate recovery from inactivation. Coexpression of β1/β2 subunit chimeras and deletion variants revealed that the functional α/β1 interaction is not mediated by a conserved molecular mechanism in IIA and hH1 channels. In contrast to the results obtained with the Na+ channel isoforms of brain (McCormick et al., 1998, 1999; this study) and skeletal muscle (Chen and Cannon, 1995), the extracellular domain of the β1 subunit was neither sufficient nor required to modulate the cardiac-specific Na+ channel isoform. Instead, the β1 membrane anchor was identified as a structural requirement for β1-like modulation of hH1. All chimeras lacking this region failed to increase current density and to accelerate recovery from inactivation.
However, the β1 membrane anchor alone did not modulate hH1 currents (see β212 in Fig. 5). To accelerate the recovery process, additional molecular regions of the β1 subunit were necessary: either the ID in β211 or the ED in β11Δ. This surprising result suggests two alternative mechanisms for the acceleration of the recovery from inactivation: one mediated by extracellular and the other by intracellular hH1/β1 interaction sites. Both mechanisms obviously require the primary interaction of the β1 membrane–spanning region with a putative intramembrane site in hH1. This interaction could then facilitate an exposure of the ID and the ED of the β1 subunit to respective interaction sites of hH1, finally resulting in a specific hH1/β1 interaction and in the observed current modulation.
In addition to the effect on the recovery of hH1 channels, a strong increase in the current density was only observed with the wild-type β1 subunit and with chimera β211 (see Fig. 3). Deletion of the β1 intracellular domain (β11Δ and β21Δ) significantly reduced the peak current amplitude, suggesting an important role of the β1 intracellular domain for an efficient hH1/β1 subunit interaction. We speculate that the absence of this domain reduces the binding affinity between β1 and hH1, finally resulting in a decreased cell surface expression of functional channels. Supporting this view, Meadows et al. (2001) recently showed by coimmunoprecipitation experiments that the deletion of 34 amino acids at the COOH terminus of the β1 subunit drastically reduced the β1 binding affinity to IIA channels in a mammalian cell line.
The intracellular β1 domain may exert its effect on hH1 channels not only by a direct subunit interaction, but also through the interaction with other proteins. Recent studies provided evidence for cytoskeletal interactions of the β1 subunit through ankyrin (Chauhan et al., 2000; Malhotra et al., 2000) and for the binding of the β1, but not the β2 subunit, to receptor tyrosine phosphatase β (Ratcliffe et al., 2000). Thus, the hH1/β1 interaction at the intracellular side might be regulated by cytoskeletal proteins or by a specific phosphorylation site in the β1 intracellular domain.
Recently, an alternative spliced variant of the β1 subunit has been reported (β1A; Kazen-Gillespie et al., 2000), which is expressed in the heart. Similar to the β2 subunit, this splice variant possesses a membrane-spanning and intracellular domain that shows no obvious sequence similarities with the respective regions of the β1 subunit (protein sequence identity of 10.5% and 8.6% of the β1A ID vs. the corresponding residues in β1 and β2, respectively). Therefore, it is likely that β1A has either no or at least altered modulating effects on hH1. Respective coexpression studies with hH1/β1A channels including β1/β1A chimeras could be a clue for the understanding of the physiological relevance of the alternative splicing of the β1 subunit in the heart.
In conclusion, our data contribute to a better understanding of the hH1/β1 interaction. We provide evidence that different molecular mechanisms underlie the β1 modulatory effects in hH1/β1 and IIA/β1 channels. Future studies using site-directed mutagenesis and protein binding assays may reveal the corresponding key amino acids both in hH1 and in the β1 subunit that determine the nature of the subunit interaction of the cardiac Na+ channel.
Acknowledgments
The authors are grateful to K. Schoknecht for her contribution to the electrophysiological recordings, and to S. Bernhardt, A. Kolchmeier, and B. Tietsch for their technical assistance.
This work was supported by grant Be1250/9-2 from the Deutsche Forschungsgemeinschaft to K. Benndorf and T. Zimmer, and by BMBF grant 01ZZ015/IZKF Jena to T. Zimmer.
Footnotes
Abbreviations used in this paper: ED, extracellular domain; ID, intracellular domain; MA, membrane anchor; UTR, untranslated region.
References
- Auld, V.J., A.L. Goldin, D.S. Krafte, J. Marshall, J.M. Dunn, W.A. Catterall, H.A. Lester, N. Davidson, and R.J. Dunn. 1988. A rat brain Na+ channel α subunit with novel gating properties. Neuron. 1:449–461. [DOI] [PubMed] [Google Scholar]
- Catterall, W.A. 1992. Cellular and molecular biology of voltage-gated sodium channels. Physiol. Rev. 72:S15–S48. [DOI] [PubMed] [Google Scholar]
- Chauhan, V.S., S. Tuvia, M. Buhusi, V. Bennett, and A.O. Grant. 2000. Abnormal cardiac Na+ channel properties and QT heart rate adaptation in neonatal ankyrinB knockout mice. Circ. Res. 86:441–447. [DOI] [PubMed] [Google Scholar]
- Chen, C., and S.C. Cannon. 1995. Modulation of Na+ channel inactivation by the β1 subunit: a deletion analysis. Pflugers Arch. 431:186–195. [DOI] [PubMed] [Google Scholar]
- Gellens, M.E., A.L. George, L. Chen, M. Chahine, R. Horn, R.L. Barchi, and R.G. Kallen. 1992. Primary structure and functional expression of the human cardiac tetrodotoxin-insensitive voltage-dependent sodium channel. Proc. Natl. Acad. Sci. USA. 89:554–558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldin, A.L. 2001. Resurgence of sodium channel research. Annu. Rev. Physiol. 63:871–894. [DOI] [PubMed] [Google Scholar]
- Higuchi, R. 1989. Using PCR to engineer DNA. In PCR Technology: Principles and Applications for DNA Amplification. H.A. Erlich, editor. Stockton Press, NY. 61–70.
- Isom, L.L. 2001. Sodium channel β subunits: anything but auxiliary. Neuroscientist. 7:42–51. [DOI] [PubMed] [Google Scholar]
- Isom, L.L., K.S. De Jongh, D.E. Patton, B.F.X. Reber, J. Offord, H. Charbonneau, K. Walsh, A.L. Goldin, and W.A. Catterall. 1992. Primary structure and functional expression of the β1 subunit of the rat brain sodium channel. Science. 256:839–842. [DOI] [PubMed] [Google Scholar]
- Isom, L.L., D.S. Ragsdale, K.S. De Jongh, R.E. Westenbroek, B.F.X. Reber, T. Scheuer, and W.A. Catterall. 1995. Structure and function of the β2 subunit of brain sodium channels, a transmembrane glycoprotein with a CAM motif. Cell. 83:433–442. [DOI] [PubMed] [Google Scholar]
- Kazen-Gillespie, K.A., D.S. Ragsdale, M.R. D'Andrea, L.N. Mattei, K.E. Rogers, and L.L. Isom. 2000. Cloning, localization, and functional expression of sodium channel β1A subunits. J. Biol. Chem. 275:1079–1088. [DOI] [PubMed] [Google Scholar]
- Liman, E.R., J. Tytgat, and P. Hess. 1992. Subunit stoichiometry of a mammalian K+ channel determined by construction of multimeric cDNAs. Neuron. 9:861–871. [DOI] [PubMed] [Google Scholar]
- Makita, N., P.B. Bennett, and A.L. George. 1994. Voltage-gated Na+ channel β1 subunit mRNA expressed in adult human skeletal muscle, heart, and brain is encoded by a single gene. J. Biol. Chem. 269:7571–7578. [PubMed] [Google Scholar]
- Makita, N., P.B. Bennett, and A.L. George. 1996. Molecular determinants of β1 subunit- induced gating modulation in voltage-dependent Na+ channels. J. Neurosci. 16:7117–7127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malhotra, J.D., K. Kazen-Gillespie, M. Hortsch, and L.L. Isom. 2000. Sodium channel β subunits mediate homophilic cell adhesion and recruit ankyrin to points of cell-cell contact. J. Biol. Chem. 275:11383–11388. [DOI] [PubMed] [Google Scholar]
- McCormick, K.A., L.L. Isom, D. Ragsdale, D. Smith, T. Scheuer, and W.A. Catterall. 1998. Molecular determinants of Na+ channel function in the extracellular domain of the β1 subunit. J. Biol. Chem. 273:3954–3962. [DOI] [PubMed] [Google Scholar]
- McCormick, K.A., J. Srinivasan, K. White, T. Scheuer, and W.A. Catterall. 1999. The extracellular domain of the β1 subunit is both necessary and sufficient for β1-like modulation of sodium channel gating. J. Biol. Chem. 274:32638–32646. [DOI] [PubMed] [Google Scholar]
- Meadows, L., J.D. Malhotra, A. Stetzer, L.L. Isom, and D.S. Ragsdale. 2001. The intracellular segment of the sodium channel β1 subunit is required for its efficient association with the channel α subunit. J. Neurochem. 76:1871–1878. [DOI] [PubMed] [Google Scholar]
- Morgan, K., E.B. Stevens, B. Shah, P.J. Cox, A.K. Dixon, K. Lee, R.D. Pinnock, J. Hughes, P.J. Richardson, K. Mizuguchi, and A.P. Jackson. 2000. β3: An additional auxiliary subunit of the voltage-sensitive sodium channel that modulates channel gating with distinct kinetics. Proc. Natl. Acad. Sci. USA. 97:2308–2313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nuss, H.B., N. Chiamvimonvat, M.T. Perez-Garcia, G.F. Tomaselli, and E. Marban. 1995. Functional association of the β1 subunit with human cardiac (hH1) and rat skeletal muscle (μ1) sodium channel α subunits expressed in Xenopus oocytes. J. Gen. Physiol. 106:1171–1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patton, D.E., L.L. Isom, W.A. Catterall, and A.L. Goldin. 1994. The adult rat brain β1 subunit modifies activation and inactivation gating of multiple sodium channel α subunits. J. Biol. Chem. 269:17649–17655. [PubMed] [Google Scholar]
- Qu, Y., L.L. Isom, R.E. Westenbroek, J.C. Rogers, T.N. Tanada, K.A. McCormick, T. Scheuer, and W.A. Catterall. 1995. Modulation of cardiac Na+ channel expression in Xenopus oocytes by β1 subunits. J. Biol. Chem. 270:25696–25701. [DOI] [PubMed] [Google Scholar]
- Qu, Y., J.C. Rogers, S.-F. Chen, K.A. McCormick, T. Scheuer, and W.A. Catterall. 1999. Functional roles of the extracellular segments of the sodium channel α subunit in voltage-dependent gating and modulation by β1 subunits. J. Biol. Chem. 274:32647–32654. [DOI] [PubMed] [Google Scholar]
- Ratcliffe, C.F., Y. Qu, K.A. McCormick, V.C. Tibbs, J.E. Dixon, T. Scheuer, and W.A. Catterall. 2000. A sodium channel signaling complex: modulation by associated receptor protein tyrosine phosphatase beta. Nat. Neurosci. 3:437–444. [DOI] [PubMed] [Google Scholar]
- Sambrook, J., E.F. Fritsch, and T. Maniatis. 1989. Molecular Cloning: A Laboratory Manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY.
- Smith, R.D., and A.L. Goldin. 1998. Functional analysis of the rat I sodium channel in Xenopus oocytes. J. Neurosci. 18:811–820. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Srinivasan, J., M. Schachner, and W.A. Catterall. 1998. Interaction of voltage-gated sodium channels with the extracellular matrix molecules tenascin-C and tenascin-R. Proc. Natl. Acad. Sci. USA. 95:15753–15757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vijayaragavan, K., M.E. O'Leary, and M. Chahine. 2001. Gating properties of Nav1.7 and Nav1.8 peripheral nerve sodium channels. J. Neurosci. 21:7909–7918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallner, M., L. Weigl, P. Meera, and I. Lotan. 1993. Modulation of the skeletal muscle sodium channel α-subunit by the β1-subunit. FEBS Lett. 336:535–539. [DOI] [PubMed] [Google Scholar]
- Xiao, Z.-C., D.S. Ragsdale, J.D. Malhotra, L.N. Mattei, P.E. Braun, M. Schachner, and L.L. Isom. 1999. Tenascin-R is a functional modulator of sodium channel β subunits. J. Biol. Chem. 274:26511–26517. [DOI] [PubMed] [Google Scholar]
- Yang, J.S., P.B. Bennett, N. Makita, A.L. George, and R.L. Barchi. 1993. Expression of the sodium channel β1 subunit in rat skeletal muscle is selectively associated with the tetrodotoxin-sensitive α subunit isoform. Neuron. 11:915–922. [DOI] [PubMed] [Google Scholar]
- Zerangue, N., B. Schwappach, Y.N. Jan, and L.Y. Jan. 1999. A new ER trafficking signal regulates the subunit stoichiometry of plasma membrane KATP channels. Neuron. 22:537–548. [DOI] [PubMed] [Google Scholar]
- Zimmer, T., C. Biskup, C. Bollensdorff, and K. Benndorf. 2002. The β1 subunit but not the β2 subunit colocalizes with the human heart Na+ channel (hH1) already within the endoplasmic reticulum. J. Membr. Biol. 186:13–21. [DOI] [PubMed] [Google Scholar]