Abstract
Feedback from horizontal cells (HCs) to cone photoreceptors plays a key role in the center-surround–receptive field organization of retinal neurons. Recordings from cone photoreceptors in newt retinal slices were obtained by the whole-cell patch-clamp technique, using a superfusate containing a GABA antagonist (100 μM picrotoxin). Surround illumination of the receptive field increased the voltage-dependent calcium current (ICa) in the cones, and shifted the activation voltage of ICa to negative voltages. External alkalinization also increased cone ICa and shifted its activation voltage toward negative voltages. Enrichment of the pH buffering capacity of the extracellular solution increased cone ICa, and blocked any additional increase in cone ICa by surround illumination. Hyperpolarization of the HCs by a glutamate receptor antagonist-augmented cone ICa, whereas depolarization of the HCs by kainate suppressed cone ICa. From these results, we propose the hypothesis that pH changes in the synaptic clefts, which are intimately related to the membrane voltage of the HCs, mediate the feedback from the HCs to cone photoreceptors. The feedback mediated by pH changes in the synaptic cleft may serve as an additional mechanism for the center-surround organization of the receptive field in the outer retina.
Keywords: retina, synapse, lateral inhibition
INTRODUCTION
The center-surround organization of the receptive field is one of the most important characteristics of vertebrate retinal neurons. Since its discovery by Kuffler (1953), many investigators have attempted to unravel the neural mechanisms underlying the center-surround antagonism. In 1971, Baylor et al. showed that the cone photoreceptors of the turtle responded by hyperpolarization to direct illumination of the recorded cone, but by delayed depolarization to surround illumination of an area comparable to the receptive field size of the horizontal cells (HCs). In a more direct experiment, Baylor et al. (1971) also showed that hyperpolarization of the HCs by extrinsic current injection depolarizes the nearby cones. Thus, it has been widely accepted that negative feedback from the HCs plays a key role in the center-surround antagonism of retinal neurons.
There is considerable evidence to suggest that the feedback from the HCs to cones is mediated by GABA. In many animal species, the HCs have been shown to be GABAergic; they have glutamic acid decarboxylase (GAD, Lam et al., 1979), a GABA-synthesizing enzyme. They accumulate GABA via a selective uptake mechanism (Lam and Steinman, 1971; Marc et al., 1978), and release (Schwartz, 1987) it when they are depolarized. It has also been shown in isolated turtle cones that these photoreceptors have a high sensitivity to the presence of GABA at their terminal (Tachibana and Kaneko, 1984; Kaneko and Tachibana, 1986). The presence of GABA receptors has also been shown in mammalian cones (Picaud et al., 1998; Pattnaik et al., 2000). On the other hand, there are several reports that argue against the GABA feedback hypothesis, because the surround response of the cone persists regardless of the presence of GABA antagonists (Thoreson and Burkhardt, 1990; Burkhardt, 1993; Verweij et al., 1996) or agonists (Thoreson and Burkhardt, 1990; Burkhardt, 1993). H3-type cone HCs in the teleost retina show a triphasic chromatic response; it is believed that the formation of the triphasic chromatic response is modulated by feedback from H2 HCs to green cones (Stell et al., 1975). However, in the goldfish retina, while GABA uptake into HCs could not be confirmed in H2/3 cone HCs, H1 HCs showed GABA uptake (Marc et al., 1978). Thus, the mediator of the triphasic feedback response of the H3 HCs remains unknown.
Modulation of the calcium current (ICa) in cones have also been proposed as a mechanism for feedback. Verweij et al. (1996) reported that surround illumination shifts the activation voltage range of the cone ICa in the goldfish retina. Feedback by modulation of the cone ICa is also consistent with the observation in the turtle retina that surround illumination causes calcium-dependent spikes in the cones (Piccolino and Gerschenfeld, 1978, 1980; Gerschenfeld and Piccolino, 1980). Furthermore, Verweij et al. (1996) also proposed that GABA is not likely to be the main mediator of this feedback mechanism, since this modulation of the cone ICa was resistant to GABA receptor and GABA transporter antagonists.
Byzov and Shura-Bura (1986) proposed an electrical (ephaptic) feedback mechanism by which a local current in the cone-HC synapse causes a voltage drop in the cone synaptic clefts and results in modulation of the transfer function between the cones and bipolar cells. More recently, Kamermans et al. (2001) suggested that an ephaptic effect of the current flowing through the hemigap channel of the dendritic tips of the HCs shifts the activation voltage of the cone ICa, based on evidence that (a) hemichannel proteins exist in the dendritic tips of HCs and (b) carbenoxolone, a blocker of hemichannels, suppresses the surround response of the cones. However, the validity of the hemichannel-mediated feedback hypothesis is still under debate, since the pharmacological specificity of carbenoxolone is uncertain.
This study was conducted with the aim of examining the mechanisms of feedback from HCs to cone photoreceptors. The surround responses of cone photoreceptors in newt retinal slices were recorded with whole-cell patch-clamp recording. We found that surround illumination shifts the activation voltage of the cone ICa toward negative voltages, as reported by Verweij et al. (1996), and that the current-voltage relation of ICa did not merely shift along the voltage axis as suggested by an ephaptic effect, but was very similar to the changes caused by alkalinization of the extracellular space. Enrichment of the pH buffering capacity of the extracellular solution increased cone ICa and suppressed the light-induced surround effect. Hyperpolarization of the HCs induced by CNQX increased cone ICa, whereas depolarization of the HCs induced by kainate decreased cone ICa. The present data thus strongly suggest that feedback from HCs to cone photoreceptors is mediated by pH changes in the synaptic cleft.
MATERIALS AND METHODS
Preparation of Retinal Slices and Superfusion
The experimental procedures conformed to the Guidelines for the Care and Use of Laboratory Animals, Keio University School of Medicine, and our experiments were approved by the University Animal Welfare Committee. Retina specimens obtained from newts (Cynops pyrrhogaster, 10–12 cm in length) were cut into slices by a method originally reported by Werblin (1978), as follows: The animals were kept in a room maintained under a natural 12 h/12 h light/dark cycle. Before the experiment, the newts were dark-adapted for 2 h. Subsequent manipulations were performed under dim red light. The animals were decapitated, the eyes enucleated and hemisected, and the frontal part, including the lens, was removed. The retina was detached from the eye-cup on to a piece of filter paper (pore size 0.45 or 0.80 μm: Toyo Roshi, Ltd.) and sectioned into 300-μm thick slices using a custom-made tissue slicer. Slices were superfused with Ringer's solution containing (in mM): 110 NaCl, 2.6 KCl, 2 MgCl2, 22 NaHCO3, 3 CaCl2, 10 glucose, adjusted to pH 7.4, saturated with a 95% O2–5% CO2 gas mixture. In this study, experiments were performed with perfusates containing 100 μM picrotoxin, to exclude any confounding effects by GABA-activated chloride currents in the cones (Kaneko and Tachibana, 1986), although picrotoxin did not change any current whose reversal potential was equivalent to the equilibrium potential of the chloride ions or suppress the surround responses of the cones in the retinal slices (three cones: unpublished data). 6-Cyano-7-nitroquinoxaline disodium (CNQX, an antagonist of non-NMDA receptors) and kainate were added directly to the superfusate in some experiments. The pH of the HEPES- and Tris-containing solutions was adjusted to the same value (within 0.1 pH unit) as that of Ringer's solution, using NaOH or HCl. The tonicity of the solutions was adjusted with sucrose. All the drugs, unless otherwise specified, were purchased from Sigma-Aldrich. The volume of the recording chamber was 0.75 ml, and the superfusate flowed continually at the rate of 0.9 ml/min. Plastic tubing (Tygon, Norton Co.) and Teflon tubing were used as conduits for the solutions, to minimize the loss of CO2. The chamber containing the retinal slices was mounted on the stage of an upright microscope (BX50WI; Olympus), which was equipped with an infrared (>800 nm) illumination system and a viewing system with a CCD camera (ICD-47AC; Ikegami Tsushinki Co., Ltd.). The microscope was mounted on a Gibraltar platform and an X-Y stage (Burleigh Instruments, Inc.). The images of the slices were monitored on a CRT display. All the experiments were conducted in a dark room maintained at a room temperature of 22°C.
Light Stimulation
Light stimuli were supplied to the retinal slices from two 100-W halogen lamps. A small spot light with a diameter of 30 μm was projected through the 40× water immersion objective lens and a diffuse light with a diameter of 3,500 μm was projected through the condenser lens. For the recordings made from the bipolar cells (BCs), the diameter of the spot light was increased to 50 μm, to cover the entire receptive field center. The timing and duration of illumination with the spot light and diffuse light were controlled by an electrical shutter (Melles Griot) interposed in each of the light paths and controlled by computer. The shutter opened from 10% to 90% in ∼20 ms. The light intensity was attenuated by inserting a neutral-density filter and by adjusting the supply voltage to the lamp. The intensity of the small spot light was 3 × 10−5 μW/μm2, which evoked a saturating response in the cones, but did not bleach them completely. The intensity of the large spot light was 5 × 10−6 μW/μm2, which evoked the maximum surround response, but did not affect the state of adaptation. The relative intensity of the 450-nm spot light was ∼27% that of the 650-nm spot light, and 10% that of the 650-nm diffuse light. The spectrum of the light was measured with a monochromator (Shimadzu), and the intensity was measured with a calibrated silicon photodiode (Centronic). To stimulate the receptive field surround, a large (3- to 4-mm diameter) spot was superimposed concentrically over a small (30-μm diameter) steady light spot. These stimuli will hereafter be referred to as surround illumination, and the responses evoked by such stimuli, as surround responses.
Newt cones express three kinds of opsins, Cp-LWS, SWS-1, and SWS-2 (Sakakibara et al., 2002). Thus, three kinds of cones (red-, blue-, and ultraviolet-sensitive cones) are expected. In this study, we identified the subtype of 39% of the cones (70/179), according to their responses to red, green, and blue light–emitting diodes (LED's; DHR6610, SBY 5710, and GNB4710; Iwasaki Electric Ltd.). The remaining 109 cones could not be classified. However, since we did not find any differences in the responses among the cone subtypes, all the cones were treated equally for the data analyses.
Whole-cell Recording
Pipettes for whole-cell recordings were fabricated from standard-wall borosilicate glass tubing, and their tips were heat-polished. The Cs+-based pipette solution contained (in mM): 90 Cs methanesulfonate (CsMeSO4), 10 TEA-Cl, 20 BAPTA, 10 HEPES, 1 MgCl2, 2.5ATP-Mg, 1 GTP-Na, 1 cGMP, 10 phosphocreatine, and 50 U creatinephosphokinase, adjusted to pH 7.3 with CsOH. Since cone photoreceptors have a high voltage–activated (HVA) Ca2+ current and Ca-activated K+ and Cl− currents (Barnes, 1994), we added 20 mM BAPTA to minimize the Ca-activated currents in the cones.
The resistance of the filled pipettes was usually in the range of 9–12 MΩ. The cells were usually voltage clamped at −40 mV. The mean input resistance of the cones in the saturating spot light, calculated from the linear leak conductance, was 686 ± 7 MΩ (mean of 47 cells analyzed). The patch pipettes were connected to an Axopatch 1-D amplifier (Axon Instruments, Inc.). No series resistance compensation was employed. Signals were low-pass filtered (Bessel filter) at 2 kHz, sampled (12 bit) at 5 kHz, and then stored on the hard disk of a personal computer.
Isolation of the Calcium Current in Cones
The ICa of the cones was identified as the current selectively blocked by 3 mM cadmium (Cd). The I-V relations of the ICa were obtained by subtracting the I-V curves recorded in a 3-mM Cd-containing solution from those recorded in control solution (four cones). Another method used to obtain the I-V relations of ICa was by subtraction of the leakage current. The leakage current was estimated by extrapolating the linear part of the I-V curve, between −50 and −36 mV. Since the I-V curve obtained by the leakage subtraction method showed good agreement with that obtained by the Cd-subtraction method (Fig. 2, boxed inset), we determined the I-V relations of ICa by the leakage subtraction method in the subsequent experiments.
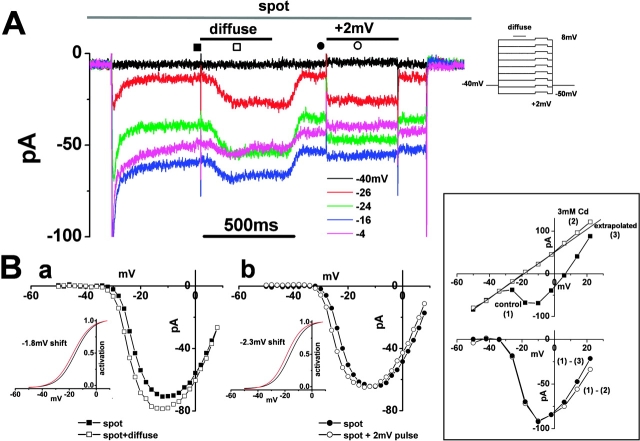
Figure 2.
Surround illumination augments the cone Ca2+ current A. The Ca2+ current (ICa) in the cone photoreceptors of the newt retinal slice was recorded under the whole-cell voltage clamp condition. The retinal slice was superfused with control Ringer's solution buffered with bicarbonate and containing 100 μM picrotoxin. The cone was held at −40 mV and polarized to voltages ranging from −50 mV to +8 mV in 2-mV steps. Five representative traces, voltage- clamped at −40, −26, −24, −16, and −4 mV, are shown. During the command voltage, surround illumination (diameter, 4,000 μm; duration, 400 ms: shorter bar) was applied every 4 s, while the spot illumination (diameter, 30 μm: top bar) was maintained. An additional 2-mV depolarization was applied to mimic an ephaptic effect (external voltage drop) after withdrawing the surround illumination. Note that at −4 mV (pink trace); surround illumination evoked an inward current, while a +2-mV pulse evoked an outward current. The current amplitude was sampled at the time indicated by the symbols, to construct the I-V curves shown in B a and B b. (B a) Leak-subtracted I-V curve of the cone ICa in the presence of the spot (filled squares) and during surround illumination (open squares). The data are from the same cone as in A. The leakage current amplitude (conductance, 0.59 nS), determined by extrapolation of the linear portion of the I-V curve between −50 and −32 mV, was subtracted from the measured current amplitude at each voltage. Inset shows activation curves fitted to the Boltzmann function derived from the I-V curves. The midpoint of the curve as obtained under the control condition (−15.3 mV; black line) was shifted by 1.8 mV in the negative direction during surround illumination (red line). The maximum conductance was calculated from the slope of the I-V curve between +2 and +8 mV and normalized to 1.0. (b) Leak-subtracted I-V curve of the cone ICa in the presence of the spot light (filled circles) and during a +2-mV depolarizing pulse (open circles). Inset shows activation curves fitted to the Boltzmann function derived from the I-V curves. The midpoint of the curve as obtained under the control condition (−16.2 mV; black line) was shifted by 2.3 mV in the negative direction during the 2-mV induced depolarization (red line). The 0.3 mV discrepancy in the curve shifting is probably due to a curve fitting error or a voltage clamp error. (Boxed inset) Isolation method of I-V relations of cone ICa. These data were obtained from a different cone in A. (Top) The I-V relations of the cone were obtained in the control solution (filled squares (1)) and in a 3-mM Cd-containing solution (open squares (2)). The leakage conductance (2.7 nS) was estimated by extrapolating the linear part of the I-V curve between −50 and −36 mV (solid line (3)). (Bottom, open circles) I-V relations of the cone ICa obtained by subtracting the I-V curve recorded in a 3-mM Cd-containing solution from that recorded in the control solution ((1) − (2)). (filled circles) I-V relations obtained by subtracting the I-V curve from the extrapolated leakage current from that recorded in the control solution ((1) − (3)).
Analysis
The digitized data were analyzed and plotted using Origin 6.1 software (Microcal Ltd.). The digitized waveform data were finally low-pass filtered (<1 kHz) with an FFT-smoothing algorithm. The statistical data are presented as means ± SEM. The statistical significance of the differences among the data was tested by Student's t test.
RESULTS
Response of Cone Photoreceptors in Newt Retinal Slices to Surround Illumination
A voltage-dependent surround response of the cones in newt retinal slices was obtained in the current-clamp mode (Fig. 1) . Spot illumination hyperpolarized the cones, while surround illumination depolarized them (Fig. 1, middle trace, no extrinsic current injection). The size of the surround response was dependent on the membrane voltage. Hyperpolarization of the cones by extrinsic current injection (−0.03 nA current injection) suppressed the surround response without reducing the amplitude of the response to spot illumination. Depolarization (+0.03 nA) of the cones also reduced the size of the surround response. The amplitude of the surround response was maximal at around −30 mV. Cones that were hyperpolarized up to −50 mV by spot illumination did not show any surround response, but the surround response appeared when the membrane voltage was brought to near −30 mV by extrinsic current injection (unpublished data).
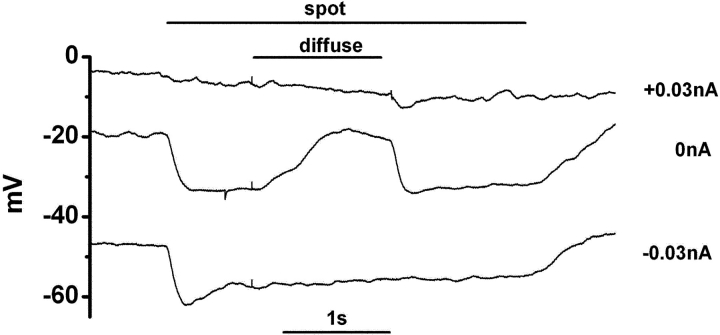
Figure 1.
The response of a newt cone photoreceptor recorded in the current-clamp mode The outer segment of the cone was illuminated by a spot (diameter, 30 μm; duration, 3,380 ms; timing indicated by the top horizontal line). A diffuse light (diameter, 4,000 μm; duration, 1,250 ms) was superimposed on the spot as indicated by the shorter horizontal line. The retinal slice was superfused with control Ringer's solution buffered with bicarbonate and containing 100 μM picrotoxin. Under control condition (when no current was injected from the recording pipette: 0 nA), illumination with the spot evoked hyperpolarization, and the surround illumination evoked depolarization in the cone. Both hyperpolarization and depolarization of the cone induced by current injection (−0.03 and +0.03 nA) from the recording pipette abolished the surround response. The vertical scale on the left indicates the absolute membrane voltage. Recovery at the spot offset was slow (1 s), probably due to blockade of the calcium feedback to the phototransduction cascade in the cones (Lamb et al., 1986; Nakatani and Yau, 1988), because the intracellular Ca2+ level was maintained at a low level due to the addition of 20 mM BAPTA in the pipette solution. Lowering the BAPTA concentration in the pipette solution (5 mM) accelerated the recovery (0.5 s; unpublished data).
A voltage-dependent calcium current (ICa) in the newt cone was activated by depolarization to voltages more positive than −30 mV, similar to activation of ICa in tiger salamander rods (Barnes et al., 1993). The I-V curve of the cone ICa was obtained by the linear leak current subtraction method. Under voltage-clamp recording, surround illumination evoked an inward current at voltages more positive than −30 mV, while no inward current was evoked at voltages more negative than −40 mV (Fig. 2 A). Surround illumination augmented the cone ICa measured in the presence of spot illumination at all holding voltages (Fig. 2 B a). This augmentation was voltage dependent; greater augmentation was seen at voltages more negative than −15 mV, at which the standing inward current was maximal, whereas little augmentation was seen at voltages between 0 and +10 mV. In 24 cones sampled, surround light illumination shifted the midpoint of the cone ICa activation curve by −2.55 ± 0.32 mV, within the range of −6.5 and −0.6 mV. These data suggest that surround illumination augmented cone ICa and shifted its activation voltage, similar to the observations in goldfish cones (Verweij et al., (1996)). The cone surround response disappeared after rundown of the cone ICa (unpublished data), which also suggests that the cone surround response is cone-ICa dependent. Even in the absence of picrotoxin, surround illumination did not evoke any current whose reversal potential was equal to the equilibrium potential of chloride ions (three cones).
It was hypothesized recently that a current flowing into HCs creates an ephaptic effect (a field effect) that causes a drop of the voltage in the intersynaptic cleft at the cone terminal, resulting in an enhancement of the cone ICa (Kamermans et al., 2001). The ephaptic effect would be expected to shift the voltage dependence of the cone ICa parallel to the voltage axis, in the negative direction. To mimic the ephaptic effect, the cones were depolarized by 2 mV after switching off the surround illumination. During the 2-mV depolarization, the I-V curve of the cone ICa clearly shifted by 2 mV in the negative direction (Fig. 2 B b). The change was clearly different from that induced by surround illumination. A difference was especially apparent at membrane voltages between −15 and +8 mV, where surround illumination increased the current amplitude (while 2-mV depolarization decreased it). The differences between these two conditions led us to wonder whether the surround response of the cones is mediated by a mechanism other than the ephaptic effect.
pH Change Modulates the Cone Current in a Voltage-dependent Manner
It has been demonstrated that extracellular acidification regulates the voltage-dependent Ca2+ current (ICa) (Tombaugh and Somjen, 1998) by several mechanisms, including (a) charge-screening effects of protons on the Ca channels (Krafte and Kass, 1988; Prod'hom et al., 1989; Klöckner and Isenberg, 1994) and (b) proton-binding effects on the Ca channels (Chen et al., 1996; Zhou and Jones, 1996). The effects of pH change on cone-ICa–influenced transmitter release have been examined (Barnes and Bui, 1991; Barnes et al., 1993; DeVries, 2001). We therefore confirmed the effects of extracellular pH change on the cone ICa in the newt retina. Pressure ejection of high pH Ringer's (pH 9.0) solution onto the outer plexiform layer enhanced an inward ICa. The high pH-induced inward current was voltage dependent; the inward current was more pronounced at voltages between −10 and −30 mV (Fig. 3 A). Thus, the external alkalinization augmented the peak of the cone ICa (Fig. 3 B, top; +39.5 ± 8.5%, n = 6), as reported in other vertebrate cones (Barnes and Bui, 1991; DeVries, 2001). Moreover, the midpoint of the activation curve of the cone ICa was shifted in the negative direction (Fig. 3 B, bottom; 8.2 ± 2.0 mV, n = 6), similar to the effects of surround illumination (Fig. 2 B a). Voltage-dependent calcium channels are localized in the terminal regions of the photoreceptors (Morgans et al., 1998; Nachman-Clewner et al., 1999), and are thus presumably localized near the center of the invaginating synapses. Therefore, it could be considered that surround illumination alkalinized the intersynaptic clefts of the invaginating synapses, to augment the cone ICa locally.
Figure 3.
Modulation of cone ICa by focal application of a high-pH solution to the cone synaptic terminal layer. Recordings were obtained from cone photoreceptors of newt retinal slices under whole-cell voltage clamp. The slices were superfused with control Ringer's solution buffered with bicarbonate and containing 100 μM picrotoxin. (A) Alkalinized Ringer's solution (pH 9.0) was focally applied to the cone synaptic terminal layer by pressure ejection (duration: 10 ms, pressure: 59 kPa; time indicated by arrow). The cell was voltage-clamped at various voltages in the range of −50 to +6 mV, in 8-mV steps. The representative four traces, voltage clamped at −42, −26, −18, and +6 mV are shown. Transient signals at the pressure ejection were artifacts produced by the valve opening. While a small inward current at −42 mV was seen in this cell, it was not seen in the other five cones tested. The current was sampled at the points marked by a symbol to construct the I-V curves shown in B. Small spot (diameter, 30 μm) illumination was maintained throughout. (B, top) Leak-subtracted I-V curve of cone ICa in normal Ringer's solution (pH 7.4, filled squares) and in response to a high-pH solution (pH 9.0, open circles). The data is from the same cell as that described in A. The leak conductance was 0.94 nS. (Bottom) Activation curves derived from the I-V curves fitted to the Boltzmann function. The midpoint of the curve (−17.7 mV; black line connecting the filled squares) as obtained in normal Ringer's solution (pH 7.4) was shifted to −28.1 mV (gray line connecting the open circles) after the application of the high-pH solution. The maximum conductance was calculated from the slope of the I-V curve in the high-pH solution, between −10 and 6 mV, and normalized to 1.0.
Strong pH Buffering Suppresses the Surround Response of the Cones
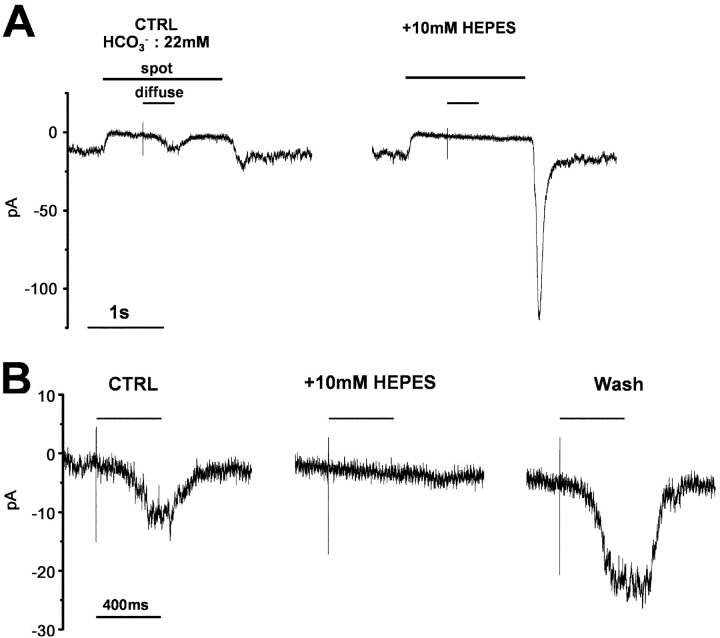
If pH changes in the synaptic clefts were considered to mediate the surround response of the cones, fixation of the extracellular pH by enrichment of the pH-buffering capacity of the external solution would be expected to prevent the generation of the cone surround response. In fact, changing the superfusate from control Ringer's solution to one supplemented with 10 mM HEPES (having an identical pH value) increased cone ICa, and prevented any additional inward current potentially caused by surround illumination (Fig. 4 A). The addition of 10 mM HEPES (pKa = 7.5) was estimated to increase the buffering capacity of the solution by 5.75 mM at pH 7.4 (Chesler, 1998). The effect of HEPES enrichment of the superfusate on the membrane current of the cones was examined at several holding voltages, from −50 mV to +6 mV. When cone ICa was not activated, however, HEPES had no significant effect on the membrane conductance (traces at −34 mV in Fig. 4 B a). The use of HEPES-enriched solution reversibly increased cone ICa and prevented any additional inward current potentially evoked by surround illumination (traces at −18 and −10 mV in Fig. 4 B a). Such changes in ICa were seen throughout the entire voltage range at which ICa was activated (Fig. 4 B b). As a result, use of HEPES (10 mM) shifted the Boltzmann-fit activation curve of cone ICa by 1.8 mV in the negative direction (Fig. 4 B c), similar to the shift induced by surround illumination of the retinal specimens in control Ringer's solution (1.6 mV). When HEPES-enriched solution was used, surround illumination induced little additional shift of the activation curve. On average, use of HEPES (10 mM)-enriched buffer shifted the activation curve of cone ICa by 1.2 ± 0.3 mV in the negative direction (n = 5), which was almost identical to the shift caused by surround illumination of the retinal specimens in control solution (1.1 ± 0.3 mV), with peak ICa increased by 15.5 ± 1.0%. HEPES did not block the light response of the HCs to diffuse light illumination (Fig. 4 A, inset, n = 4). In 10-mM HEPES-enriched buffer, the surround responses were suppressed completely in three out of eight cones, and partially in the remaining five cones at −26 mV (Fig. 4 B b, inset, Vh = −26 mV). In 20-mM HEPES-enriched buffer, the surround response was suppressed in the four cones tested.
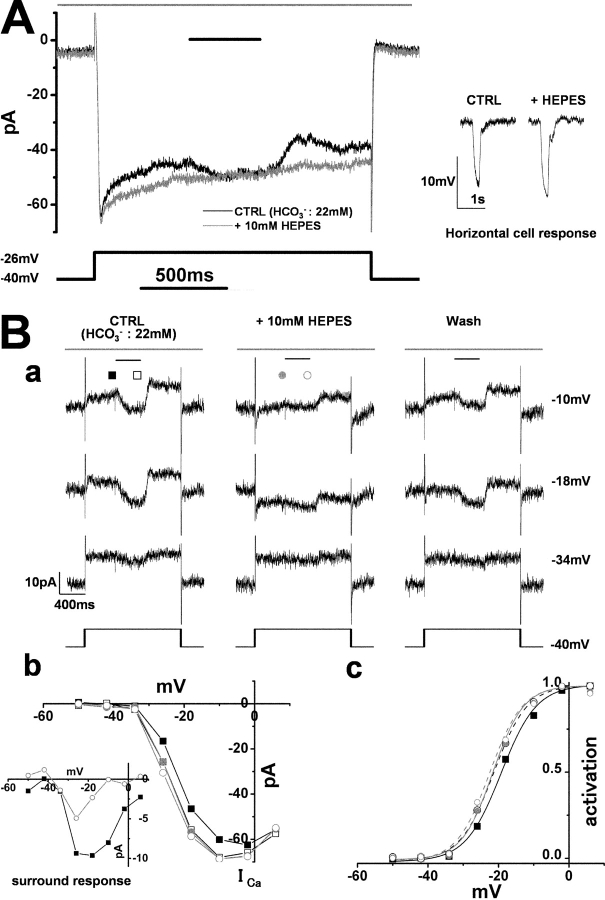
Figure 4.
The cone ICa and its surround response recorded in a superfusate enriched with HEPES. A cone photoreceptor in the retinal slice was recorded under whole-cell voltage clamp. The slice was superfused with control Ringer's solution (buffered with bicarbonate) and the solution enriched with HEPES to elevate the pH buffering capacity. (A) Effects of the 10-mM HEPES-enriched buffer on the cone ICa and surround response. The cone photoreceptor was depolarized from the holding voltage of −40 to −26 mV. Diffuse light (duration, 400 ms) illumination was given during the step depolarization (shorter bar) in the presence of a small spot light (diameter, 30 μm; top bar). (Black trace) Current recorded in the control solution (bicarbonate buffer alone). (Gray trace) Current recorded in the external solution with bicarbonate buffer plus 10 mM HEPES. The leakage conductance was 0.53 nS. Inset shows the horizontal cell responses to a large light spot (diameter, 4,000 μm; duration 100 ms), in the control solution and in the solution enriched with HEPES. (B a) Reversible effects of 10-mM HEPES-enriched buffer on the cone ICa and surround response. The small spot light (diameter, 30 μm) was kept on throughout (top bars). Diffuse light (duration, 400 ms) illumination was given during the step depolarization (shorter bar) in the presence of the small spot (diameter, 30 μm; top bar). The cone was held at −40 mV and polarized to voltages ranging from −50 to +6 mV in 8-mV steps. Representative traces, clamped at −34, −18, and −10 mV, are shown. (From the left through the middle to the right column) The current traces before (external HCO3 −: 22 mM), during, and after application of bicarbonate solution plus 10 mM HEPES. All the recordings were from the same cell. The recording sequence was left column (−34, −18, and −10 mV) followed by the middle column (the same command voltage sequence as that for the recording in the left column), and finally the right column. The leak conductance of 2.26 nS did not change either in the HEPES-containing solution or during the washout (see, for example the current traces at −34 mV). In the HEPES-containing solution, ICa in darkness was reversibly increased and the surround response was reversibly suppressed (see the current traces at −18 and −10 mV). Symbols denote the sampling points for calculation of the I-V curves of ICa (B b). In the control solution, the inward current produced by ICa was counterbalanced by the outward leak current (at −18 and −10 mV in the control and washout solutions). (b) I-V curves of the cone ICa recorded in B a. The leak conductance was subtracted. Filled squares, in the control solution without surround illumination; open squares, in the control solution during surround illumination; filled gray circles, in the HEPES-containing solution without surround illumination; open gray circles, in the HEPES-containing solution during surround illumination. Inset shows the voltage dependence of the surround response in the control solution (filled squares) and in the HEPES-containing solution (open circles). (c) Activation curves fitted to the Boltzmann function derived from the data in B b. The midpoint of activation curve shifted from −19.2 mV (control, black solid line) to −21.4 mV (surround illumination in control: black broken line), to −21.6 mV (no surround illumination in the HEPES-containing solution, gray solid line), and to −22.5 mV (surround illumination in the HEPES-containing solution, gray broken line). The maximum conductance was determined from the linear part of the curve, between −2 and 6 mV, obtained in the control solution during illumination with diffuse light, and normalized to 1.0.
HEPES, MES, and TAPS, commonly used “Good buffers,” have an aminosulfonate moiety, and it has been reported that these protonated aminosulfonate compounds decrease the permeability of connexin-26 hemichannels (Bevans and Harris, 1999). To exclude the possibility that HEPES closes the connexin-26 hemichannels on HCs to cause blockade of the ephaptic effect (Kamermans et al., 2001), the effects of Tris buffer that does not possess an aminosulfonate moiety were also examined. Tris (15 mM)-supplemented external solution as the superfusate also enhanced cone ICa and suppressed any potential additional increment of ICa caused by surround illumination (Fig. 5 A a), indicating that the effects of pH buffering on cone ICa and surround response were not unique to HEPES. When large surround responses of 20 pA were recorded in the control solution, diffuse light elicited a current dip at the time of switch-off of the illumination (but not at the beginning) when the specimens were superfused with enriched buffer solution (arrow in Fig. 5 A). The addition of 15 mM Tris (pKa = 7.8) was estimated to increase the buffering capacity by 8.59 mM at pH 7.4. On average, Tris-enriched buffer as the perfusate shifted the activation curve of the cone ICa by 3.7 ± 0.7 mV, which was almost identical to the shift caused by surround illumination of the retinal specimens in control solution (3.3 ± 0.6 mV) (Fig. 5 A, c), with peak ICa increased by 26.6 ± 1.8% (n = 4). In Tris-enriched buffer, surround light illumination did not evoke any additional shift of the I-V curve of cone ICa. Tris-enriched buffer solution increased the amplitude of the light response of the HCs (by 32 ± 11%: n = 3) (Fig. 5 B).
Figure 5.
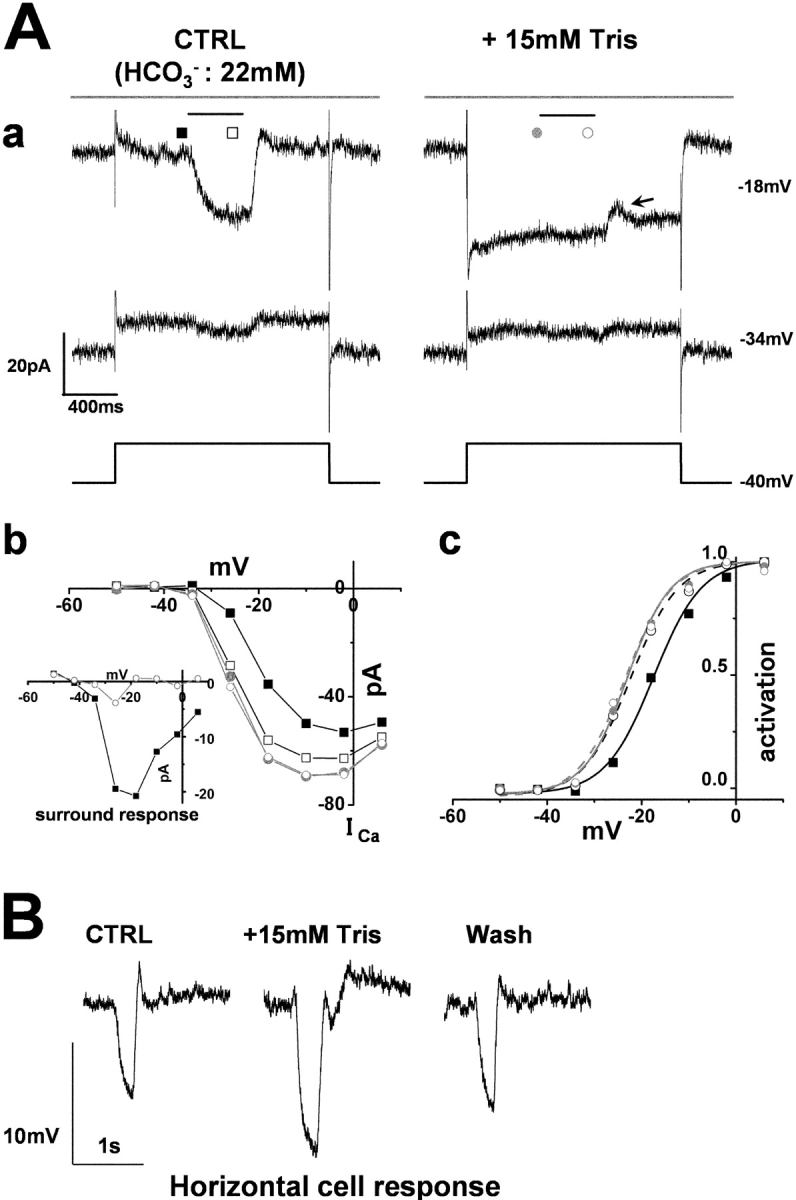
The cone ICa and its surround response recorded in a superfusate enriched with Tris A cone photoreceptor in the retinal slice was recorded under whole-cell voltage-clamp. The slice was superfused with control Ringer's solution (buffered with bicarbonate) and a solution enriched with Tris to elevate the pH-buffering capacity. (A a) Effects of 15-mM Tris-enriched buffer (with 15 mM Tris) on the cone ICa and surround response. Diffuse light (duration, 400 ms) illumination was given during the step depolarization (shorter bar) in the presence of a small spot light (diameter, 30 μm; top bar). The cone was held at −40 mV and polarized to voltages ranging from −50 to +6 mV in 8-mV steps. Representative traces at −34 and −18 mV are shown. (From the left to the right column) The current traces before (external HCO3 −: 22 mM) and during application of bicarbonate solution plus 15 mM Tris. All the recordings were from the same cell. The recording sequence was: the left column (−34 mV, −18 mV) followed by the right column (the same command voltage sequence as for the recording in the left column). The leak conductance of 2.0 nS did not change in the Tris-containing solution (see, e.g., the current traces at −34 mV). In the Tris-containing solution, the ICa in darkness increased and the surround response was suppressed (see the current traces at −18 mV). Symbols denote the sampling points for the calculation of the I-V curves of ICa (B b). In the control solution, the inward current produced by ICa was counterbalanced by the outward leak current (at −18 mV in control). A current dip appeared toward the time of switch-off of the surround illumination (arrow). (b) I-V curve of the ICa of the same cone photoreceptor shown described in A a. Filled squares, in the control solution without surround illumination; open squares, in the control solution during surround illumination; filled gray circles, in the Tris-containing solution without surround illumination; open gray circles, in the Tris-containing solution during surround illumination. Inset shows the voltage dependence of the surround response in the control solution (filled squares) and in 15 mM Tris-containing solution (open circles). (c) Activation curves fitted to the Boltzmann function derived from the data in A b. (B) Effect of Tris on the response of a HC to the flash of a large (diameter, 4,000 μm; duration, 100 ms) light spot. The recording in the left column was taken in the control Ringer, that in the middle column was recorded during application of 15 mM Tris-containing solution, and the right record that in the right column was recorded after the return of the slice to the control Ringer's solution.
When the leak conductance of the cones exceeded 2 nS, the inward ICa could be masked by an outward leak current. Thus, in some cones, the net current before subtraction appeared to be moderately outward (Fig. 4 B a, control [at −18 mV]), or slightly inward (Fig. 5 A a, control [at −18 mV]).
HEPES did not change the spatial summation of the HCs. The ratios of the amplitudes of the HC light responses to spot (50-μm diameter) versus diffuse lights (4,000-μm diameter) were 0.78 ± 0.08 in control Ringer's solution, and 0.77 ± 0.10 in HEPES-enriched buffer (n = 4) (unpublished data). Tris had no significant effect on the spatial summation of the HCs, either. Thus, suppression of the surround response of the cones by solutions with a high buffering capacity cannot be due to a reduction of the spatial summation of the HCs.
Hare and Owen (1998) studied the effects of substituting bicarbonate for HEPES in the perfusate solution on the light responses of the retinal neurons. The substitution with an external pH buffer could have changed the intracellular pH-buffering capacity in several retinal neurons, because HEPES buffer is membrane impermeable. In contrast to the experiments conducted by Hare and Owen (1998), we examined the effects of supplementation of either HEPES or Tris (with identical pH), using an identical protocol, only allowing for a change of the pH-buffering capacity in the extracellular space.
Horizontal Cells Mediate the Surround Response of Cones
It is widely accepted that the surround response of cones has a close relationship with the membrane voltage of the HCs. The preceding experiments strongly suggest that the pH in the invaginating synaptic clefts is modified by the membrane voltage of the HCs. To verify this hypothesis, we performed two experiments. First, we examined the effect of a small (0.25 mm) diameter surround light, since it is known that the response amplitude of HCs depends on the illuminated area of the retina. In the presence of a small spot (30-μm diameter), the surround response of the cones to a small diffuse light became smaller than that to a large (4 mm) diffuse light (Fig. 6 A). As a result, cone ICa became smaller (Fig. 6 B) and the activation curve of ICa shifted in the positive direction (Fig. 6 C). The response of the HCs to a small surround light also became smaller (n = 2: boxed inset). This observation is a good indication that the surround response of the cones depends strongly on the membrane voltage of the HCs (Baylor et al., 1971).
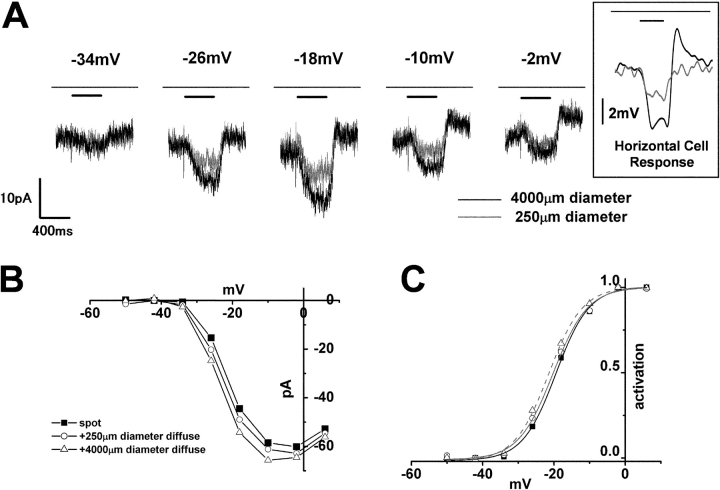
Figure 6.
Effects of the diameter of illumination on the surround response of a cone photoreceptor. (A) Surround response of a cone photoreceptor to small (diameter, 250 μm; gray trace) and large (diameter, 4,000 μm; black trace) surround illumination recorded at various holding voltages (indicated on each trace). Diffuse light (duration, 400 ms) illumination was given during the step depolarization (shorter bar) in the presence of small spot light (diameter, 30 μm; top bar). (Boxed inset) Voltage response of a HC to small (diameter, 250 μm; gray trace) and large (diameter, 4,000 μm; black trace) surround illumination. The voltage trace was low-pass filtered at 20 Hz. Diffuse light illumination was given during the step depolarization (shorter bar) in the presence of small spot light (top bar). (B) I-V curve of the ICa of the cone shown in A. Filled squares, without surround illumination; open circles, during small (diameter, 250 μm) surround illumination; open triangles, during large (diameter, 4,000 μm) surround illumination. The current was measured at its peak. (C) Activation curves fitted to the Boltzmann function derived from the data in B, showing a lateral shift of the mid point from −17.4 mV (no surround illumination, black solid line) to −20.3 mV (small surround illumination, thin line), and −21.5 mV (large surround illumination, broken line). The maximum conductance was determined from the linear part of the data curve, between −2 and 6 mV, obtained following diffuse light illumination in the control solution, and was normalized to 1.0.
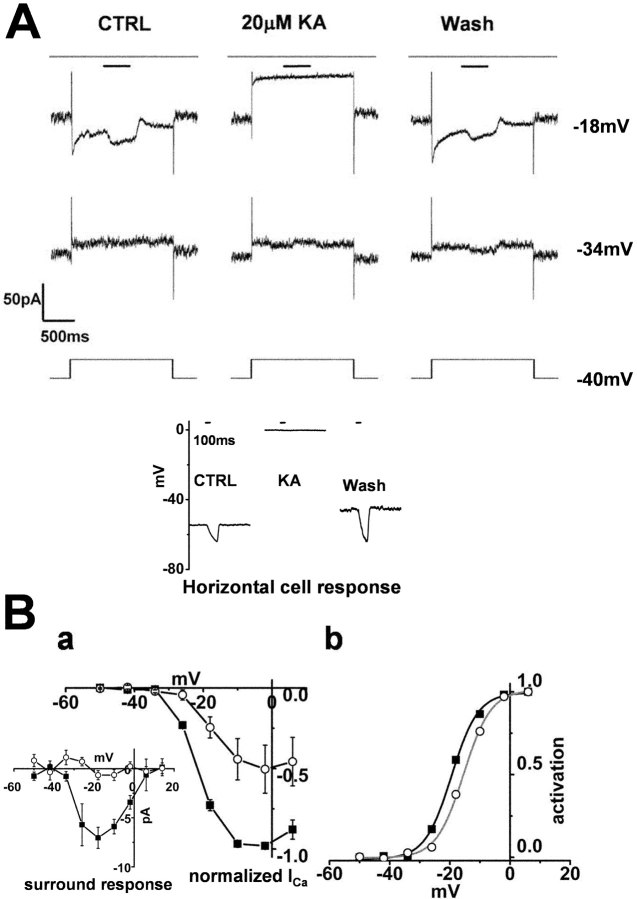
In the second experiment, we examined the effects of pharmacological depolarization and hyperpolarization of the HCs. 20 μM kainate, an agonist of the postsynaptic glutamate receptors of the HCs, depolarized the HCs from their dark membrane voltage (−49.7 ± 6.4 mV) to −1.3 ± 0.8 mV, and blocked their light responses (bottom Fig. 7 A, n = 3), similar to the responses of HCs of the tiger salamander (Yang and Wu, 1989).
Figure 7.
Effect of kainate on the surround response of a cone photoreceptor and on a horizontal cell. Whole-cell recordings from a cone photoreceptor and a horizontal cell in a retinal slice superfused with control Ringer's solution (buffered with bicarbonate alone). (A) Effects of 20 μM kainate on the cone ICa and the surround response. Diffuse light (duration 400 ms) illumination was given during the step depolarization (shorter bar) in the presence of a small spot light (diameter, 30 μm: top bar). The cell was held at −34 and −18 mV from the initial holding voltage of −40 mV. The current traces in the left column were recorded in the control solution, those in the middle column were recorded in solution containing 20 μM kainate, and those in the right column were recorded after washout of kainate. All the recordings were from the same cell. The recording sequence was left column (−34 mV, −18 mV), middle column (the same command voltage sequence as that for the recording in the left column), and finally the right column. The leak conductance was 3.7 nS. (Inset) Effect of kainate on the light-evoked HC voltage response. The recording in the left column was obtained in control Ringer's solution, that in the middle column was obtained after addition of 20 μM kainate, and that in the right column was obtained after washout of kainate. A large (diameter, 4,000 μm; duration 100 ms) light spot was flashed, the timing of which is indicated by the short bar above each column. (B a) I-V curves of ICa of four cones recorded under the same conditions as those described in A and averaged after normalization to each peak. Filled squares, current recorded in the control solution. Open circles, current recorded in the solution containing 20 μM kainate. Error bars indicate the SEM. (Inset) Changes in the ICa induced by surround illumination recorded in the control solution (filled squares) and in the solution containing 20 μM kainate (open circles). Average of four cones. (b) Activation curves fitted to the Boltzmann function derived from B a. Kainate shifted the midpoint of the curve from −19.4 mV (black line) to −15.5 mV (gray line). The maximum conductance was determined from the linear part of the curve, between −2 and 6 mV, obtained after diffuse light illumination in control Ringer's solution, and was normalized to 1.0.
Kainate reversibly suppressed cone ICa (Fig. 7 A) without any significant effect on the leak conductance (from 2.0 ± 0.8 nS to 2.2 ± 0.5 nS; P = 0.42 in four cones). Kainate also suppressed the surround response of the cones (Fig. 7 B a, inset). The midpoint of the Boltzmann-fit activation curve of cone ICa shifted by 3.9 mV in the positive direction (Fig. 7 B b). The effect of kainate on cone ICa cannot be a direct effect on the Ca channels of the cones, since subsequent application of CNQX in the presence of kainate not only restored cone ICa, but eventually increased it to more than its original value (n = 2). Furthermore, subsequent application of 10 mM HEPES in the presence of kainate also restored the cone ICa (n = 2), lending support to the assumption that the effect of kainate is due to extracelluar acidification.
In contrast to the effects of kainate, 20 μM CNQX hyperpolarized the HCs from −42.5 ± 7.8 mV to −59.7 ± 9.0 mV (n = 3: bottom of Fig. 8 A), and blocked their light responses. In cones, CNQX augmented ICa to such an extent that surround illumination did not induce any additional increase in ICa (Fig. 8 B a). Similar results with CNQX have also been reported in goldfish cones (Verweij et al., 1996). CNQX had no significant effect on the linear leak conductance. CNQX shifted the midpoint of the Boltzmann-fit activation curve of the cone ICa by 6.0 mV in the negative direction (Fig. 8 B b).
Figure 8.
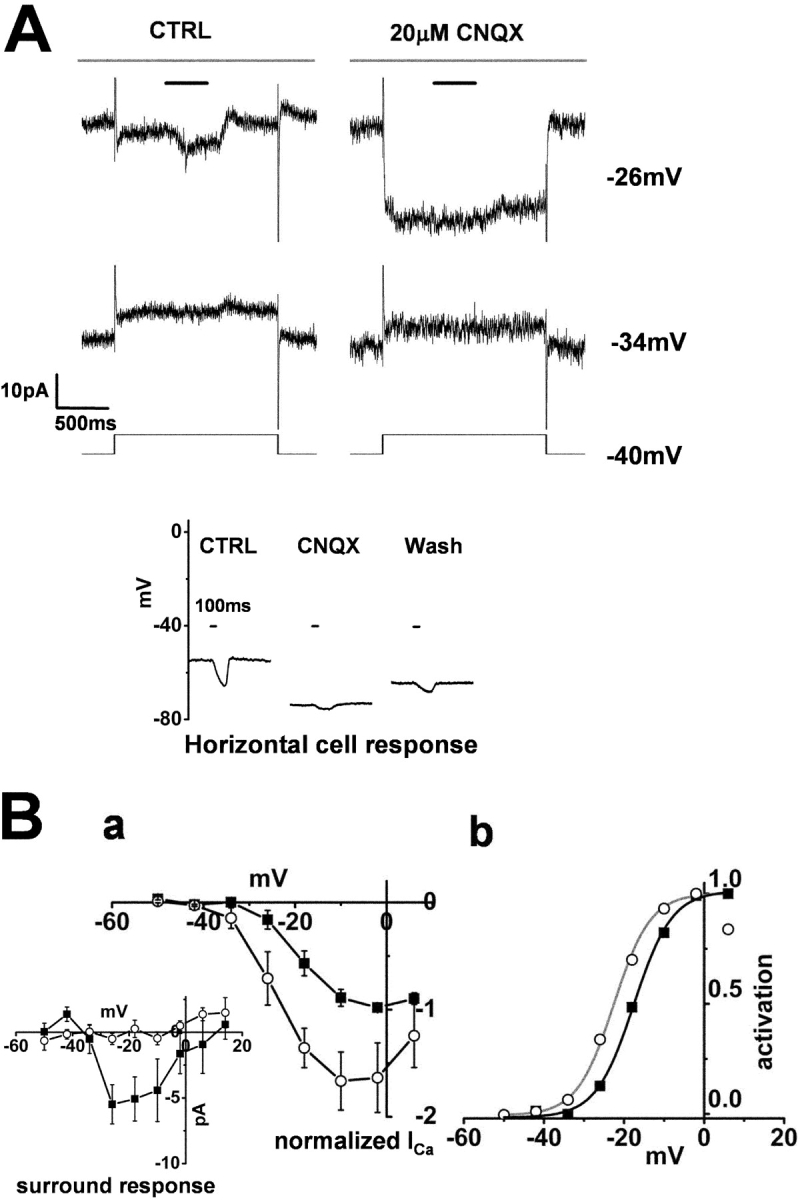
Effect of CNQX on the surround response of a cone photoreceptor and on a horizontal cell. Whole-cell recordings from a cone photoreceptor and a horizontal cell in a retinal slice superfused with control Ringer's solution (buffered with bicarbonate alone). (A) Effects of 20 μM CNQX on the ICa in a cone photoreceptor and its surround response. Diffuse light (duration, 400 ms) illumination was given during the step depolarization (shorter bar) in the presence of a small spot light (diameter, 30 μm; top bar). The cell was held at −34 and −26 mV from the initial holding voltage of −40 mV. The current traces shown in the left column were recorded in the control solution, and those shown in the right column were recorded in the solution containing 20 μM CNQX. All the recordings were from the same cell. The recording sequence was: left column (−34 mV, −18 mV), right column (the same command voltage sequence as that for the recording shown in the left column). The leak conductance was 1.6 nS. (Inset) Effect of CNQX on the light-evoked HC voltage response. The recording shown in the left column was obtained in control Ringer's solution, that in the middle column was obtained following the addition of 20 μM CNQX, and that in the right column was obtained after washout of the CNQX. A large (diameter, 4,000 μm; duration 100 ms) light spot was flashed, the timing of which is indicated by the short bar above each column. (B a) I-V curves of the ICa of 3 cones recorded under the same conditions as those described in A and averaged after normalization to each peak. Filled squares, current recorded in the control solution. Open circles, current recorded in the solution containing 20 μM CNQX. Error bars indicate the SEM. (Inset) Changes in the ICa induced by surround illumination recorded in the control solution (filled squares) and in the solution containing 20 μM CNQX (Open circles). Average from three cones. (b) Activation curves fitted to the Boltzmann function derived from B a. CNQX shifted the midpoint of the curve from −17.5 mV (black line) to −22.5 mV (gray line). The maximum conductance was determined from the linear part of the curve, between −2 and 6 mV, obtained after diffuse light illumination in control Ringer's solution, and was normalized to 1.0. The data point at +6 mV in CNQX was omitted for curve fitting.
These observations support the contention that the surround response of cones is closely related to the membrane voltage of HCs. Depolarization of the HCs had the effect of suppressing cone ICa, and hyperpolarization of the HCs had the effect of enhancing cone ICa. Taken together with the results of experiments in which the membrane voltage of the HCs was controlled, it is tempting to speculate that the membrane voltage of the HCs controls the pH in the synaptic clefts of the cone terminal.
Strong pH Buffering Suppresses the Surround Responses of the Bipolar Cells
It is widely considered that feedback from the HCs to the cones is involved in the center-surround organization of the bipolar cell (BC) receptive field. Do pH changes in the synaptic clefts also generate receptive field organization in the BCs?
To answer this question, we examined the effects of HEPES-enriched buffer on the surround response of off-type BCs under voltage-clamp. When retinal slice preparations were superfused with control Ringer's solution, spot illumination (50-μm diameter) evoked a sustained outward current in the BCs held at −40 mV. Surround illumination elicited an inward current, suggesting a surround-induced depolarization of the off-type BCs (Kaneko, 1970). Changing the superfusate to HEPES-enriched buffer solution slightly increased the holding inward current in the dark (mean increase by −2.6 ± 0.7 pA; n = 3), and suppressed the surround response (Fig. 9 A). The transient inward current at the time of switch-off of the spot illumination was prominently increased (n = 3). This effect of the HEPES-enriched buffer on the surround response was reversible (Fig. 9 B). Similar results were obtained from three off-type BCs.
Figure 9.
Center and surround responses of an off-type bipolar cell recorded in a solution enriched with HEPES. (A) The current responses to a spot light (50 μm in diameter, indicated by the long horizontal bar above the response trace, duration 1,560 ms) and to surround illumination (diffuse light, 4,000 μm in diameter, superimposed on the spot light, duration 400 ms) of an off-type bipolar cell recorded under the voltage-clamp conditions (holding voltage, −40 mV). In control Ringer's solution, spot illumination evoked an outward current (suppression of the maintained inward current), while the surround illumination evoked an inward current (enhancement of the maintained inward current). The HEPES (10 mM)-enriched solution enhanced the maintained inward current (from −12 to −14 pA) and abolished the surround response. The transient inward current at the time of switch-off of spot illumination was prominently increased in HEPES buffer. The two traces were obtained from the same cell. (B) The same recordings as those shown in A in an expanded time and amplitude scale. Horizontal bars indicate the timing of the surround illumination.
DISCUSSION
We examined the mechanisms underlying the generation of the surround response in cone photoreceptors in retinal slice preparations of the newt. Under current-clamp recording, a surround response appearing at around −20 mV was suppressed by both depolarization and hyperpolarization of the membrane voltage, which is consistent with a number of previously published reports that have suggested that the surround response of the cone photoreceptors is voltage dependent (turtle, O'Bryan, 1973; tiger salamander cones, Skrzypek and Werblin, 1983; Wu, 1991). Current flow through gap junctions between neighboring cones (DeVries et al., 2002) did not generate a surround response in the present study, because the amplitude of the surround response was independent of the difference between the membrane voltage and the presumed resting potential of neighboring cones. In the case of voltage-clamp recording, the surround response appeared in the voltage range in which the cone ICa was activated, which indicates that the surround response of the cones is closely related to ICa. The contribution of cone ICa to the surround response has been demonstrated previously in turtle (Piccolino and Gerschenfeld, 1978, 1980; Gerschenfeld and Piccolino, 1980; Thoreson and Burkhardt, 1991) and goldfish (Verweij et al., 1996; Kraaij et al., 2000) retinas. In this study, we focused on the mechanism by which surround illumination triggers activation of cone ICa.
We found that surround illumination increased cone ICa and shifted the activation voltage of ICa in the negative direction. External alkalinization also evoked a similar effect on cone ICa. Thus, we considered that pH changes in the cone synaptic clefts might mediate feedback from the HCs to cones by modulating Ca2+ channels.
Feedback Mediated by pH Changes in the Outer Retina
Several factors have been proposed as candidate feedback messengers from the HCs to cones. These include substances that modulate ICa of the cones, such as nitric oxide, glutamate, chloride, protons, and the ephaptic effect caused by current flowing into the HCs (for review see Kamermans and Spekreijse, 1999). Enrichment of the pH buffering capacity of the extracellular solution increased cone ICa and prevented any additional increase in ICa produced by surround illumination. Thus, we consider that protons are the most likely candidates among these substances. Moreover, HC depolarization by kainate suppressed cone ICa, whereas HC hyperpolarization by CNQX increased it. Therefore, it is highly plausible that the pH in the cone synaptic cleft is tightly controlled by the membrane voltage of the HCs.
The most plausible interpretation for the effects of enrichment of the pH buffering capacity is that the surround response of the cones is modulated by pH changes in the invaginating synaptic clefts, and thus the following sequences are considered. In a normal bicarbonate-buffer solution, pH in the synaptic cleft is slightly more acidic (by about pH 0.2; estimated in discussion) than the pH in the large extracellular pool (pH 7.4). Thus, cone ICa is relatively suppressed. The HC hyperpolarization caused by surround illumination neutralizes this acidic condition, thus restoring cone ICa and the cone surround response. On the other hand, in an external solution with a high buffering capacity, pH in the synaptic cleft is already fixed to the same pH as that of the extracellular pool (pH 7.4) even in darkness, and thus the enhancement of cone ICa by surround illumination does not occur.
The assumed pH change of 0.2 U may be a large enough change for pH electrode to detect. Therefore, we measured the pH in the outer plexiform layer by inserting a pH-selective microelectrode (tip diameter: 2 μm) into the outer synaptic region in the retinal slices. The fabrication method of the microelectrode used was that described by Smith et al. (1999) and Molina et al. (2000). The smallest pH change that our pH micropipette could detect was 0.06 pH U. However, no pH changes were detected after the addition of kainate (synaptic clefts should be acidified) and CNQX (the clefts should be alkalinized) to the superfusate in 23 slices examined. However, the absence of detectable pH change may not mean that the pH of the synaptic clefts is fixed. Insertion of the pH-sensitive micropipette may open up the narrow space at the invaginating synapse and make a wide artificial channel to the bath of the recording chamber. Through this artificial channel, any local pH change, that might be large enough within a narrow space, may become diluted and become undetectable.
Recently, DeVries (2001) demonstrated that exocytosed protons from the cone terminal mediate a feedback to block the cone ICa. The exocytosed protons can form a negative feedback loop to control glutamate release in a sustained manner. In contrast, synaptic acidification caused by HC depolarization can depend on the illumination area of the surround light, and thus contribute to receptive field surround formation in the outer retina.
If the pH in the synaptic cleft is controlled by the membrane voltage of the HCs, the mechanism by which surround illumination augmented the cone ICa can be interpreted easily. Kamermans et al. (2001) proposed hemichannel-mediated ephaptic feedback in the goldfish retina, based on following evidence. (a) The presence of hemichannel proteins in the dendrite tips of the HCs, and (b) the blocking effect of carbenoxolone, a blocker of hemichannels, on the feedback responses. If the ephaptic effect modulates cone ICa, surround illumination should be expected to shift the I-V curve of the cone ICa at all voltages (like in Fig. 2 B b). However, importantly, surround illumination never induced a parallel shift of the I-V curve of the cone ICa in the negative direction; rather it increased ICa even in the region of the positive slope of the I-V curve of the cone ICa (between −10 and 10 mV; Fig. 2 B a), which is inconsistent with the ephaptic feedback hypothesis.
Protonated aminosulfonate compounds, including HEPES, have been reported to decrease the permeability of connexin-26 hemichannels, but compounds without an aminosulfonate moiety, such as Tris, maleate, and bicarbonate, do not decrease the permeability of these channels (Bevans and Harris, 1999). The effects of HEPES on the surround response are not likely to be due to blockade of the connexin-26 hemichannels in HCs (Kamermans et al., 2001), because Tris also suppressed the surround responses. Furthermore, there are other phenomena which seem to lend greater support to the pH-feedback hypothesis. First, in five of eight cones, 10 mM HEPES partially blocked the surround response at −26 mV (inset in Fig. 4 B b). Since cone ICa was most sensitive to external pH change at −26 mV (Fig. 3), even a slight change in pH was detectable. Second, when large surround responses of 20 pA were recorded in the control solution, diffuse light occasionally elicited a current dip at the offset of illumination (but not at the beginning) in the enriched buffer solution (Fig. 5 A, arrow). This may be interpreted to mean that the rate of proton buffering by Tris did not match up well with the rate of the sudden increment of protons at the time of switch-off of the surround light.
In this study, surround illumination shifted the activation curve of the cone ICa by ∼2.5 mV, which corresponded to the alkalinization-mediated shift of pH by ∼0.2 (estimated from Barnes et al., 1993) in a pH 7.4 external solution. In retinal slices, the surround response may be weakened due to the reduction of the receptive field size. Thus, the pH change by surround illumination in the retinal slice may be an underestimate of the value prevailing in vivo. In an isolated retina of the goldfish, surround illumination shifted the I-V curve of the cone ICa by 7.5 mV (Verweij et al., 1996), which corresponds to an alkalinization of ∼0.7 pH U. It is possible that the surround illumination induced alkalinization by 0.2 pH U in the bicarbonate buffer solution. In the retina, light stimulation evokes alkalinization of up to 0.2 pH U in the intraretinal extracellular space (Yamamoto et al., 1992), probably due to the change of H+ release caused by the energy metabolism in retinal cells. The activity-dependent changes in the external pH can play an active role in neuronal activity, as well as in the basal metabolism of retinal systems.
External protons also inhibit the glutamate response of AMPA receptors by increasing steady-state desensitization (Ihle and Patneau, 2000). However, the IC50 values for proton inhibition exceed the physiological range (from pH 5.7 to 6.3; Traynelis and Cull-Candy, 1991). Thus, during the generation of the cone surround response, pH changes in the cone synaptic clefts may affect the presynaptic cone calcium channels selectively, but not the postsynaptic AMPA receptors on the HCs.
In off-type BCs, 10 mM HEPES augmented the inward current response at the offset of a light spot, in addition to suppressing the surround response (Fig. 9). This is probably due to augmentation of transmitter release from the photoreceptors, and is consistent with the fact finding that HEPES augmented the cone ICa (Fig. 4). The sustained inward current in darkness, however, was not significantly augmented and this was probably due to desensitization of postsynaptic kainate receptors on off-BCs (DeVries and Schwartz, 1999). Under spot illumination (glutamate release is stopped), the kainate receptors would be released from a desensitized state. Thus, at light offset, the postsynaptic current evoked by abrupt glutamate release can be marked. A picrotoxin-resistant surround response of off-type BCs has also been reported in the tiger salamander retina (Hare and Owen, 1996). Thus, pH-mediated feedback to cones, rather than a GABAergic feedforward input to BCs, may be mainly involved in the receptive field organization of the BCs, at least in the amphibian retina. Modulation of the cone ICa is very strategic for direct control of the amount of transmitter release. Thus, the modulatory effect of pH on ICa at the cone synaptic terminal has a dual role, of contributing to the formation of the receptive field surround in the cones, and of controlling transmitter release from the cones to the BCs.
A Possible Mechanism of Control of the Extracellular pH Associated with Voltage Change in the HCs
There are several reports that neuronal activities evoke pH changes in the extracellular spaces in neuronal tissue (Kaila and Chesler, 1998). However, the mechanism by which HC depolarization acidifies the extracellular space in the cone synaptic clefts remains to be elucidated. An applicable model for the present hypothesis has been proposed as the model of neuron–glial cell interaction (Ransom, 2000). In this model, depolarization of glial cells caused by neuronal excitation activates Na+-HCO3 − cotransport (stoichiometry of 2HCO3 −: Na+) in the glia, resulting in the cellular uptake of extracellular HCO3 – and acidification of the extracellular space (Ransom, 2000). If extracellular protons are increased in darkness, the extracellular space in a tightly packed invaginating synapse might be easily acidified. Also in isolated HCs of the skate retina, Na+- and HCO3 −-dependent ion transport regulates the intracellular pH (Haugh-Schmedt and Ripps, 1998). Besides Na+-HCO3 − cotransport in HCs, other ionic transporters or exchangers, such as acid loaders or extruders must also be considered. Moreover, proton release via vesicular release of GABA, which has been suggested in mammalian HCs (Cueva et al., 2002), must also be considered.
Molina et al. (2000) reported that application of glutamate to isolated HCs of the all-rod skate retina reduces the number of hydrogen ions on its surface, indicating that glutamate causes proton influx into the cytoplasm of the HCs. This glutamate-mediated proton influx into the HCs is consistent with the evidence that L-glutamate raises the intracellular proton concentration in isolated HCs (Dixon et al., 1993). The results of Molina et al. (2000) and Dixon et al. (1993) appear to be inconsistent with our conclusion that the extracellular space should be acidified in darkness (when glutamate is tonically released). However, since the experiments by Molina et al. (2000) and Dixon et al. (1993) were performed with an external solution buffered with HEPES (without bicarbonate), the bicarbonate-dependent system may not be applicable in their studies. Moreover, Molina et al. (2000) performed their study using rod-driven HCs, whereas our discussion is on pH regulation in cone systems. Therefore, these two studies are not necessarily inconsistent with our present results obtained using bicarbonate buffer in the external solution.
Contribution of GABAergic Feedback to Receptive Field Organization
Our present analysis was focused on GABA-independent components; therefore, all recordings were made in the presence of 100 μM picrotoxin, to exclude any possible effects of GABA. It has been a matter of debate whether GABA is also involved in the formation of the receptive field surround of cone photoreceptors. Our observations indicate clearly that there is a large component that does not require GABA. In fact, in preliminary studies, we found that the amplitude of cone surround response was not reduced by GABA antagonists (picrotoxin, bicculine, SR95531). Moreover, the GABA antagonists did not evoke any current change whose reversal potential was equal to the equilibrium potential of chloride ions (unpublished data). Thus, it is unlikely that GABA plays a major role as a mediator of the feedback in the outer retina.
GABA may, however, play a substantial role in modulating the feedback response in the outer retina, rather than being the main mediator of the feedback. GABA can change membrane voltages of the HCs by modulating GABA-gated chloride currents and GABA transporter currents in the HCs (Kamermans and Werblin, 1992; Takahashi et al., 1995a,b), besides modulating GABA-gated chloride currents in cones. Thus, it is assumed that GABA may play some role in the information processing in the outer retina, but its role has to be reexamined in light of new data on the surround response of cones.
The synaptic structure of the invaginating synapse may be specialized for evoking pH changes in the intersynaptic clefts, because its highly packed structure can promote accumulation of protons. In contrast, GABAergic synapses between amacrine cells and bipolar and ganglion cells are conventional synapses, much different from the invaginating type of synapse. Thus, it may be reasonable to assume that lateral inhibition in the inner retina is mediated by GABA (Cook and McReynolds, 1998).
In summary, we have proposed an alternative hypothesis for the mechanism underlying the generation of the receptive field surround in the outer retina. We propose that intersynaptic pH changes in the invaginating synaptic clefts at the cone terminal contribute to the generation of the receptive field surround in the outer retina. Although the mechanism by which HCs regulate the extracellular pH is still uncertain, the present pH hypothesis should serve as the simplest description, so far, for the mechanism of feedback in the outer retina.
Acknowledgments
We thank Dr. Charles Edwards for editing the English language in the earlier edition.
This work was partly supported by the Keio University Grant-in-Aid for Encouragement of Young Medical Scientists (to H. Hirasawa), and Grants-in-Aid for Scientific Research (B) from JSPS (No. 14380378 to A. Kaneko) and Neuroinformatics Research in Vision (PI: Shiro Usui) under the Target-Oriented R&D for Brain Science at the MEXT.
Olaf S. Andersen served as editor.
Akimichi Kaneko's present address is Department of Rehabilitation, Seijoh University, Tokai-city, Aichi 476-8588, Japan.
Abbreviations used in this paper: CNQX, 6-cyano-7-nitroquinoxaline disodium; GABA, γ-aminobutyric acid; HC, horizontal cell; ICa, calcium current.
References
- Barnes, S., and Q. Bui. 1991. Modulation of calcium-activated chloride current via pH-induced changes of calcium channel properties in cone photoreceptors. J. Neurosci. 11:4015–4023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes, S. 1994. After transduction: response shaping and control of transmission by ion channels of the photoreceptor inner segments. Neuroscience. 58:447–459. [DOI] [PubMed] [Google Scholar]
- Barnes, S., V. Merchant, and F. Mahmud. 1993. Modulation of transmission gain by protons at the photoreceptor output synapse. Proc. Natl. Acad. Sci. USA. 90:10081–10085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baylor, D.A., M.G. Fuortes, and P.M. O'Bryan. 1971. Receptive fields of cones in the retina of the turtle. J. Physiol. 214:265–294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bevans, C.G., and A.L. Harris. 1999. Regulation of connexin channels by pH. Direct action of the protonated form of taurine and other aminosulfonates. J. Biol. Chem. 274:3711–3719. [DOI] [PubMed] [Google Scholar]
- Burkhardt, D.A. 1993. Synaptic feedback, depolarization, and color opponency in cone photoreceptors. Vis. Neurosci. 10:981–989. [DOI] [PubMed] [Google Scholar]
- Byzov, A.L., and T.M. Shura-Bura. 1986. Electrical feedback mechanism in the processing of signals in the outer plexiform layer of the retina. Vision Res. 26:33–44. [DOI] [PubMed] [Google Scholar]
- Chen, X.H., I. Bezprozvanny, and R.W. Tsien. 1996. Molecular basis of proton block of L-type Ca2+ channels. J. Gen. Physiol. 108:363–374. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesler, M. 1998. Principles and practical aspects of pH buffering. pH and Brain Function. K. Kaila and B.R. Ransom, editors. John Wiley & Sons, New York. 11–20.
- Cook, P.B., and J.S. McReynolds. 1998. Lateral inhibition in the inner retina is important for spatial tuning of ganglion cells. Nat. Neurosci. 1:714–719. [DOI] [PubMed] [Google Scholar]
- Cueva, J.G., S. Haverkamp, R.J. Reimer, R. Edwards, H. Wässle, and N.C. Brecha. 2002. Vesicular gamma-aminobutyric acid transporter expression in amacrine and horizontal cells. J. Comp. Neurol. 445:227–237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dixon, D.B., K. Takahashi, and D.R. Copenhagen. 1993. L-glutamate suppresses HVA calcium current in catfish horizontal cells by raising intracellular proton concentration. Neuron. 11:267–277. [DOI] [PubMed] [Google Scholar]
- DeVries, S.H. 2001. Exocytosed protons feedback to suppress the Ca2+ current in Mammalian cone photoreceptors. Neuron. 32:1107–1117. [DOI] [PubMed] [Google Scholar]
- DeVries, S.H., and E.A. Schwartz. 1999. Kainate receptors mediate synaptic transmission between cones and ‘Off’ bipolar cells in a mammalian retina. Nature. 397:157–160. [DOI] [PubMed] [Google Scholar]
- DeVries, S.H., X. Qui, R. Smith, W. Makous, and P. Sterling. 2002. Electrical coupling between mammalian cones. Curr. Biol. 12:1900–1907. [DOI] [PubMed] [Google Scholar]
- Gerschenfeld, H.M., and M. Piccolino. 1980. Sustained feedback effects of L-horizontal cells on turtle cones. Proc. R. Soc. Lond. B. Biol. Sci. 206:465–480. [DOI] [PubMed] [Google Scholar]
- Hare, W.A., and W.G. Owen. 1996. Receptive field of the retinal bipolar cell: a pharmacological study in the tiger salamander. J. Neurophysiol. 76:2005–2019. [DOI] [PubMed] [Google Scholar]
- Hare, W.A., and W.G. Owen. 1998. Effects of bicarbonate versus HEPES buffering on measured properties of neurons in the salamander retina. Vis. Neurosci. 15:263–271. [DOI] [PubMed] [Google Scholar]
- Haugh-Schmedt, L., and H. Ripps. 1998. pH regulation in horizontal cells of the skate retina. Exp. Eye Res. 66:449–463. [DOI] [PubMed] [Google Scholar]
- Ihle, E.C., and D.K. Patneau. 2000. Modulation of α-amino-3-hydroxyl-5-methyl-4-isoxazolepropionic acid receptor desensitization by extracellular protons. Mol. Pharmacol. 58:1204–1212. [DOI] [PubMed] [Google Scholar]
- Kaila, K., and M. Chesler. 1998. Activity-evoked changes in intracellular pH. pH and Brain Function. K. Kaila and B.R. Ransom, editors. John Wiley & Sons, New York. 291–308.
- Klöckner, U., and G. Isenberg. 1994. Calcium channel current of vascular smooth muscle cells: extracellular protons modulate gating and single channel conductance. J. Gen. Physiol. 103:665–678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans, M., and F. Werblin. 1992. GABA-mediated positive autofeedback loop controls horizontal cell kinetics in tiger salamander retina. J. Neurosci. 12:2451–2463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamermans, M., and H. Spekreijse. 1999. The feedback pathway from horizontal cells to cones. A mini review with a look ahead. Vision Res. 39:2449–2468. [DOI] [PubMed] [Google Scholar]
- Kamermans, M., I. Fahrenfort, K. Schultz, U. Janssen-Bienhold, T. Sjoerdsma, and R. Weiler. 2001. Hemichannel-mediated inhibition in the outer retina. Science. 292:1178–1180. [DOI] [PubMed] [Google Scholar]
- Kaneko, A. 1970. Physiological and morphological identification of horizontal, bipolar and amacrine cells in goldfish retina. J. Physiol. 207:623–633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneko, A., and M. Tachibana. 1986. Effects of gamma-aminobutyric acid on isolated cone photoreceptors of the turtle retina. J. Physiol. 373:443–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraaij, D.A., H. Spekreijse, and M. Kamermans. 2000. The nature of surround-induced depolarizing responses in goldfish cones. J. Gen. Physiol. 115:3–16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krafte, D.S., and R.S. Kass. 1988. Hydrogen ion modulation of Ca channel current in cardiac ventricular cells. Evidence for multiple mechanisms. J. Gen. Physiol. 91:641–657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuffler, S.W. 1953. Discharge patterns and functional organization of mammalian retina. J. Neurophysiol. 16:37–68. [DOI] [PubMed] [Google Scholar]
- Lam, D.M., Y.Y. Su, L. Swain, R.E. Marc, C. Brandon, and J.Y. Wu. 1979. Immunocytochemical localisation of L-glutamic acid decarboxylase in the goldfish retina. Nature. 278:565–567. [DOI] [PubMed] [Google Scholar]
- Lam, D.M., and L. Steinman. 1971. The uptake of (-3 H) aminobutyric acid in the goldfish retina. Proc. Natl. Acad. Sci. USA. 68:2777–2781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lamb, T.D., H.R. Matthews, and V. Torre. 1986. Incorporation of calcium buffers into salamander retinal rods: a rejection of the calcium hypothesis of phototransduction. J. Physiol. 372:315–349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marc, R.E., W.K. Stell, D. Bok, and D.M. Lam. 1978. GABA-ergic pathways in the goldfish retina. J. Comp. Neurol. 182:221–244. [DOI] [PubMed] [Google Scholar]
- Molina, A.J., P.J. Smith, and R.P. Malchow. 2000. Hydrogen ion fluxes from isolated retinal horizontal cells: modulation by glutamate. Biol. Bull. 199:168–170. [DOI] [PubMed] [Google Scholar]
- Morgans, C.W., O. El Far, A. Berntson, H. Wässle, and W.R. Taylor. 1998. Calcium extrusion from mammalian photoreceptor terminals. J. Neurosci. 18:2467–2474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nachman-Clewner, M., R. St. Jules, and E. Townes-Anderson. 1999. L-type calcium channels in the photoreceptor ribbon synapse: localization and role in plasticity. J. Comp. Neurol. 415:1–16. [PubMed] [Google Scholar]
- Nakatani, K., and K.W. Yau. 1988. Calcium and light adaptation in retinal rods and cones. Nature. 334:69–71. [DOI] [PubMed] [Google Scholar]
- O'Bryan, P.M. 1973. Properties of the depolarizing synaptic potential evoked by peripheral illumination in cones of the turtle retina. J. Physiol. 235:207–223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattnaik, B., A. Jellali, J. Sahel, H. Dreyfus, and S. Picaud. 2000. GABAC receptors are localized with microtubule-associated protein 1B in mammalian cone photoreceptors. J. Neurosci. 20:6789–6796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Picaud, S., B. Pattnaik, D. Hicks, V. Forster, V. Fontaine, J. Sahel, and H. Dreyfus. 1998. GABAA and GABAC receptors in adult porcine cones: evidence from a photoreceptor-glia co-culture model. J. Physiol. 513:33–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Piccolino, M., and H.M. Gerschenfeld. 1978. Activation of a regenerative calcium conductance in turtle cones by peripheral stimulation. Proc. R. Soc. Lond. B. Biol. Sci. 201:309–315. [DOI] [PubMed] [Google Scholar]
- Piccolino, M., and H.M. Gerschenfeld. 1980. Characteristics and ionic processes involved in feedback spikes of turtle cones. Proc. R. Soc. Lond. B. Biol. Sci. 206:439–463. [DOI] [PubMed] [Google Scholar]
- Prod'hom, B., D. Pietrobon, and P. Hess. 1989. Interactions of protons with single open L-type calcium channels. Location of protonation site and dependence of proton-induced current fluctuations on concentration and species of permeant ion. J. Gen. Physiol. 94:23–42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ransom, B.R. 2000. Glial modulation of neural excitability mediated by extracellular pH: a hypothesis revisited. Prog. Brain Res. 125:217–228. [DOI] [PubMed] [Google Scholar]
- Sakakibara, S., H. Hiramatsu, Y. Takahashi, O. Hisatomi, Y. Kobayashi, S. Sakami, T. Saito, and F. Tokunaga. 2002. Opsin expression in adult, developing, and regenerating newt retinas. Brain Res. Mol. Brain Res. 103:28–35. [DOI] [PubMed] [Google Scholar]
- Schwartz, E.A. 1987. Depolarization without calcium can release gamma-aminobutyric acid from a retinal neuron. Science. 238:350–355. [DOI] [PubMed] [Google Scholar]
- Skrzypek, J., and F. Werblin. 1983. Lateral interactions in absence of feedback to cones. J. Neurophysiol. 49:1007–1016. [DOI] [PubMed] [Google Scholar]
- Smith, P.J., K. Hammar, D.M. Porterfield, R.H. Sanger, and J.R. Trimarchi. 1999. Self-referencing, non-invasive, ion selective electrode for single cell detection of trans-plasma membrane calcium flux. Microsc. Res. Tech. 46:398–417. [DOI] [PubMed] [Google Scholar]
- Stell, W.K., D.O. Lightfood, T.G. Wheeler, and H.F. Leeper. 1975. Goldfish retina: functional polarization of cone horizontal cell dendrites and synapses. Science. 190:989–990. [DOI] [PubMed] [Google Scholar]
- Tachibana, M., and A. Kaneko. 1984. γ-Aminobutyric acid acts at axon terminals of turtle photoreceptors: difference in sensitivity among cell types. Proc. Natl. Acad. Sci. USA. 81:7961–7964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi, K., S. Miyoshi, and A. Kaneko. 1995. a. GABA-induced chloride current in catfish horizontal cells mediated by non-GABAA receptor channels. Jpn. J. Physiol. 45:437–456. [DOI] [PubMed] [Google Scholar]
- Takahashi, K., S. Miyoshi, A. Kaneko, and D.R. Copenhagen. 1995. b. Actions of nipecotic acid and SKF89976A on GABA transporter in cone-driven horizontal cells dissociated from the catfish retina. Jpn. J. Physiol. 45:457–473. [DOI] [PubMed] [Google Scholar]
- Thoreson, W.B., and D.A. Burkhardt. 1990. Effects of synaptic blocking agents on the depolarizing responses of turtle cones evoked by surround illumination. Vis. Neurosci. 5:571–583. [DOI] [PubMed] [Google Scholar]
- Thoreson, W.B., and D.A. Burkhardt. 1991. Ionic influences on the prolonged depolarization of turtle cones in situ. J. Neurophysiol. 65:96–110. [DOI] [PubMed] [Google Scholar]
- Tombaugh, G.C., and G.G. Somjen. 1998. pH modulation of voltage-gated ion channels. pH and Brain Function. K. Kaila and B.R. Ransom, editors. John Wiley & Sons, New York. 395–416.
- Traynelis, S.F., and S.G. Cull-Candy. 1991. Pharmacological properties and H+ sensitivity of excitatory amino acid receptor channels in rat cerebellar granule neurones. J. Physiol. 433:727–763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verweij, J., M. Kamermans, and H. Spekreijse. 1996. Horizontal cells feed back to cones by shifting the cone calcium-current activation range. Vision Res. 36:3943–3953. [DOI] [PubMed] [Google Scholar]
- Werblin, F.S. 1978. Transmission along and between rods in the tiger salamander retina. J. Physiol. 280:449–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu, S.M. 1991. Input-output relations of the feedback synapse between horizontal cells and cones in the tiger salamander retina. J. Neurophysiol. 65:1197–1206. [DOI] [PubMed] [Google Scholar]
- Yamamoto, F., G.A. Borgula, and R.H. Steinberg. 1992. Effects of light and darkness on pH outside rod photoreceptors in the cat retina. Exp. Eye Res. 54:685–697. [DOI] [PubMed] [Google Scholar]
- Yang, X.L., and S.M. Wu. 1989. Effects of CNQX, APB, PDA, and kynurenate on horizontal cells of the tiger salamander retina. Vis. Neurosci. 3:207–212. [DOI] [PubMed] [Google Scholar]
- Zhou, W., and S.W. Jones. 1996. The effects of external pH on calcium channel currents in bullfrog sympathetic neurons. Biophys. J. 70:1326–1334. [DOI] [PMC free article] [PubMed] [Google Scholar]