Abstract
In an earlier investigation, we demonstrated that the likelihood of interaction of a positively charged ryanoid, 21-amino-9α-hydroxyryanodine, with the sarcoplasmic reticulum Ca2+-release channel (ryanodine receptor, RyR) is dependent on holding potential (Tanna, B., W. Welch, L. Ruest, J.L. Sutko, and A.J. Williams. 1998. J. Gen. Physiol. 112:55–69) and suggested that voltage dependence could result from either the translocation of the charged ligand to a site within the voltage drop across the channel or a voltage-driven alteration in receptor affinity. We now report experiments that allow us to assess the validity of these alternate mechanisms. Ryanodol is a neutral ryanoid that binds to RyR and induces modification of channel function. By determining the influence of transmembrane potential on the probability of channel modification by ryanodol and the rate constants of ryanodol association and dissociation, we demonstrate that the influence of voltage is qualitatively the same for both the neutral and positively charged ryanoids. These experiments establish that most, if not all, of the modification of ryanoid interaction with RyR by transmembrane holding potential results from a voltage-driven alteration in receptor affinity.
Keywords: ryanodine receptor, sarcoplasmic reticulum, calcium channel, ryanodine, ryanodol
INTRODUCTION
Ryanodine is a plant alkaloid that binds with high affinity and specificity to a class of intracellular membrane Ca2+-release channels (Sutko et al. 1997). As a consequence of this interaction, these channels are commonly referred to as ryanodine receptors (RyRs). Ryanodine has proven to be a useful tool in establishing the molecular identity of the Ca2+-release channel (Lai et al. 1988a,Lai et al. 1988b), in the characterization of the function of the receptor as a channel, and in the elucidation of its role in Ca2+ signaling processes in a wide range of systems (Bazotte et al. 1991; Foskett and Wong 1991; Swann 1992; Walz et al. 1995; Ullmer et al. 1996). The binding of ryanodine to its high affinity site on RyR initiates a marked change in channel function; rates of permeant ion translocation are altered and open probability (P o) is increased dramatically. The modification of RyR ion handling by ryanodine is the consequence of the alteration of a number of processes governing cation translocation. The reduction in single-channel conductance observed on the binding of ryanodine to the RyR channel with either divalent or monovalent inorganic cations as the permeant species reflects alterations in both the relative permeability of ions and the affinity of sites within the conduction pathway for these ions (Lindsay et al. 1994). It seems likely that the binding of ryanodine to the channel induces a conformational change in the protein that in turn modifies the manner in which permeant ions interact with the conduction pathway (Lindsay et al. 1994).
The kinetic parameters of the ryanodine–RyR interaction are traditionally determined by monitoring the binding of [3H]ryanodine to populations of receptors, either in intact membrane vesicles, or after receptor purification (Fleischer et al. 1985; Pessah et al. 1986; Lai et al. 1989; Chu et al. 1990; Holmberg and Williams 1990; Needleman and Hamilton 1997; Murayama et al. 1999). We have recently developed methods that permit the investigation of the kinetic parameters of the interaction of ryanoids with individual RyR channel proteins under voltage clamp conditions. These experiments are based on the observation that (a) the interaction of a ryanoid with the channel induces a distinct modification of channel function and (b) alterations in ryanoid structure produce variations in binding kinetics. Consistent with the very slow rates of dissociation monitored for [3H]ryanodine in binding assays, the interaction of ryanodine with a single RyR channel is irreversible on the timescale of a single-channel experiment (Rousseau et al. 1987; Lindsay et al. 1994; Tinker et al. 1996); ryanodine “locks” the channel in a high-P o, modified-conductance state. Modification of the structure of ryanodine yields ryanoids that compete with ryanodine for the high affinity site on the RyR channel (Welch et al. 1994, Welch et al. 1996; Bidasee and Besch 1998; Humerickhouse et al. 1994) and induce high-P o, modified-conductance states. However, the kinetics of the interaction of some of these ligands with single RyR channels is much faster than those of ryanodine. In the continued presence of the ryanoid, we observe transitions between periods of normal channel gating and ion translocation and periods of modified channel function during which the ryanoid is bound (Tinker et al. 1996; Tanna et al. 1998). We have characterized the interaction of one such ryanoid, 21-amino-9α-hydroxyryanodine, with the sheep cardiac isoform of the RyR channel and have identified a number of novel features (Tanna et al. 1998). Variations in 21-amino-9α-hydroxyryanodine binding to single channels with changing concentrations of the ryanoid indicate that modifications of RyR channel function induced by this ryanoid result from the interaction of a single molecule of the ryanoid with each channel protein. Related experiments demonstrated that 21-amino-9α-hydroxyryanodine has access to its binding site only in open conformations of the channel protein, that the ryanoid binding site can only be reached from the cytosolic side of the channel, and that the interaction of the ryanoid with its binding site is influenced strongly by transmembrane voltage (Tanna et al. 1998).
The quantitative determination of the voltage dependence of the interaction of 21-amino-9α-hydroxyryanodine with RyR indicates that approximately two positive charges move through the voltage drop across the channel during the activation of 21-amino-9α-hydroxyryanodine binding by voltage (Tanna et al. 1998). Both the rate of association of the ryanoid with the receptor and the rate of dissociation from the receptor are influenced by voltage. A likely source of the voltage dependence of this reaction would involve the translocation of 21-amino-9α-hydroxyryanodine (net charge +1) to a binding site within the voltage drop across the channel. If all the voltage dependence of the interaction arises in this way, either two molecules of the ryanoid would need to be translocated across the entire voltage drop, or several ryanoid molecules could interact with sites located nearer the cytosolic entry to the voltage drop. Neither of these possibilities is consistent with our observation that other aspects of the interaction of 21-amino-9α-hydroxyryanodine with RyR can be described in terms of a bimolecular reaction (Tanna et al. 1998). An alternative explanation is that the measured voltage dependence results not from the movement of the charged ryanoid into an electric field, but from a voltage-driven movement of charge within the RyR channel protein that produces a conformational change in the channel protein and switches the ryanoid binding site between two states with different affinities (Tanna et al. 1998). The elucidation of the mechanism underlying the dependence on transmembrane voltage of the interaction of 21-amino-9α-hydroxyryanodine with RyR is of interest in its own right, but may also provide information on the location of the ryanodine binding site in RyR. For example, translocation of 21-amino-9α-hydroxyryanodine into the voltage drop across the channel would suggest that the binding site is within the conduction pathway of the RyR channel.
In this communication we have addressed these issues by investigating the influence of transmembrane voltage on the interaction of a neutral ryanoid, ryanodol, with single RyR channels. Our experiments demonstrate that ryanodol displays the same qualitative voltage dependence as the positively charged 21-amino-9α-hydroxyryanodine and are consistent with the proposal that most, if not all, of the influence of voltage on the interaction of ryanoids with the RyR channel is derived from a voltage-driven alteration in the affinity of the receptor.
MATERIALS AND METHODS
Materials
Phosphatidylethanolamine was supplied by Avanti Polar Lipids, Inc. and phosphatidylcholine by Sigma-Aldrich. [3H]Ryanodine was purchased from New England Nuclear Ltd. Aqueous counting scintillant was purchased from Packard. Standard chemicals were obtained as the best available grade from BDH Ltd. or Sigma-Aldrich. Ryanodol was synthesized as described earlier (Wiesner 1972; Deslongchamps et al. 1990) and stored as a stock solution in 50% ethanol at −20°C.
Isolation of Sheep Cardiac Heavy Sarcoplasmic Reticulum Membrane Vesicles and Solubilization and Separation of the Ryanodine Receptor
Heavy sarcoplasmic reticulum (HSR) membrane vesicles were prepared using procedures described earlier (Sitsapesan and Williams 1990). Sheep hearts were collected from a local abattoir in ice-cold cardioplegic solution (Sitsapesan and Williams 1990). A mixed membrane fraction was obtained by differential centrifugation after homogenization of the ventricular septum and left ventricle free wall. The mixed membrane vesicles were further fractionated by sucrose density gradient centrifugation and the HSR fraction was collected at the 30/40% (wt/vol) interface. The HSR fraction was resuspended in 0.4 M KCl before sedimentation at 100,000 g. The resulting pellet was resuspended in 0.4 M sucrose, 5 mM HEPES, titrated to pH 7.2 with hydroxymethyl methylamine (Tris). HSR membrane vesicles were solubilized with 3-[(3-cholamidopropyl)-dimethylammonio]-1-propane sulfonate (CHAPS) and RyR was isolated and reconstituted into unilamellar liposomes for incorporation into planar phospholipid bilayers, as described previously (Lindsay and Williams 1991).
Planar Phospholipid Bilayers
Phospholipid bilayers were formed from suspensions of phosphatidylethanolamine in n-decane (35 mg/ml) across a 200-μm diameter hole in a polystyrene copolymer partition that separated two chambers referred to as cis (0.5 ml) and trans (1.0 ml). The trans chamber was held at virtual ground while the cis chamber could be clamped at holding potentials relative to ground. Current flow across the bilayer was monitored using an operational amplifier as a current–voltage converter (Miller 1982). Bilayers were formed with solutions containing 600 mM KCl, 20 mM HEPES, titrated to pH 7.4 with KOH, resulting in a solution containing 610 mM K+ in both chambers. An osmotic gradient was created by the addition of an aliquot (50–100 μl) of 3 M KCl to the cis chamber. Proteoliposomes were added to the cis chamber and stirred. Under these conditions, channels usually incorporated into the bilayer within 2–3 min. If channels did not incorporate, a second aliquot of 3 M KCl could be added to the cis chamber. After channel incorporation, further fusion was prevented by perfusion of the cis chamber with 610 mM K+. Channel proteins incorporate into the bilayer in a fixed orientation so that the cytosolic face of the channel is exposed to the solution in the cis chamber and the luminal face of the channel to the solution in the trans chamber. Single channel P o was increased by the addition of up to 200 μM EMD 41000 to the cytosolic face of the channel (McGarry and Williams 1994; Tanna et al. 1998). Only bilayers containing a single channel were used in the experiments described in this communication. Experiments were carried out at room temperature (21 ± 2°C). The interactions of ryanodol with the channel were studied by adding the indicated concentration to the solution at the cytosolic face of the bilayer.
Single Channel Data Acquisition
Single channel current fluctuations were displayed on an oscilloscope and stored on Digital Audio Tape. For analysis, data were replayed, filtered at 1 kHz with an eight-pole Bessel filter, and digitized at 4 kHz using Satori V3.2 (Intracel). Single channel current amplitudes and lifetimes were measured from digitized data. The representative traces shown in the figures were obtained from digitized data acquired with Satori V3.2 and transferred as an HPGL graphics file to a graphics software package (CorelDraw; Corel Systems Corp.) for annotation and printing.
Monitoring the Interaction of Ryanodol with Single Channels
Ryanodol binds to the high affinity ryanodine binding site on the SR Ca2+-release channel and induces modifications of channel function; channel conductance is reduced and P o increases (Tinker et al. 1996). The interaction kinetics of ryanodol are sufficiently rapid that, in the continued presence of the ryanoid, we observe repeated transitions between periods of modified channel function and periods of normal gating and conductance. In previous studies, we have established that the interaction of 21-amino-9α-hydroxyryanodine and the resulting modification of channel function can be described by a simple bimolecular reaction scheme (Tanna et al. 1998). Consistent with this scheme, dwell times of the RyR channel in the ryanodol-modified and -unmodified gating states are described by single exponentials, the rate of association of ryanodol with its receptor varies with ryanodol concentration while the rate of dissociation of ryanodol from its receptor is independent of ligand concentration (data not shown). As a consequence, apparent rate constants for the association (k on) and dissociation (k off) of ryanodol can be determined from the mean dwell times in the unmodified and modified conductance states ( and ):
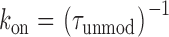 |
1 |
and
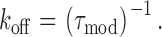 |
2 |
Dwell times and the probability that the channel is in the ryanoid-modified state (P mod) were determined using a pattern recognition program as described in Tanna et al. 1998. To obtain sufficient events, these parameters were obtained from steady state recordings lasting at least 6 min.
The Modification of the Interaction of Ryanodol with RyR by Voltage
If the transition between the normal gating state of the RyR and the modified state resulting from the interaction of ryanodol is dependent on holding potential, P mod will be determined by the Boltzmann distribution,
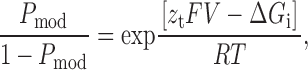 |
3 |
where F is the Faraday constant, V is the transmembrane voltage, R is the gas constant, T is temperature (°K), z t is the voltage dependence of the occurrence of the ryanoid modified state, ΔG i is the difference in free energy of the unmodified and ryanoid-modified states, and ΔG i /RT is an expression of the equilibrium of the reaction at a holding potential of 0 mV.
For such a relationship, the rate constants at a given voltage will be described as follows:
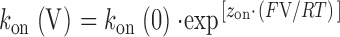 |
4 |
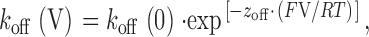 |
5 |
where k(V) and k(0) are the rate constants at a particular voltage and at 0 mV, respectively, and z is the valence of the appropriate reaction. Plots of the natural logarithm of k on and k off against holding potential should be linear with slopes z on F/RT and −z off F/RT and intercepts ln[k on(0)] and ln[k off(0)], respectively. The total voltage dependence (z total) of the reaction is then z on + z off.
The Probability of the Channel Being Open
While the rates of dissociation of ryanodol and 21-amino-9α-hydroxyryanodine from RyR are independent of P o, the rates of association of both ryanodol (data not shown) and 21-amino-9α-hydroxyryanodine (Tanna et al. 1998) are directly proportional to channel P o. For this reason, it is necessary to measure P o in all experiments. This was done by monitoring this parameter in the sections of the recorded data during which no ryanoid was bound; i.e., with transitions only between the open and closed conductance levels. P o was determined by 50% threshold analysis as described previously (Sitsapesan and Williams 1994). To minimize variability in P o, all experiments were carried out in the presence of cytosolic EMD 41000. As kinetic parameters determined for ryanodol are to be compared with equivalent parameters determined in an earlier investigation for 21-amino-9α-hydroxyryanodine (Tanna et al. 1998), it is important to correct for variations in k on arising from unavoidable differences in P o between the populations of channels used in the two sets of experiments. The kon values quoted for both ryanodol and 21-amino-9α-hydroxyryanodine have been normalized to a P o of 1.0.
RESULTS
Is the Interaction of Ryanodol with the RyR Channel Influenced by Transmembrane Holding Potential?
In previous studies, we demonstrated that the interaction of 21-amino-9α-hydroxyryanodine with the high affinity ryanodine binding site on the RyR channel is influenced by transmembrane voltage (Tanna et al. 1998). We observed a marked dependence of P mod on voltage with a value of z t obtained from best fit Boltzmann distributions () and from variations in k on and k off ( and ) of ∼2. As outlined in the introduction, this dependence on holding potential could arise from the translocation of the positively charged ryanoid into the voltage drop across the RyR channel. Alternatively, voltage dependence could be derived from a voltage-driven movement of charge within the RyR channel that results in a conformational change that switches the ryanoid binding site between two states of different affinity (Tanna et al. 1998). In such a scheme, the location of the ryanoid binding site could influence the measured voltage dependence of a charged ryanoid such as 21-amino-9α-hydroxyryanodine. If the site were located outside the voltage drop across the channel, all of the voltage dependence would be derived from the proposed voltage-driven conformational change. However, if the site were located within the voltage drop, a proportion of the total voltage dependence would arise from the movement of the charged ryanoid into and out of the voltage drop. These alternatives can be distinguished by monitoring the voltage dependence of a ryanoid such as ryanodol that carries no ionic groups.
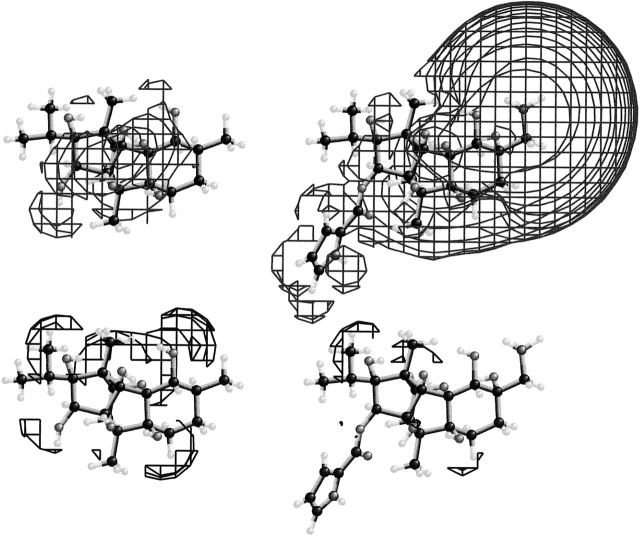
Fig. 1 presents the electrical potential of 21-amino-9α-hydroxyryanodine (right, net charge +1) and ryanodol (left, no ionic groups) together with ball and stick models of the molecules. The top shows positive and the bottom shows negative electrostatic fields. 21-amino-9α-hydroxyryanodine is shown with the pyrrole ring at the bottom left. Ryanodol, which lacks the pyrrole ring, is in the same orientation. The wire frames in all diagrams represent the surface where the electrostatic potential equals 10 kcal/mol. Note the extensive electrical potential of 21-amino-9α-hydroxyryanodine (top right) due to the ammonium ion (located in the upper right of the ball and stick diagram). In comparison with ryanodol (top left), the far more extensive electrical interactions of 21-amino-9α-hydroxyryanodine with other molecules is clearly visible. While ryanodol is neutral (has no net charge), the presence of hydroxyl groups creates a nonuniform distribution of potential in the molecule. The hydroxyl groups on one surface mean that there will be excess negative electrostatic potential localized to a specific surface region of ryanodol. The resulting microscopic dipoles sum to yield the electrical fields shown on the left hand figures. Note that, as for the positive electrostatic field, the distribution of the negative electrostatic potential (bottom) is much different for 21-amino-9α-hydroxyryanodine and ryanodol. Fig. 1 visualizes the large difference in the macroscopic dipole moments of these two molecules. The dipole (24.2 Debye) of 21-amino-9α-hydroxyryanodine runs along the long axis of the cationic ryanoid (the long axis is parallel to the plane of Fig. 1). In contrast, the dipole (3.3 Debye) of the neutral ryanoid is at right angles to that of 21-amino-9α-hydroxyryanodine. Therefore, these two ryanoids will experience considerably different torsional forces within any electrical potential gradient, including the applied transmembrane voltage. The large permanent dipole will tend to orient 21-amino-9α-hydroxyryanodine with the long axis parallel to the electric field.
Figure 1.
(Top) The positive electrostatic fields of 21-amino-9α-hydroxyryanodine (right) and ryanodol (left). (Bottom) The negative electrostatic fields of the same compounds. In all cases, the wire frames indicate the surface where the electrical field strength is 10 kcal/mol. Ball and stick models of 21-amino-9α-hydroxyryanodine (right) and ryanodol (left) are shown in the same alignment. Referring to the models of 21-amino-9α-hydroxyryanodine, the pyrrole group is at the bottom left and the 21-amino group is at the top right.
Fig. 2 shows current fluctuations of a single RyR channel at holding potentials ranging from −50 to 20 mV in the presence of 20 μM ryanodol. As is the case with 21-amino-9α-hydroxyryanodine, ryanodol interacts reversibly with the RyR channel, inducing modifications to both channel gating and ion handling; however, the fractional conductance of the modified state (Tinker et al. 1996) differs for the two ryanoids. The fractional conductance of ryanodol is independent of holding potential, being 0.65 ± 0.01, 0.64 ± 0.02, 0.66 ± 0.01, 0.66 ± 0.01, 0.66 ± 0.01, 0.65 ± 0.02, 0.68 ± 0.02, and 0.68 ± 0.01 at −60, −50, −40, −30, −20, −10, 10, and 20 mV. Fractional conductance for 21-amino-9α-hydroxyryanodine under these conditions is ∼0.45 (Tanna et al. 1998).
Figure 2.
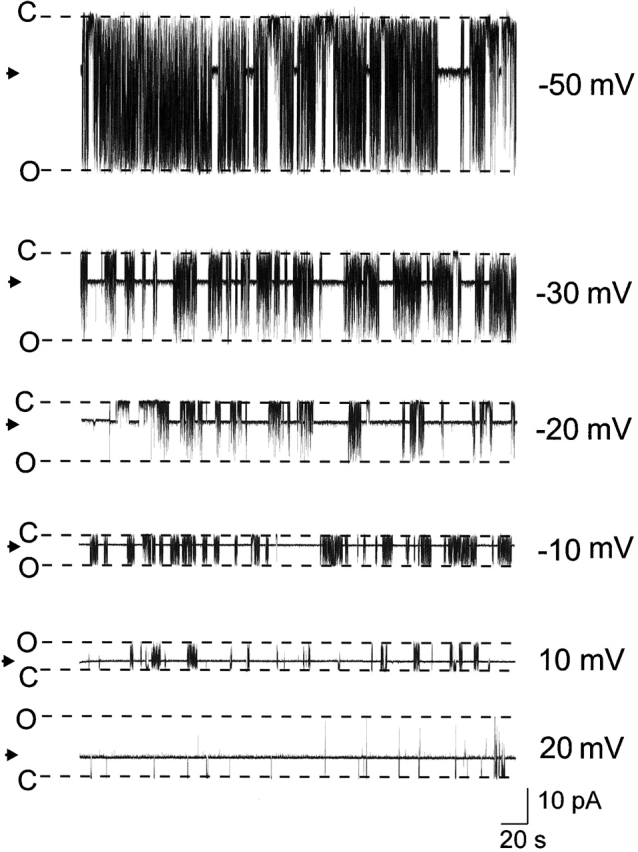
The influence of holding potential on the probability of modification of RyR channel function by ryanodol. Traces were obtained from a single RyR channel in symmetrical 610 mM K+ with 20 μM ryanodol in the solution at the cytosolic face of the channel. O, open; C, closed; →, modified.
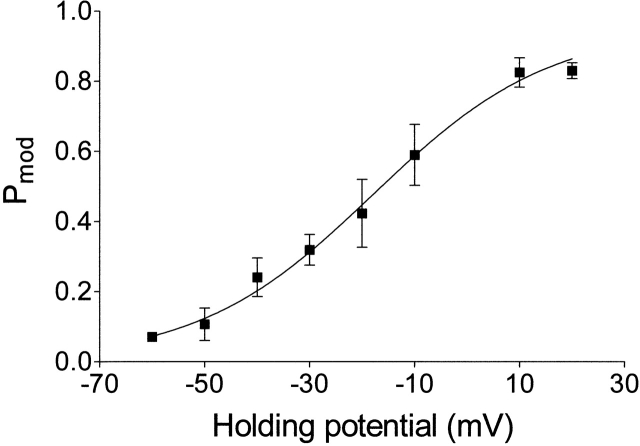
Inspection of the traces in Fig. 2 highlights the first novel finding of these studies. The probability of RyR channel modification by a neutral ryanoid, ryanodol, varies markedly with holding potential, and the effect of voltage is qualitatively the same as that observed with 21-amino-9α-hydroxyryanodine: P mod for both ryanoids rises as holding potential is shifted to more positive values. The relationship between P mod and holding potential, in the range −60 to 20 mV, for several channels in the presence of 20 μM ryanodol is shown in Fig. 3. The solid line is the best fit Boltzmann distribution () obtained by nonlinear regression with a value for z t of 1.54 (r = 1.0).
Figure 3.
The relationship between P mod by ryanodol and holding potential. P mod was determined by monitoring dwell times in the unmodified and modified conductance states in 6-min recordings with 20 μM ryanodol in the solution at the cytosolic face of the channel. Each point is the mean ± SEM of 4–10 experiments. The curve is the best fit Boltzmann distribution obtained by nonlinear regression with the parameters quoted in the text.
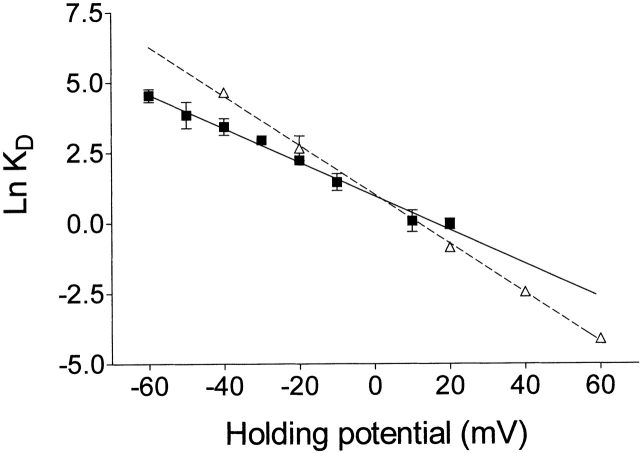
Fig. 4 shows the influence of holding potential on k on (a) and k off (b) of ryanodol together with equivalent data for 21-amino-9α-hydroxyryanodine taken from Tanna et al. 1998. Both the rates of association and dissociation of ryanodol vary with applied holding potential; k on increases and k off decreases as the holding potential is shifted to more positive values. The line of best fit obtained by linear regression for the k on plot has a slope of 0.041 ± 0.003 (r = 1.0), yielding a value of z on of 1.03 (). The equivalent plot for k off has a slope of −0.019 ± 0.002 (r = 0.98), yielding a value for z off of 0.48 (). Together, these produce a z total of 1.51. Values for k on and k off at 0 mV, obtained from the lines of best fit in Fig. 4, a and b, are 0.035 μM−1 s−1 and 0.095 s−1, respectively. Fig. 5 shows the relationship of the ryanodol dissociation constant [K d = k off (s−1)/k on (μM−1 s−1)] to transmembrane holding potential; 21-amino-9α-hydroxyryanodine data are again included for comparison.
Figure 4.
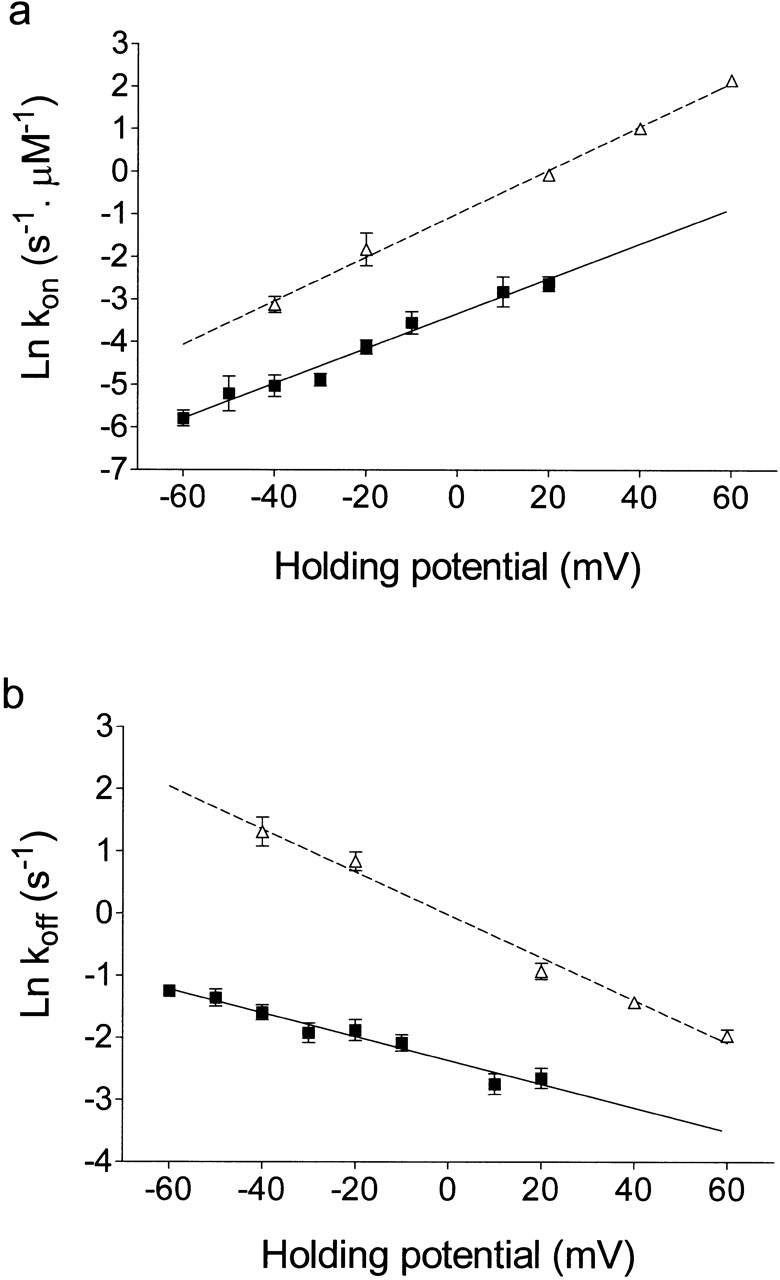
(a) The dependence of rates of association of ryanodol (▪) and 21-amino-9α-hydroxyryanodine (▵) on holding potential. 20 μM ryanodol was present in the solution at the cytosolic face of the channel. Each point is the mean ± SEM of 4–10 experiments. 21-amino-9α-hydroxyryanodine data is from Tanna et al. 1998, with all values of k on normalized for a P o of 1.0. (b) The dependence of rates of dissociation of ryanodol (▪) and 21-amino-9α-hydroxyryanodine (▵) on holding potential. 20 μM ryanodol was present in the solution at the cytosolic face of the channel. Each point is the mean ± SEM of 4–10 experiments. 21-amino-9α-hydroxyryanodine data is from Tanna et al. 1998. The solid lines in both a and b were obtained by linear regression with the parameters quoted in the text.
Figure 5.
The relationship of the dissociation constant [K d = k off (s−1)/k on (μM−1 s−1)] of ryanodol (▪) and 21-amino-9α-hydroxyryanodine (▵) to the transmembrane holding potential.
The ryanodol data are summarized, together with equivalent parameters for 21-amino-9α-hydroxyryanodine (Tanna et al. 1998), in Table .
Table 1.
The Influence of holding Potential on the Interaction of Ryanodol and 21-Amino-9α-Hydroxyryanodine with Sheep RYR2
| Ryanodol | 21-Amino-9α-Hydroxyryanodine | |
|---|---|---|
| Slope (z on F/RT) | 0.041 ± 0.003 (r = 1.0) | 0.051 ± 0.002 (r = 1.0) |
| z on | 1.03 | 1.29 |
| Slope (−z on F/RT) | −0.019 ± 0.002 (r = 1.0) | −0.0344 ± 0.002 (r = 1.0) |
| z off | 0.48 | 0.87 |
| z total | 1.51 | 2.16 |
| k on at 0 mV (μM−1 s−1) | 0.035 | 0.365 |
| k off at 0 mV (s−1) | 0.095 | 0.990 |
| K d at 0 mV (μM) | 2.81 | 2.79 |
Data for 21-amino-9α-hydroxyryanodine are from Tanna et al. 1998 with values of k on normalized for a P o of 1.0. Values of k on, k off, and K d at 0 mV are calculated from the regression lines fitted to data in Fig. 4 and Fig. 5.
DISCUSSION
Our earlier investigations of the interaction of 21-amino-9α-hydroxyryanodine with individual cardiac muscle RyR channels demonstrated for the first time that both the association of a ryanoid with its receptor and dissociation of a ryanoid from its receptor could be sensitive to transmembrane voltage (Tanna et al. 1998). While the mechanism underlying the observed voltage dependence was not established, we proposed that it might result from either the translocation of the charged ryanoid into the voltage drop across the channel or a voltage-driven conformational change of RyR resulting in altered receptor affinity. We have tested these proposals in this report by monitoring the influence of voltage on the likelihood of interaction of ryanodol, a neutral ryanoid, with the RyR channel.
These experiments establish that transmembrane voltage influences the likelihood of a ryanoid being bound to the receptor on the RyR channel by a mechanism that is independent of the translocation of a charged moiety into the voltage drop across the channel.
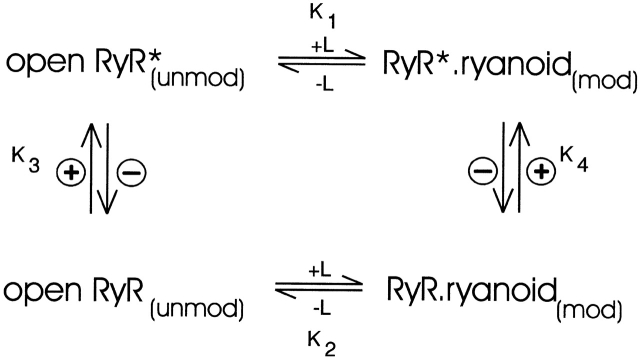
A likely mechanism for the modulation of ryanoid interaction with the receptor on RyR would then involve a voltage-dependent equilibrium between receptor states of different affinity (Tanna et al. 1998). In such a scheme (Fig. 6), the transmembrane potential would provide an energy source for the movement of a charged residue, or domain, of RyR within the electric field that would produce a conformational change and result in altered receptor affinity. The difference in voltage dependence of k on and k off for ryanodol indicates a degree of asymmetry in the energy profile for the transition between the different affinity states of the receptor; the electrical distance covered by the charged residue in the forward and reverse reactions may be different, or the amount of charge moved in the forward and reverse reactions may be different due to screening (Moczydlowski 1986).
Figure 6.
Scheme summarizing the influence of transmembrane voltage on the interaction of ryanoids with the high affinity binding site of RyR (based on information presented in this communication and Tanna et al. 1998). The ryanoid binding site is only available when the channel is open and only accessible from the cytosolic side of the channel. L represents the ligand (any ryanoid), and + and − indicate the application of positive and negative potential to the cytosolic side of the channel. A minimum of two forms of the vacant receptor exist in this model (low affinity, open RyR; high affinity, open RyR*). Increasing positive potential shifts the equilibrium toward open RyR* by increasing the value of the equilibrium constants K3 and K4. If the ryanoid binding site is located within the voltage drop across the channel, then charged ryanoids such as 21-amino-9α-hydroxyryanodine will experience an additional influence of voltage.
While the experiments reported here provide an unequivocal demonstration that modulation of ryanoid binding by voltage is not dependent on the ryanoid carrying a positive charge, they do suggest that the net charge of the ryanoid may produce small quantitative differences in the influence of transmembrane voltage. The total voltage dependence, monitored from variations in k on and k off, with voltage, of 21-amino-9α-hydroxyryanodine is slightly greater than that of ryanodol, and this produces a significant difference in the influence of voltage on the resulting K ds for the two ryanoids. How could the net charge of the ryanoid alter the influence of voltage on the interaction of the ligand with its receptor? The binding of 21-amino-9α-hydroxyryanodine may induce a different conformer of the ligand–RyR complex diagrammed in Fig. 6 than that induced by ryanodol. The difference in fractional conductance of the ryanodol–RyR and 21-amino-9α-hydroxyryanodine–RyR complexes is evidence of such a difference. The binding of the two ryanoids may induce different distributions of charge in the ryanoid–RyR complex, either directly by addition of another ionic charge or indirectly through alteration of one or more of the RyR acid dissociation constants by interaction with the electric charge of 21-amino-9α-hydroxyryanodine. The small difference in voltage dependence of the neutral and cationic ryanoid indicates that movement of charge is involved in the voltage-induced transition; however, the small magnitude of the effect makes interpretation difficult. While not eliminating the possibility of allosteric interactions between the ryanodine binding site and the ion conduction pathway, a mechanism where the ryanoid binding site is placed within the voltage drop across the channel is appealing because of its simplicity. Under these circumstances, the small increase in voltage dependence arises from the movement of a charged ryanoid into and/or out of the electric field. A location of the ryanoid binding site within the voltage drop of RyR would be consistent with the proposal that the receptor site is within the conduction pathway of the channel. The conduction pathway of the RyR channel is almost certainly formed from residues within the carboxyl terminal domains of the four RyR monomers that together make up the functional homotetramer (Bhat et al. 1997; Zhao et al. 1999) and investigations involving the proteolytic digestion of channels labeled with [3H]ryanodine or a [3H]photoactivated derivative of ryanodine have established that the high affinity ryanodine binding site on the skeletal muscle isoform of RyR (RyR1) is located at the carboxyl terminus of the molecule (Callaway et al. 1994; Witcher et al. 1994). Very recent experiments indicate that mutations around the probable pore-forming region of the RyR channel produce reductions or abolition of the binding of [3H]ryanodine and could be interpreted as indicating this region as a component of the ryanoid binding site (Zhao et al. 1999).
In addition, several features of the interaction of ryanoids with the RyR channel are consistent with the proposal that the high-affinity binding site for these ligands is located within the conduction pathway of the channel. The magnitude of equilibrium [3H]ryanodine binding is altered by interventions that modify RyR channel P o. Activating ligands such as Ca2+, caffeine, and ATP increase binding while ligands that lower P o, such as Mg2+ and ruthenium red, reduce binding (Chu et al. 1990; Holmberg and Williams 1990; Hawkes et al. 1992; Meissner and El-Hashem 1992). These observations have given rise to the proposal that the high affinity binding site for [3H]ryanodine is only accessible when the channel is open and, as a consequence, equilibrium [3H]ryanodine binding is used routinely as a method of assessing the P o of RyR channels. A direct demonstration that the high-affinity binding of a ryanoid to the RyR channel requires the channel to be open was provided in the studies of Tanna et al. 1998, in which a linear dependence of the rate of association of 21-amino-9α-hydroxyryanodine on RyR channel P o was demonstrated.
The dissociation constants monitored in these single-channel experiments are in the same range as those determined for RyR in intact cardiac sarcoplasmic reticulum membrane vesicles in competition binding studies with [3H]ryanodine [2 μM 21-amino-9α-hydroxyryanodine (Welch, W., unpublished results) and 1 μM ryanodol (Welch et al. 1997)]. The good agreement of the dissociation constants determined by two very different methods indicates that the parameters determined for individual RyRs in planar bilayers are relevant to the receptor in the native SR membrane. Using the values in Table , the difference in binding energy of the two ryanoids is only 4 cal/mol. The small difference in binding energy demonstrates that the differences in channel function observed with 21-amino-9α-hydroxyryanodine and ryanodol are more likely to result from the detailed differences in electrical and steric interactions between the receptor and ligand rather than from differences in the global ryanoid–RyR interaction energy. Note that compared with ryanodine (K d = 2 nM; Welch et al. 1997), the high dissociation constant of 21-amino-9α-hydroxyryanodine arises from a 4 kcal/mol unfavorable interaction between the ligand and receptor (either electrostatic repulsion, poor solvation, or both). Steric interactions can be discounted since placing the bulky BODIPY derivative at the 21 position caused only a small perturbation in binding (Welch et al. 1994). In contrast, the 4 kcal/mol reduction in binding energy of ryanodol compared with ryanodine is due to the loss of the pyrrole carbonyl. That is, while relative to ryanodine, both ryanodol and 21-amino-9α-hydroxyryanodine have 4 kcal/mol less binding energy; this results in one case from the omission of an important interaction, while in the other case it is due to the addition of an unfavorable interaction.
Independent of voltage-induced changes, there are very marked differences in both the rates of association and dissociation for ryanodol and 21-amino-9α-hydroxyryanodine with RyR. For example, at a holding potential of 0 mV, ryanodol is ∼10× less likely to associate (ΔΔG = 1.4 kcal/mol) with the ryanodine binding site on RyR than 21-amino-9α-hydroxyryanodine; once bound, ryanodol leaves this site ∼10× slower (ΔΔG = 1.4 kcal/mol) than 21-amino-9α-hydroxyryanodine. Therefore, the difference in energy barriers limiting association with and dissociation from the ryanoid binding site is considerably greater than the difference in total binding energy of the two ryanoids.
In summary, the experiments described in this report were designed to examine the mechanisms involved in the striking influence of transmembrane holding potential on the interaction of ryanoids with the high affinity binding site on the RyR channel. Our earlier observation of a strong influence of voltage on the interaction of a positively charged ryanoid with RyR could be explained either by the movement of the charged ligand into the voltage drop of the channel to reach its binding site and/or a voltage-driven conformational alteration in RyR leading to altered affinity of the receptor. Here we demonstrate that the apparent dissociation constant for the neutral ryanoid, ryanodol, decreases as the potential at the cytosolic face of the channel is made increasingly positive, and that this results from alterations to both the rates of association of ryanodol with its receptor and dissociation of ryanodol from its receptor. This observation provides very strong evidence in support of a voltage-driven alteration in ryanoid receptor affinity as the major determining factor in the influence of transmembrane holding potential on the interactions of ryanoids with RyR (Fig. 6).
While this mechanism underlies the influence of voltage on the interaction of both neutral and positively charged ryanoids, our experiments indicate that a small additional voltage-dependent effect can be observed with the positively charged 21-amino-9α-hydroxyryanodine. This may reflect the movement of the charged moiety from the cytosolic bulk solution to a binding site within the voltage drop across the channel, presumably in the conduction pathway of the channel.
Acknowledgments
This work was supported by grants from the Biotechnology and Biological Sciences Research Council, The British Heart Foundation, The Wellcome Trust, the National Institutes of Health (HL53677), and the National Science Foundation (MCB-9817605).
Footnotes
Abbreviation used in this paper: RyR, ryanodine receptor.
References
- Bazotte R.B., Pereira B., Higham S., Shoshan-Barmatz V., Kraus-Friedmann N. Effects of ryanodine on calcium sequestration in the rat liver. Biochem. Pharmacol. 1991;42:1799–1803. doi: 10.1016/0006-2952(91)90518-a. [DOI] [PubMed] [Google Scholar]
- Bhat M.B., Zhao J.Y., Takeshima H., Ma J.J. Functional calcium release channel formed by the carboxyl-terminal portion of ryanodine receptor. Biophys. J. 1997;73:1329–1336. doi: 10.1016/S0006-3495(97)78166-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bidasee K.R., Besch H.R., Jr. Structure–function relationships among ryanodine derivativespyridyl ryanodine definitively separates activation potency from high affinity. J. Biol. Chem. 1998;273:12176–12186. doi: 10.1074/jbc.273.20.12176. [DOI] [PubMed] [Google Scholar]
- Callaway C., Seryshev A., Wang J.-P., Slavik K.J., Needleman D.H., Cantu C., III, Wu Y., Jayaraman T., Marks A.R., Hamilton S.L. Localization of the high and low affinity [3H]ryanodine binding sites on the skeletal muscle Ca2+ release channel. J. Biol. Chem. 1994;269:15876–15884. [PubMed] [Google Scholar]
- Chu A., Diaz-Munoz M., Hawkes M.J., Brush K., Hamilton S.L. Ryanodine as a probe for the functional state of the skeletal muscle sarcoplasmic reticulum calcium release channel. Mol. Pharmacol. 1990;37:735–741. [PubMed] [Google Scholar]
- Deslongchamps P., Belanger A., Berney D.J.F., Borschberg H.J., Brousseau R., Doutheau A., Durand R., Katayama H., Lapalme R., Leturc D.M. The total synthesis of (+)-ryanodol. Can. J. Chem. 1990;68:115–192. [Google Scholar]
- Fleischer S., Ogunbunmi E.M., Dixon M.C., Fleer E.A.M. Localization of Ca2+ release channels with ryanodine in junctional terminal cisternae of sarcoplasmic reticulum of fast skeletal muscle. Proc. Natl. Acad. Sci. USA. 1985;82:7256–7259. doi: 10.1073/pnas.82.21.7256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foskett J.K., Wong D. Free cytoplasmic Ca2+ concentration oscillations in thapsigargin-treated parotid acinar cells are caffeine- and ryanodine-sensitive. J. Biol. Chem. 1991;266:14535–14538. [PubMed] [Google Scholar]
- Hawkes M.J., Nelson T.E., Hamilton S.L. [3H]Ryanodine as a probe of changes in the functional state of the Ca2+-release channel in malignant hyperthermia. J. Biol. Chem. 1992;267:6702–6709. [PubMed] [Google Scholar]
- Holmberg S.R.M., Williams A.J. The cardiac sarcoplasmic reticulum calcium-release channelmodulation of ryanodine binding and single-channel activity. Biochim. Biophys. Acta. 1990;1022:187–193. doi: 10.1016/0005-2736(90)90113-3. [DOI] [PubMed] [Google Scholar]
- Humerickhouse R.A., Bidasee K.R., Gerzon K., Emmick J.T., Kwon S., Sutko J.L., Ruest L., Besch H.R., Jr. High affinity C10-Oeq ester derivatives of ryanodine. Activator-selective agonists of the sarcoplasmic reticulum calcium release channel. J. Biol. Chem. 1994;269:30243–30253. [PubMed] [Google Scholar]
- Lai F.A., Erickson H.P., Rousseau E., Liu Q.-Y., Meissner G. Evidence for a Ca2+ channel within the ryanodine receptor complex from cardiac sarcoplasmic reticulum Biochem. Biophys. Res. Commun. 151 1988. 441 449a [DOI] [PubMed] [Google Scholar]
- Lai F.A., Erickson H.P., Rousseau E., Liu Q.-Y., Meissner G. Purification and reconstitution of the Ca release channel from skeletal muscle Nature. 331 1988. 315 319b [DOI] [PubMed] [Google Scholar]
- Lai F.A., Misra M., Xu I., Smith H.A., Meissner G. The ryanodine receptor–Ca2+ release channel complex of skeletal muscle sarcoplasmic reticulum. Evidence for a cooperatively coupled, negatively charged homotetramer. J. Biol. Chem. 1989;264:16776–16785. [PubMed] [Google Scholar]
- Lindsay A.R.G., Tinker A., Williams A.J. How does ryanodine modify ion-handling in the sheep cardiac sarcoplasmic reticulum Ca2+–release channel? J. Gen. Physiol. 1994;104:425–447. doi: 10.1085/jgp.104.3.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lindsay A.R.G., Williams A.J. Functional characterisation of the ryanodine receptor purified from sheep cardiac muscle sarcoplasmic reticulum. Biochim. Biophys. Acta. 1991;1064:89–102. doi: 10.1016/0005-2736(91)90415-5. [DOI] [PubMed] [Google Scholar]
- McGarry S.J., Williams A.J. Activation of the sheep cardiac sarcoplasmic reticulum Ca2+–release channel by analogues of sulmazole. Br. J. Pharmacol. 1994;111:1212–1220. doi: 10.1111/j.1476-5381.1994.tb14874.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meissner G., El-Hashem A. Ryanodine as a functional probe of the skeletal muscle sarcoplasmic reticulum Ca2+ release channel. Mol. Cell. Biochem. 1992;114:119–123. doi: 10.1007/BF00240306. [DOI] [PubMed] [Google Scholar]
- Miller C. Open-state substructure of single chloride channels from Torpedo electroplax. Phil. Trans. R. Soc. Lond. B Biol. Sci. 1982;299:401–411. doi: 10.1098/rstb.1982.0140. [DOI] [PubMed] [Google Scholar]
- Moczydlowski E. Single-channel enzymology. In: Miller C., editor. Ion Channel Reconstitution. Plenum Publishing Corp; New York, NY: 1986. pp. 75–113. [Google Scholar]
- Murayama T., Oba T., Katayama E., Oyamada H., Oguchi K., Kobayashi M., Otsuka K., Ogawa Y. Further characterization of the type 3 ryanodine receptor (RyR3) purified from rabbit diaphragm. J. Biol. Chem. 1999;274:17297–17308. doi: 10.1074/jbc.274.24.17297. [DOI] [PubMed] [Google Scholar]
- Needleman D.H., Hamilton S.L. Factors influencing [3H]ryanodine binding to the skeletal muscle Ca2+ release channel. Anal. Biochem. 1997;248:173–179. doi: 10.1006/abio.1997.2125. [DOI] [PubMed] [Google Scholar]
- Pessah I.N., Francini A.O., Scales D.J., Waterhouse A.L., Casida J.E. Calcium-ryanodine receptor complex. Solubilization and partial characterization from skeletal muscle junctional sarcoplasmic reticulum vesicles. J. Biol. Chem. 1986;261:8643–8648. [PubMed] [Google Scholar]
- Rousseau E., Smith J.S., Meissner G. Ryanodine modifies conductance and gating behavior of single Ca2+ release channel. Am. J. Physiol. 1987;253:C364–C368. doi: 10.1152/ajpcell.1987.253.3.C364. [DOI] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A.J. Mechanisms of caffeine activation of single calcium-release channels of sheep cardiac sarcoplasmic reticulum. J. Physiol. 1990;423:425–439. doi: 10.1113/jphysiol.1990.sp018031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sitsapesan R., Williams A.J. Gating of the native and purified cardiac SR Ca2+–release channel with monovalent cations as permeant species. Biophys. J. 1994;67:1484–1494. doi: 10.1016/S0006-3495(94)80622-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutko J.L., Airey J.A., Welch W., Ruest L. The pharmacology of ryanodine and related compounds. Pharmacol. Rev. 1997;49:53–98. [PubMed] [Google Scholar]
- Swann K. Different triggers for calcium oscillations in mouse eggs involve a ryanodine-sensitive calcium store. Biochem. J. 1992;287:79–84. doi: 10.1042/bj2870079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanna B., Welch W., Ruest L., Sutko J.L., Williams A.J. Interactions of a reversible ryanoid (21-amino-9α-hydroxy-ryanodine) with single cardiac ryanodine receptor channels. J. Gen. Physiol. 1998;112:55–69. doi: 10.1085/jgp.112.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tinker A., Sutko J.L., Ruest L., Deslongchamps P., Welch W., Airey J.A., Gerzon K., Bidasee K.R., Besch H.R., Jr., Williams A.J. Electrophysiological effects of ryanodine derivatives on the sheep cardiac sarcoplasmic reticulum calcium-release channel. Biophys. J. 1996;70:2110–2119. doi: 10.1016/S0006-3495(96)79777-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ullmer C., Boddeke H.G.W.M., Schmuck K., Lübbert H. 5-HT2B receptor-mediated calcium release from ryanodine-sensitive intracellular stores in human pulmonary artery endothelial cells. Br. J. Pharmacol. 1996;117:1081–1088. doi: 10.1111/j.1476-5381.1996.tb16700.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walz B., Baumann O., Zimmermann B., Ciriacy-Wantrup E.V. Caffeine- and ryanodine-sensitive Ca2+-induced Ca2+ release from the endoplasmic reticulum in honeybee photoreceptors. J. Gen. Physiol. 1995;105:537–567. doi: 10.1085/jgp.105.4.537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W., Ahmad S., Airey J.A., Gerzon K., Humerickhouse R.A., Besch H.R., Jr., Ruest L., Deslongchamps P., Sutko J.L. Structural determinants of high-affinity binding of ryanoids to the vertebrate skeletal muscle ryanodine receptora comparative molecular field analysis. Biochemistry. 1994;33:6074–6085. doi: 10.1021/bi00186a006. [DOI] [PubMed] [Google Scholar]
- Welch W., Sutko J.L., Mitchell K.E., Airey J.A., Ruest L. The pyrrole locus is the major orienting factor in ryanodine binding. Biochemistry. 1996;35:7165–7173. doi: 10.1021/bi9527294. [DOI] [PubMed] [Google Scholar]
- Welch W., Williams A.J., Tinker A., Mitchell K.E., Deslongchamps P., Lamothe J., Gerzon K., Bidasee K.R., Besch H.R., Jr., Airey J.A., Sutko J.L., Ruest L. Structural components of ryanodine responsible for modulation of sarcoplasmic reticulum calcium channel function. Biochemistry. 1997;36:2939–2950. doi: 10.1021/bi9623901. [DOI] [PubMed] [Google Scholar]
- Wiesner K. The structure of ryanodine. Adv. Org. Chem. 1972;8:295–316. [Google Scholar]
- Witcher D.R., McPherson P.S., Kahl S.D., Lewis T., Bentley P., Mullinnix M.J., Windass J.D., Campbell K.P. Photoaffinity labeling of the ryanodine receptor/Ca2+ release channel with an azido derivative of ryanodine. J. Biol. Chem. 1994;269:13076–13079. [PubMed] [Google Scholar]
- Zhao M.C., Li P., Li X.L., Zhang L., Winkfein R.J., Chen S.R.W. Molecular identification of the ryanodine receptor pore-forming segment. J. Biol. Chem. 1999;274:25971–25974. doi: 10.1074/jbc.274.37.25971. [DOI] [PubMed] [Google Scholar]