Abstract
The roles of Ser775 and Glu779, two amino acids in the putative fifth transmembrane segment of the Na,K -ATPase α subunit, in determining the voltage and extracellular K + (K + o) dependence of enzyme-mediated ion transport, were examined in this study. HeLa cells expressing the α1 subunit of sheep Na,K -ATPase were voltage clamped via patch electrodes containing solutions with 115 mM Na+ (37°C). Na,K -pump current produced by the ouabain-resistant control enzyme (RD), containing amino acid substitutions Gln111Arg and Asn122Asp, displayed a membrane potential and K + o dependence similar to wild-type Na,K -ATPase during superfusion with 0 and 148 mM Na+-containing salt solutions. Additional substitution of alanine at Ser775 or Glu779 produced 155- and 15-fold increases, respectively, in the K + o concentration that half-maximally activated Na,K -pump current at 0 mV in extracellular Na+-free solutions. However, the voltage dependence of Na,K -pump current was unchanged in RD and alanine-substituted enzymes. Thus, large changes in apparent K + o affinity could be produced by mutations in the fifth transmembrane segment of the Na,K -ATPase with little effect on voltage-dependent properties of K + transport. One interpretation of these results is that protein structures responsible for the kinetics of K + o binding and/or occlusion may be distinct, at least in part, from those that are responsible for the voltage dependence of K + o binding to the Na,K -ATPase.
Keywords: Na,K -pump current; HeLa cells; voltage clamp; point mutation
INTRODUCTION
Active transport of Na+ and K + across the cell membrane of mammalian cells by the Na,K -ATPase involves a series of reaction steps that include ion binding, occlusion, and release. Each ion transport reaction is associated with specific conformational changes in the enzyme that are driven by the free energy derived from ATP hydrolysis (Glynn 1985). While these reactions have been extensively characterized, our understanding of the protein structures participating in these reactions remains sketchy.
After extensive proteolysis of the Na,K -ATPase, the resulting membrane-embedded protein fragments, referred to as “19-kD fragments” are still capable of occluding the K + congener, Rb+, and Na+ (Karlish et al. 1990). More recent studies suggest that several transmembrane segments of the enzyme participate in these ion binding and occlusion reactions (Lingrel and Kuntzweiler 1994; Andersen and Vilsen 1995; Liu and Askari 1997; Shainskaya et al. 1998). In fact, the resistance of 19-kD fragments to additional proteolysis or thermal inactivation is ion sensitive (Or et al. 1993; Shainskaya and Karlish 1994), with the disposition of the putative fifth and sixth transmembrane regions of the α subunit, often referred to as the H5–H6 hairpin, being particularly dependent on the presence of K + or Rb+ (Lutsenko et al. 1995; Shainskaya et al. 1998). Mutagenesis of specific amino acid residues in the H5–H6 hairpin region also has large effects on the apparent affinity for K +-dependent activation of enzyme function (Argüello and Lingrel 1995; Blostein et al. 1997) and K + binding (Nielsen et al. 1998; Pedersen et al. 1998). These data suggest that the fifth and sixth transmembrane domains of the Na,K -ATPase may be directly involved in ion coordination and occlusion reactions.
The involvement of two amino acids in the H5–H6 region, Ser775 and Glu779, in determining the ion transport properties of the Na,K -ATPase has been examined in several laboratories. Coordination of the side-chain carboxylic acid on Glu779 with the coumarin derivative, DEAC, inactivates the enzyme and inhibits ion binding (Argüello and Kaplan 1994). Amino acid substitutions of Glu779 have been reported to produce small changes in apparent affinity for K + activation (Feng and Lingrel 1995; Vilsen 1995; Argüello et al. 1996; Koster et al. 1996); however, the replacement Glu779Ala leads to a marked increase in electrogenic Na+–Na+ exchange, dramatic changes in the membrane potential (V M) dependence of Na,K -pump current (Argüello et al. 1996), and alterations in ion occlusion (Nielsen et al. 1998). Amino acid substitutions at Ser775 have also been reported to greatly decrease the apparent K + affinity for activation of Na,K -ATPase activity (Argüello and Lingrel 1995) and K + influx (Blostein et al. 1997), as well as to disrupt ion occlusion (Blostein et al. 1997; Pedersen et al. 1998). These data raise intriguing questions regarding the role of Ser775 and Glu779 in ion binding and occlusion reactions, as well as their possible role in V M-dependent reaction steps associated with extracellular ion binding.
To address these questions, enzyme function was studied in HeLa cells expressing heterologous Na,K -ATPase carrying point mutations at Ser775 and Glu779. The effect of amino acid substitutions at these residues on K +-dependent reactions was examined by determining the extracellular K + (K + o) and V M dependence of steady state Na,K -pump current. In the accompanying paper (Peluffo et al. 2000), extracellular Na+ (Na+ o)-dependent reactions are investigated during electrogenic Na+–Na+ exchange in enzyme containing the amino acid substitution Glu779Ala. The results of both studies are used to explain our previous observations (Argüello et al. 1996; Peluffo et al. 1997) that amino acid substitutions at Glu779 can greatly alter the V M dependence of Na,K -pump current.
MATERIALS AND METHODS
Mutagenesis and Cell Culture
HeLa cells expressing the α subunit of sheep Na,K -ATPase with amino acid substitutions Ser775Ala, Glu779Ala, and Glu779Gln were produced in a ouabain-resistant form (RD) as previously described (Price and Lingrel 1988; Argüello and Lingrel 1995; Feng and Lingrel 1995). Cells were grown on glass coverslips in Dulbecco's modified Eagle's medium (DMEM) containing 10% calf serum, 5.4 mM K Cl, and 1 μM ouabain until ∼50% confluence was observed. Cells expressing Ser775Ala-substituted enzyme were unable to grow in DMEM unless extracellular K Cl concentration was increased to 20 mM (Argüello and Lingrel 1995).
Electrophysiology and Solutions
Glass coverslips containing HeLa cells were placed in an experimental chamber at 37°C on the stage of an inverted microscope and superfused with a HEPES-buffered Tyrode's solution (Argüello et al. 1996). Cells were whole-cell voltage-clamped using single patch electrodes (∼1.5 MΩ), back-filled with a K +-free, high-Na+ intracellular electrode solution containing (mM): 85 sodium sulfamate, 20 TEA chloride, 3 MgCl2, 10 MgATP, 5 sodium pyruvate, 5 Tris2-creatine phosphate, 5.5 dextrose, 10 EGTA/Tris, 10 HEPES, pH 7.40 with NaOH, 22°C. Total Na+ concentration in the patch electrode solution was ∼115 mM. After establishing a gigaohm seal, the cells were superfused with a solution containing (mM): 145 tetramethylammonium (TMA) chloride, 2.3 MgCl2, 0.2 CdCl2, 0.5 4,4′-diisothiocyanato-stilbene-2,2′-disulfonic acid (DIDS), 5.5 dextrose, 10 HEPES/Tris, pH 7.35, 22°C. DIDS was added to block an outwardly rectifying chloride current. In Na+-containing superfusion solutions, TMA chloride was substituted with equimolar NaCl and 2 mM BaCl2 was included. TEA, Ba2+, and Cd2+ were added to block contaminating ionic currents (Gadsby and Nakao 1989; Argüello et al. 1996). All superfusion solutions contained 1 μM ouabain to block endogenous Na,K -ATPase (Jewell and Lingrel 1991). Voltage-clamped cells were exposed to these blocking agents for 5 min before additional manipulations. Na,K -pump current due to heterologous Na,K -ATPase activity was defined as the difference current measured in the presence of 1 μM and 10 mM ouabain.
Current data were normalized to total capacitance (picoamperes/picofarad) calculated from the integral of current elicited by 5-mV depolarizations. Whole-cell currents and voltage signals were sampled at 2,500 Hz and low-pass filtered at 875 Hz.
Protocols for Measurement of Na,K -pump Current
To activate the Na,K -pump, the K Cl concentration in the superfusion solution was increased from 0 to 0.02–80 mM ([K +] + [TMA] = 145 mM). In a typical experiment, the Na,K -pump was activated for 25 s with a K + o-containing superfusion solution separated by 1.5–2 min in K + o-free solution to avoid intracellular Na+ depletion. Activation of heterologous Na,K -ATPase was then measured as the K + o-sensitive difference current. However, in some experiments, ouabain concentration in K + o-containing solutions was increased from 1 μM to 10 mM to inhibit heterologous enzyme. To study the V M dependence of the K + o-activated difference current, a voltage-clamp protocol was applied before, during, and after superfusion with K + o-containing solutions. The V M dependence of ouabain-sensitive current was determined before and 2 min after increasing ouabain concentration. During these protocols, clamp pulses of 100-ms duration were elicited from the holding potential of −40 mV to various potentials over the range of −100 to +60 mV at 2 Hz. Current–voltage relationships obtained before the application of K + and after returning to K +-free solution were usually quite similar. Nonetheless, the average of the two bracketing I-V relationships obtained in K +-free solution was subtracted from that obtained in K +-containing solution. In those experiments using 40 or 80 mM K + o, application of K + o was accompanied by the development of an observable junction potential. To avoid junction potentials, Na,K -pump current in these experiments was measured as the outward current inhibited by increasing ouabain concentration from 1 μM to 10 mM in the presence of K + o.
Typically, an experiment lasted ∼15–25 min with only small (if any) changes in holding current. Experiments were typically begun and completed with measurements of K + o-sensitive current in the presence of saturating K + o so that any rundown of K + o-sensitive current density (0–40%) could be corrected by linear interpolation. It was notable that the V M dependence of this current did not change as a result of rundown.
Curve Fitting
The apparent affinity for K + o was calculated as the concentration that produced half-maximal activation of Na,K -pump current (K 0.5) using a Hill Equation {I=I max1+K 0.5K+ o γK, where γK is the Hill coefficient for K + o}. The V M dependence of K 0.5 was analyzed with a pseudo three-state model that assumes that a V M-dependent reaction occurs during K + transport. With this model, the fraction of the membrane dielectric dissipated during V M-dependent reactions involving K + o (λK) and Na+ o (λNa) could be determined by the following equation adapted from Sagar and Rakowski 1994:
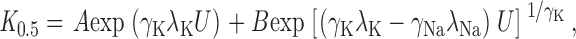 |
1 |
where U = V M F/RT, γNa is the Hill coefficient for Na+ o, and the parameters A and B are lumped V M-independent rate constants. At 0 mV, it is clear that K 0.5 (K 0 0.5) equalsA+B 1γK. The parameter B is proportional to Na+ o concentration (Sagar and Rakowski 1994) so that, in the absence of Na+ o, further simplifies to:
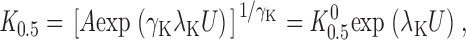 |
2 |
where K 0 0.5 is equal to A 1γK. These equations do not imply a particular mechanism for the V M-dependent step, aside from the assumption that ion binding to the enzyme is rapid (Hansen et al. 1981; Läuger 1991).
Curve-fitting was carried out using commercial software (SigmaPlot; Jandel Scientific). When the error for a given variable was heteroscedastic (unequal error variance), curve fitting was carried out with statistical weights proportional to the inverse of SEM squared.
Statistics
Data are displayed as mean ± SEM. One-way analysis of variance and linear regressions were performed with commercial software. Pair-wise comparisons were performed using a Student's t test with the level of significance set at P < 0.05.
RESULTS
Identification of Na,K -pump Current in HeLa Cells Expressing Heterologous Enzyme
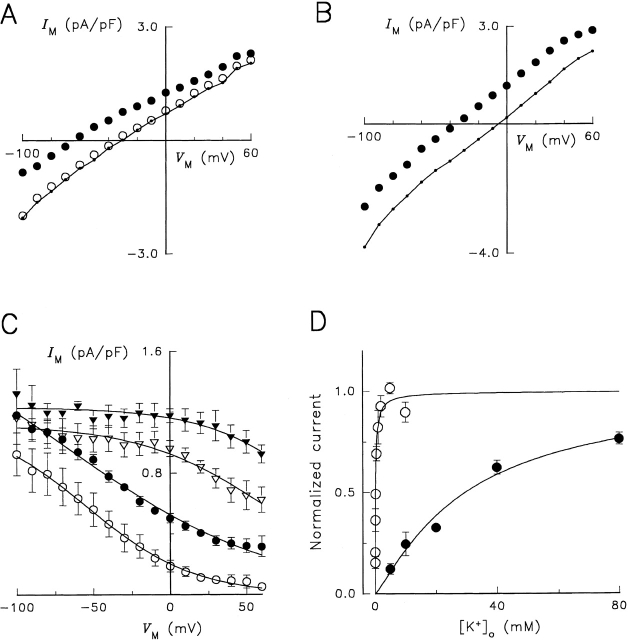
Cells were voltage clamped with wide-tipped patch electrodes containing 115 mM Na+ and superfused in a Na+- and K +-free solution containing 1 μM ouabain to block endogenous Na,K -ATPase. To activate the heterologous Na,K -pump, K + concentration in the superfusion solution was increased from 0 to 5 mM (Fig. 1 A). The increase in K + o concentration was accompanied by a maintained outward shift in current. After returning to K + o-free solution for ∼2 min, the cell was exposed to a K + o-containing solution that included 10 mM ouabain to block activity of the heterologous enzyme. After a brief outward current shift, indicating the time of the solution change, current returned to basal values in the continued presence of 5 mM K + o. Thus, 10 mM ouabain appeared to block K + o-activated current at the holding potential. The arrows in Fig. 1 A indicate the times at which I-V relationships were generated by producing voltage clamp pulses from −100 to +60 mV. In the presence of 1 μM ouabain, increasing K + o concentration to 5 mM produced an outward shift in the steady state I-V relationship at each V M; however, in the presence of 10 mM ouabain (for 2 min), a K + o-dependent shift in current was not observed (Fig. 1 B). In some cells, the converse experiment was performed in which ouabain concentration was increased from 1 μM to 10 mM in the continuous presence of elevated K + o. The amplitude of this ouabain-sensitive current was the same as the K + o-activated current (not shown). The finding that the I-V relationships obtained in the presence of 10 mM ouabain/K +-containing solution practically superimposed on the I-V relationships in K +-free solution enabled us to define K + o-activated current as Na,K -pump current for RD enzyme.
Figure 1.
Electrophysiological characterization of RD control enzyme. (A) Continuous current record during typical experimental manipulations. The cell was voltage clamped at the holding potential of −40 mV and superfused with the Na+- and K +-free, TMA-containing solution described in materials and methods. After 5 min in the presence of 1 μM ouabain, the concentration of K + o was increased to 5 mM for 25 s. K + o-free solution followed for 1–2 min before switching to a solution containing 5 mM K + plus 10 mM ouabain for 2 min. Current was low-pass filtered at 10 Hz and sampled at 25 Hz. Arrows over the trace indicate when voltage clamp pulses from −100 to +60 mV were elicited. (B) Steady state I-V relationships in the presence of 0 K + o (○), 5 mM K + o (•), and 5 mM K + o plus 10 mM ouabain (•–•). Symbols represent the average current measured during the last 50 ms of 100-ms-long voltage-clamp pulses. (Inset) Superimposed difference current records calculated by subtracting currents measured in K +-free solution from those in 5 mM K + o-containing solution. Traces were recorded during voltage-clamp pulses, as indicated by the brief current spikes, to −100, −60, −20, 0, +20, and +60 mV. Current records were not averaged. The dashed line represents zero-current level. Cell capacitance was 40 pF. (C) V M and K + o dependence of Na,K -pump current. The maneuvers described in A and B were applied during superfusion with solutions containing 0.02 (○), 0.2 (•), 1 (▿), and 5 mM K Cl (▾). K + o-activated difference currents were obtained for each K + o concentration. Symbols represent the mean ± SEM of 14 experiments. Curves through the symbols are best-fit functions to an equation derived for a pseudo two-state model of the Na,K -ATPase (see equation A20 in Sagar and Rakowski 1994).
Extracellular K + and VM Dependence of Na,K -pump Current for RD Enzyme
The magnitude of the 5 mM K + o-activated outward current shift in cells expressing RD enzyme, calculated as the K + o-sensitive difference current at various membrane potentials (Fig. 1 B, inset), showed little V M dependence between −100 and +60 mV. To study the effect of V M and extracellular ion concentration on Na,K -pump current in more detail, the protocol described above in Fig. 1 A was repeated at eight K + o concentrations between 0.02 and 10 mM. Fig. 1 C shows Na,K -pump current at selected K + o concentrations as a function of V M. As K + o concentration was decreased, current density decreased and became dependent on V M. The negative slope in the I-V relationship for Na,K -pump current at lower K + o concentrations is similar to the V M dependence of Na,K -pump current observed with wild-type Na,K -ATPase (Rakowski et al. 1991; Sagar and Rakowski 1994; Berlin and Peluffo 1997).
To further characterize the effect of K + o and V M on Na,K -pump current in cells expressing RD enzyme, data similar to those in Fig. 1 C were fitted with a Hill equation at each V M. The maximum current density calculated with this fitting procedure showed little variation with V M and, as a result, this parameter was averaged over the range of V M tested to yield a value of 1.15 ± 0.16 pA/pF (n = 17). Given the maximal turnover rate previously calculated for RD enzyme (Argüello et al. 1996), this current density corresponds to a functional enzyme expression level of ∼500 μm−2. The apparent affinity for K + o activation of Na,K -pump current, defined as the K + o concentration that produced half-maximal activation of Na,K -pump current (K 0.5), is plotted in Fig. 2 as a function of V M (•). With more positive V M, the K 0.5 for K + o increased progressively, similar to previous reports with wild-type enzyme (Sagar and Rakowski 1994). Finally, the Hill coefficient for K + o activation of Na,K -pump current (γK) was not significantly different over the range of V M tested. As a result, γK was averaged over all V M to yield a value of 0.79 ± 0.08 (n = 17), similar to γK reported previously for K + o activation of wild-type Na,K -pump current in Na+ o-free conditions (Sagar and Rakowski 1994).
Figure 2.
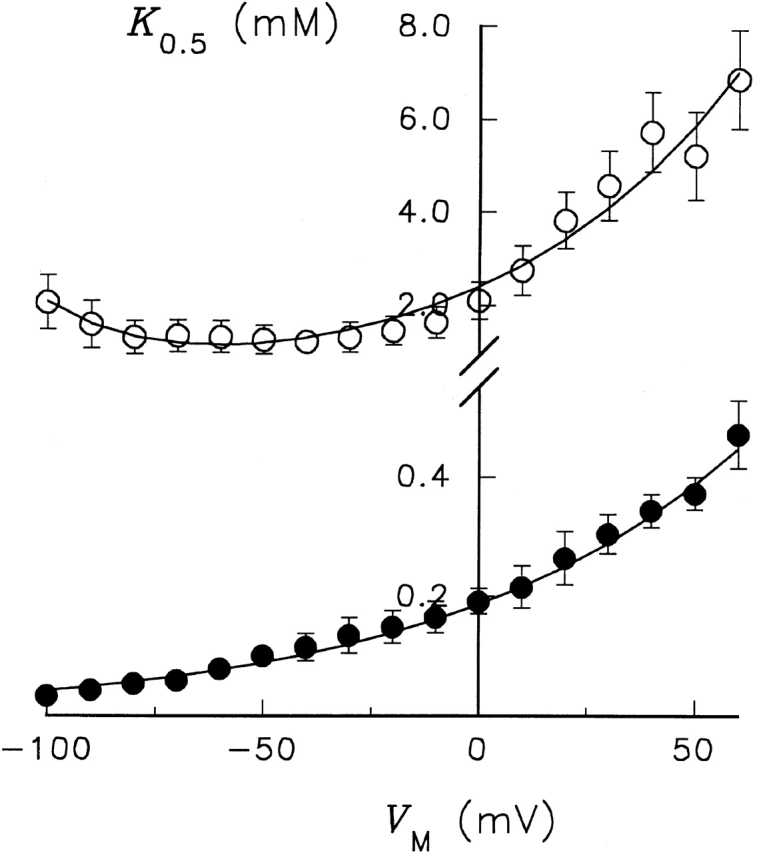
Effect of Na+ o on the membrane potential dependence of K 0.5 for Na,K -pump current activation with RD enzyme. K 0.5 values were obtained by fitting a Hill equation to data similar to those shown in Fig. 1 C and data taken from Argüello et al. 1996. The best-fit functions (solid curves) for K 0.5 vs. V M in the presence of 0 (•) and 148 (○) mM Na+ o were calculated with and , respectively. Fitting parameters are given in the text.
The most parsimonious description of Na,K -pump current shown in Fig. 1 C is a pseudo two-state model that assumes a V M-dependent step occurs during K + o transport. Using this model, the data showing the V M dependence of K 0.5 in Na+ o-free solutions (Fig. 2) could be fitted with . With this fitting procedure, K 0.5 at 0 mV (K 0 0.5) was calculated to be 0.19 ± 0.01 mM (n = 14) and the fractional distance for K + o binding in the membrane dielectric (λK) was equal to 0.39 ± 0.02 (n = 14). These values are quite similar to those observed for wild-type Na,K -ATPase in cardiac myocytes (Berlin and Peluffo 1997).
In wild-type Na,K -ATPase, the Na,K -pump is activated at higher K + o concentrations in Na+ o-containing solutions than in Na+ o-free solutions (Nakao and Gadsby 1989; Sagar and Rakowski 1994). To determine whether RD enzyme showed similar properties, the V M dependence of Na,K -pump current activation by K + o was compared in Na+ o-free and Na+ o-containing solutions. Data for Na,K -pump current density measured in 148 mM Na+ o-containing solutions with various K + o concentrations were taken from Argüello et al. 1996 and fitted by the same procedure described for the data in Fig. 1C to make this comparison. The value of γK , averaged over all V M, was found to be 1.00 ± 0.09 (n = 17). A larger value of γK in Na+ o-containing solutions has previously been reported for Na,K -pump current recorded in Xenopus oocytes (Sagar and Rakowski 1994). Values of K 0.5 calculated with the Hill equation are plotted in Fig. 2 (○). As expected from previous reports with wild-type Na,K -ATPase, the V M dependence of K 0.5 in Na+-containing solution had a “U” shape with a minimum at approximately −60 mV, indicative of competition between Na+ and K + at extracellular ion binding sites located in the membrane dielectric (Sagar and Rakowski 1994). Using the pseudo three-state model, these data were fitted with , where γK was fixed at the average value determined above to yield a value of K 0 0.5 equal to 2.41 ± 0.18 mM. Thus, the apparent K + o affinity was decreased in Na+ o-containing solutions and the magnitude of the change in K 0 0.5 was comparable with that previously reported in wild-type enzyme (Nakao and Gadsby 1989; De Weer 1992; Sagar and Rakowski 1994).
The values of additional parameters derived from fitting data in Fig. 2 with were 0.48 ± 0.08 and 1.57 ± 0.45 (n = 14) for the products γK λK and γNaλNa, respectively. Because γK is determined independently, the portion of the membrane dielectric dissipated during K + o-dependent reactions, λK , was calculated to equal 0.48 ± 0.09. This value of λK was not significantly different than λK calculated in Na+ o-free solutions. Thus, K + o-dependent reaction steps dissipate between a third and a half of the membrane electric field in RD enzyme.
Effects of Glu779 Substitutions on the K + o Dependence of Na,K -pump Current
The V M dependence of Na,K -pump current in HeLa cells expressing Na,K -ATPase enzyme variant with the substitution Glu779Ala (Glu779Ala enzyme) is quite different than in cells expressing RD enzyme (Argüello et al. 1996). In Na+ o-containing solution, K + o-activated current in cells expressing Glu779Ala enzyme displays little V M dependence over a broad range of membrane potentials (−100 to +60 mV) and K + o concentrations (1–50 mM). In addition, the apparent affinity for K + o activation of Na,K -pump current (at 0 mV) is reduced threefold. These data might suggest that both the V M dependence of enzyme reactions and the kinetics of K + o-dependent reactions are altered by this amino acid substitution.
Interpretation of these results was complicated because our experiments were conducted in Na+- and K +-containing superfusion solutions. As a result, interactions between Na+ and K + at extracellular sites could not be ruled out as being responsible for the observed changes in V M and K + o dependence of Na,K -pump current. In addition, under the conditions of these previous experiments, K + o-activated and ouabain-sensitive currents produced by Glu779Ala enzyme were not the same due to the presence of a significant component of electrogenic Na+–Na+ exchange (Argüello et al. 1996). Thus, the reported changes in the V M dependence of K + o-activated current in Glu779Ala enzyme could actually reflect the contribution of this unusually large Na+ exchange process. To circumvent these two issues, the present experiments were conducted in Na+ o-free superfusion solutions.
We have previously shown that, in cells expressing Glu779Ala enzyme, electrogenic Na+–Na+ exchange appears to be lost in Na+ o-free solutions (Argüello et al. 1996). Fig. 3 A shows this result over the full range of V M tested. The I-V relationship measured with 20 mM K + o and 1 μM ouabain was shifted outward with respect to the I-V relationship observed in K + o-free solution. On the other hand, the I-V relationship measured in the presence of 20 mM K + o and 10 mM ouabain almost superimposed on the I-V relationship measured in K + o-free conditions. As before, the converse experiment, increasing ouabain concentration in the presence of 20 mM K + o, gave comparable results (not shown). Thus, in Na+ o-free conditions, K + o-activated and ouabain-inhibitable currents appeared to be quite similar so that Na,K -pump current in Na+ o-free solutions could be measured as a K + o-sensitive difference current with Glu779Ala enzyme.
Figure 3.
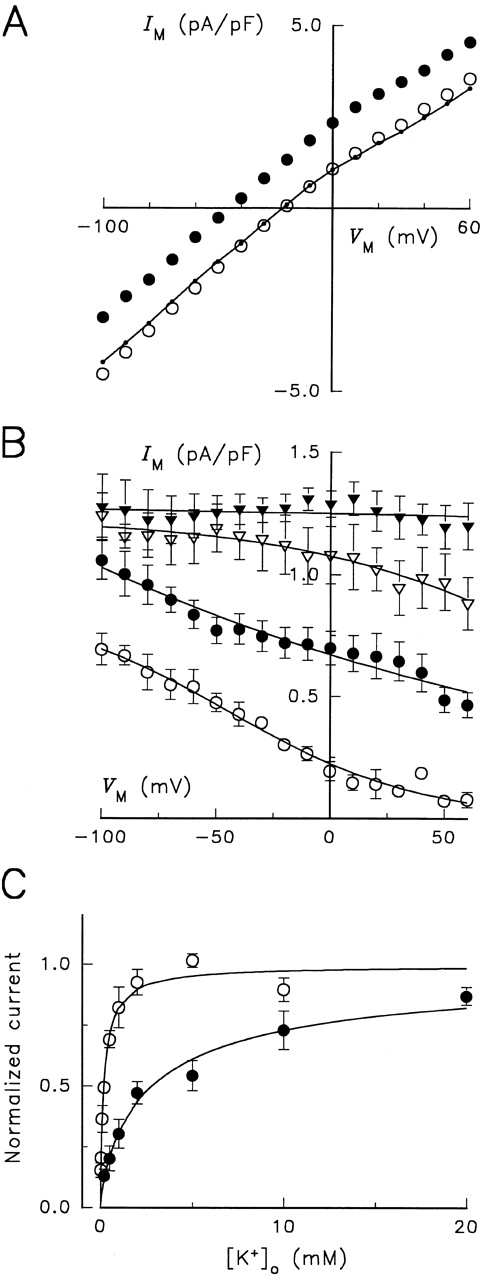
Membrane potential and K + o dependence of Na,K -pump current in Glu779Ala substituted enzyme under Na+ o-free conditions. (A) Steady state I-V relationships from a cell superfused with solutions containing 0 mM K + o (○), 20 mM K + o (•), and 20 mM K + o plus 10 mM ouabain (•–•). Cell capacitance was 81 pF. (B) V M dependence of Na,K -pump current activated by K + o. Cells were superfused with solutions containing 0.2 (○), 2 (•), 10 (▿), and 20 mM K Cl (▾), and K + o-activated difference currents were obtained (n = 12). Continuous curves represent fitting of an equation derived from a pseudo two-state model as indicated in the legend to Fig. 1. (C) K + o stimulation of Na,K -pump current at 0 mV in RD control (○) and Glu779Ala-substituted (•) enzyme. Data were normalized to the value of maximal current amplitude calculated by fitting a Hill equation (continuous curves) to each data set. The best-fit parameters for RD enzyme were K 0.5 = 0.19 ± 0.02 mM and γK = 0.92 ± 0.08 (n = 14). The fitted parameters for Glu779Ala enzyme are given in the text.
Fig. 3 B shows Na,K -pump currents measured in the presence of a variety of K + o concentrations with cells expressing Glu779Ala enzyme. With 20 mM K + o, Na,K -pump current showed little V M dependence between −100 and +60 mV. The maximum current density averaged over all V M, 1.48 ± 0.18 pA/pF (n = 17), was not significantly different than that measured in cells expressing RD enzyme. Given that the in vitro turnover rates of Glu779Ala and RD enzymes are similar (Argüello et al. 1996), these data suggest that the density of expression of heterologous enzyme is also similar.
In the presence of lower K + o concentrations, Na,K -pump current density decreased and, more importantly, also showed a monotonic decrease with increasing V M (Fig. 3 B), similar to RD enzyme. Thus, Glu779Ala enzyme displayed V M-dependent behavior in Na+ o-free conditions, a clear indication that the electrogenic properties of K + o-dependent reactions were preserved in this variant enzyme.
The K + o concentration needed to activate maximal Na,K -pump current in cells expressing Glu779Ala enzyme was higher than in cells expressing RD enzyme. This point is illustrated in Fig. 3 C, where current density at 0 mV is plotted as a function of K + o and fitted with a Hill equation. The K 0.5 for current activation was calculated to be 2.79 ± 0.27 mM and γK equal to 0.78 ± 0.06 (n = 12). This value of K 0.5 is ∼15-fold higher than the value for RD enzyme, an indication that alterations in the side chain of residue 779 can produce large changes in the apparent affinity for K + o.
To gain some understanding of the structural changes that underlie the marked effect of the substitution Glu779Ala on the K 0.5 for K + o, the properties of Na,K -pump current with a more conservative substitution, Glu779Gln, were examined. Like Glu779Ala, this mutation removes the carboxylic moiety in the side chain of residue 779; however, the bulk of the side chain group is maintained. Fig. 4 A shows a typical set of I-V relationships measured from a cell expressing Glu779Gln enzyme during superfusion in Na+ o-free solutions with 0 and 5 mM K + o and with 5 mM K + o in the presence of 10 mM ouabain. As with RD enzyme, the K + o-dependent outward shift in the I-V relationship was entirely blocked in the presence of 10 mM ouabain, so that K + o-activated current was again used to measure Na,K -pump current.
Figure 4.
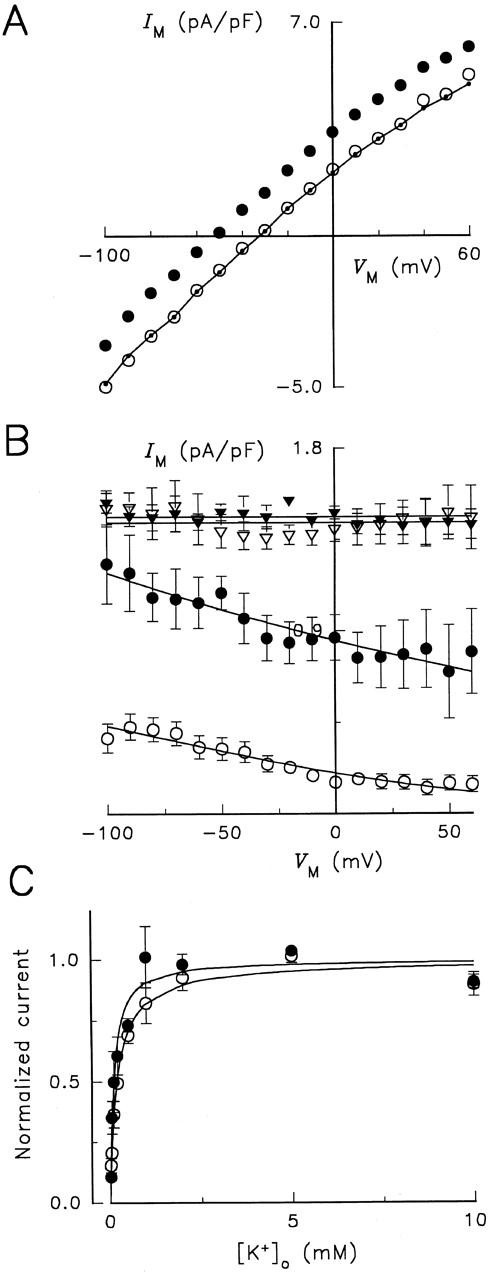
Membrane potential and K + o dependence of Na,K -pump current in Glu779Gln-substituted enzyme under Na+ o-free conditions. (A) Steady state I-V relationships from a cell superfused with solutions containing 0 mM K + o (○), 5 mM K + o (•), and 5 mM K + o plus 10 mM ouabain–containing solution (•–•). Cell capacitance was 52 pF. (B) V M dependence of Na,K -pump current activated by K + o. Cells were superfused with solutions containing 0.02 (○), 0.2 (•), 2 (▿), and 5 mM K Cl (▾), and K + o-activated difference currents were obtained in 13 cells. Continuous curves represent fitting of an equation derived from a pseudo two-state model as indicated in the legend for Fig. 1. (C) K + o stimulation of Na,K -pump current at 0 mV in RD control (○) and Glu779Gln-substituted (•) enzyme. Best-fit functions derived from Hill equations are shown as continuous curves for normalized current data. The fitting parameters are given in the text and in the legend of Fig. 3 for RD enzyme.
Fig. 4 B shows the V M and K + o dependence of Na,K -pump current measured in Na+ o-free conditions with HeLa cells expressing the Glu779Gln enzyme variant. At the highest K + o concentration, Na,K -pump current was V M independent; however, as with RD enzyme, current became V M dependent as K + o concentration was lowered. The range of K + o concentrations that activated measurable Na,K -pump current was similar to RD enzyme. This point was confirmed by plotting current density at 0 mV vs. K + o concentration (Fig. 4 C). Fitting the data with a Hill equation showed that the K 0.5 was 0.11 ± 0.02 mM with γK equal to 1.03 ± 0.15 (n = 13). These data suggest that the carboxyl moiety per se is not critical for determining the apparent K + o affinity of the enzyme. However, the 25-fold range of K 0.5 values observed with enzymes containing substitutions at residue 779 may suggest that changes in either the bulk of the side chain or loss of the carbonylic oxygen alter K + o binding reactions.
The maximum current density averaged over all V M was calculated to be 1.43 ± 0.15 pA/pF (n = 17). This current density was also not significantly different than that measured in cells expressing RD enzyme.
Effects of Ala Substitution at Ser775 on the K + o Dependence of Na,K -pump Current
The change in K 0.5 for K + o observed with Glu779Ala enzyme is much larger than in previous reports (Feng and Lingrel 1995; Vilsen 1995; Argüello et al. 1996; Koster et al. 1996). Thus, the present results might suggest that Glu779 plays a more important role in K + o binding reactions than previously thought. However, the indirect nature of our experiments with respect to K + o binding led us to examine another amino acid in the putative fifth transmembrane region of the Na,K -ATPase α subunit, Ser775, mutations of which have been reported to have dramatic effects on K + binding and/or occlusion (Argüello and Lingrel 1995; Blostein et al. 1997; Pedersen et al. 1998).
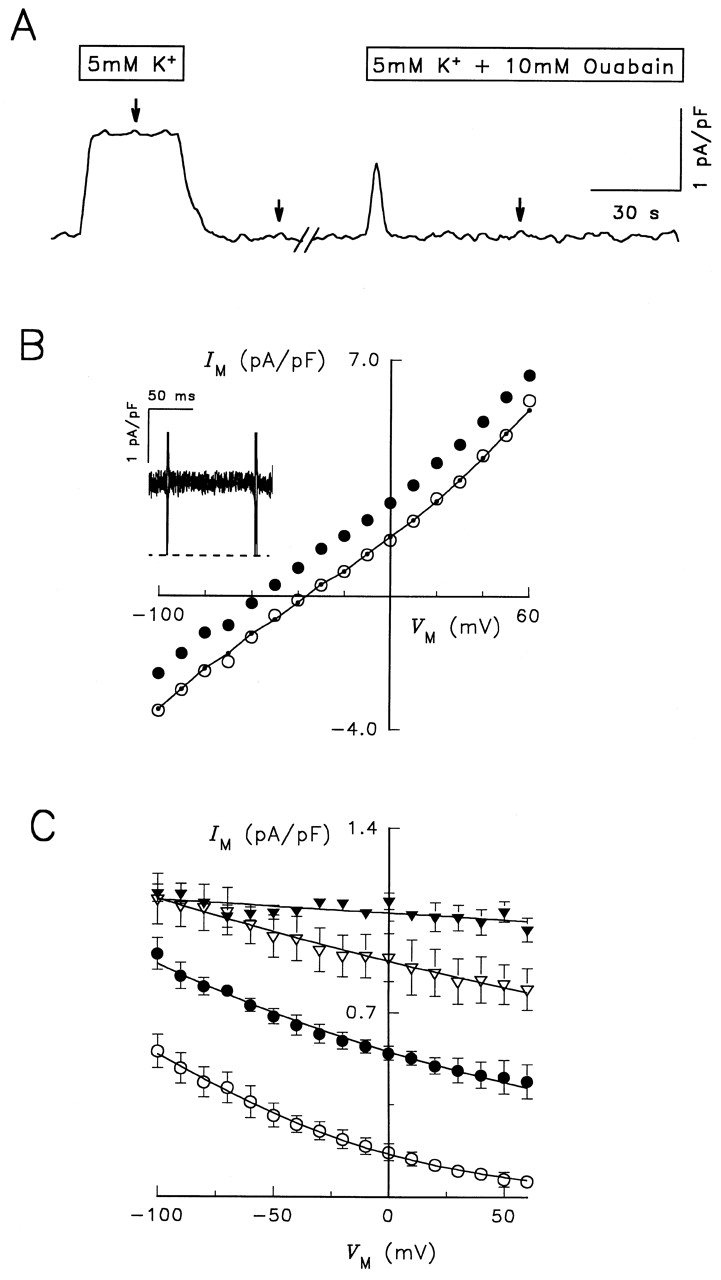
Na,K -pump current was examined in cells expressing enzyme with the Ser775Ala substitution during superfusion with Na+ o-free solutions. Fig. 5 A shows current measured in K + o-free solution as well as in the presence of 20 mM K + o with 1 μM and 10 mM ouabain. Increasing K + o concentration produced an outward shift in current that was inhibited by 10 mM ouabain. Increasing K + o concentration beyond 20 mM produced an observable junction potential that interfered with accurate measurement of K + o-sensitive current. Therefore, Na,K -pump current was measured as a 10 mM ouabain-inhibitable current during superfusion in solutions containing 40 and 80 mM K Cl. Fig. 5 B shows one such experiment in which current was measured in the presence of 80 mM K + o-containing solutions with either 1 μM or 10 mM ouabain. Under these conditions, no junction potentials were observed, so that Na,K -pump current generated by Ser775Ala enzyme could be accurately measured.
Figure 5.
Membrane potential and K + o dependence of Na,K -pump current in Ser775Ala-substituted enzyme under Na+ o-free conditions. (A) Steady state I-V relationships from a cell superfused with 0 K + o (○), 20 mM K + o (•) and 20 mM K + o plus 10 mM ouabain-containing solution (•–•). Cell capacitance was 67 pF. (B) Steady state I-V relationships in another cell superfused with 80 mM K + o (•) and 80 mM K + o plus 10 mM ouabain-containing solution (•–•). Cell capacitance was 83 pF. (C) K + o dependence of Na,K -pump current activation as a function of V M. Cells were superfused with solutions containing 5 (○), 20 (•), 40 (▿), and 80 mM K Cl (▾) plus either 1 μM or 10 mM ouabain (n = 15). Continuous curves are functions fitted to the data as indicated in the legend of Fig. 1. (D) K + o stimulation of Na,K -pump current at 0 mV in RD control (○) and Ser775Ala-substituted (•) enzyme. Data were normalized to the value of maximal current amplitude calculated by fitting a Hill equation (continuous curves) to each data set. The best-fit parameters for Ser775Ala enzyme are given in the text and in the legend of Fig. 3 for RD enzyme.
Fig. 5 C shows the V M dependence of Na,K -pump current measured in the presence of several K + o concentrations. The negative slopes of the I-V relationships indicated that V M-dependent reaction steps were maintained with this amino acid substitution. However, K + o concentrations needed to activate Na,K -pump current were much higher than those for RD enzyme. Even at the highest K + o concentration tested (80 mM), current still displayed a negative slope at positive potentials, an indication that K + o binding sites were not saturated in this variant enzyme. A Hill plot of the current density at 0 mV showed that the K 0.5 for current activation was 29.4 ± 2.2 mM and γK was 1.19 ± 0.12 (n = 15). This K 0.5 value is over 150-fold larger than that for RD enzyme (Fig. 5 D). On the other hand, this mutation had little effect on the Hill coefficient. Thus, the substitution Ser775Ala greatly reduced the apparent affinity for K + activation of Na,K -ATPase activity, consistent with the hypothesis that this residue is important in K + binding and/or occlusion (Argüello and Lingrel 1995; Blostein et al. 1997; Pedersen et al. 1998).
Effect of Amino Acid Substitutions on the VM-dependent Properties of Na,K -pump Current
The results above show that substitutions at residues Ser775 and Glu779 significantly decrease the apparent affinity of the Na,K -ATPase for K + o. To determine how these substitutions affect the V M-dependent properties of Na,K -pump current, the analysis shown in Fig. 2 was performed for each variant enzyme. As above, the K 0.5 and γK for K + o activation were determined at each V M. For all variant enzymes, γK was found to have no relation to V M, so that Table shows the average values for the range of V M tested.
Table 1.
Summary of Data for RD and Variant Enzymes Containing Substitutions at Ser775 and Glu779
| Enzyme | RD control | Ser775Ala | Glu779Ala | Glu779Gln |
|---|---|---|---|---|
| K 0 0.5 (mM) | 0.19 ± 0.01 | 29.5 ± 0.50 | 2.73 ± 0.06 | 0.11 ± 0.00 |
| λK | 0.39 ± 0.02 | 0.38 ± 0.01 | 0.37 ± 0.01 | 0.24 ± 0.01 |
| γK | 0.79 ± 0.08‡ | 0.88 ± 0.34§ | 0.67 ± 0.05‡ | 1.12 ± 0.14‡ |
The values of K 0.5 for enzymes containing an Ala mutation at Ser775 and Glu779 are plotted in Fig. 6 as a function of V M along with K 0.5 values for control RD enzyme. The apparent affinity for activation of Na,K -pump current by K + o is much lower with these variant enzymes than with RD enzyme at all V M. Like RD enzyme, K 0.5 increased as V M progressed from negative to positive values, an indication that K + o-dependent reactions remain electrogenic in the variant enzymes. To calculate the fraction of the electric field (λK) dissipated during K + o-dependent reactions, each set of data was fitted with . The best-fit parameters from this analysis for λK as well as K 0 0.5 are shown in Table . This analysis shows that λK was not significantly affected by the Ala substitutions at either residue. Values of K 0 0.5, on the other hand, were as much as 155-fold larger for the Ala-substituted enzymes. Thus, the apparent affinity for activation of Na,K -pump current can be changed over a 150-fold range by these mutations without affecting the V M dependence of K + o-related reactions.
Figure 6.
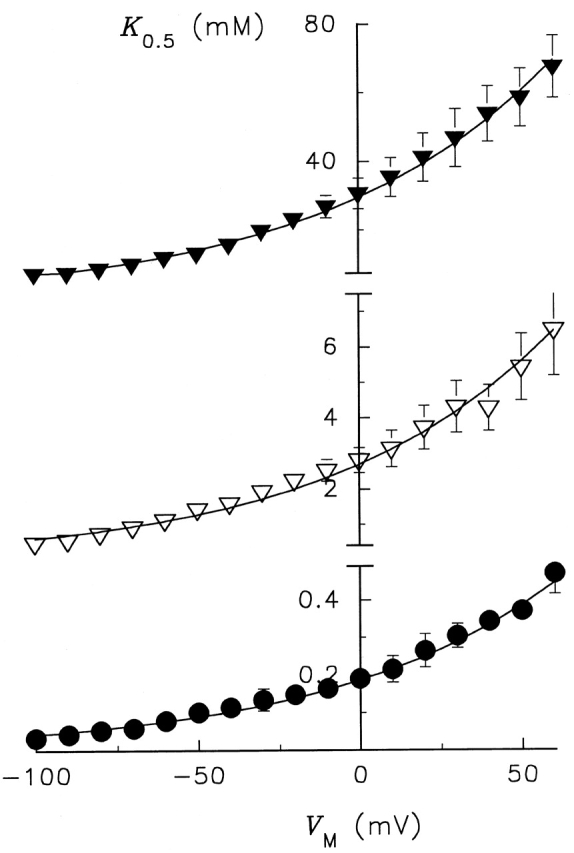
Membrane potential dependence of the K 0.5 for K + o activation of Na,K -pump current under Na+ o-free conditions. K 0.5 values obtained from fitting a Hill equation to the current density data are plotted as a function of V M for RD control (•), Glu779Ala (▿), and Ser775Ala (▾)-substituted enzymes. Continuous curves are best-fit functions for . The fitting parameters are given in Table .
On the other hand, changes in side chain structure could affect the V M-dependent properties of Na,K -pump current, as was shown with the variant enzyme Glu779Gln. The apparent affinity for K + o with this enzyme was similar to RD enzyme, although λK was significantly decreased (Table ). Thus, the more conservative mutation at residue 779 appeared to have little effect on K + o binding, but it could perturb V M-dependent reaction steps.
The data show that the three amino acid substitutions tested in this study altered either the V M-dependent properties of Na,K -pump current or the kinetics of reactions related to K + o binding. However, none of these mutations affected both the V M dependence and the kinetics of K + o-dependent reactions. One interpretation of these results is that elements of protein structure that determine ion binding affinity can be separated from those that control V M dependence of ion transport.
DISCUSSION
Electrogenic Properties of RD Enzyme
Previous work showed that Na,K -pump current in HeLa cells expressing RD enzyme retains some of the essential V M-dependent properties of wild-type enzyme (Argüello et al. 1996). The present study extended these observations by examining the ion and V M-dependent properties of RD enzyme in Na+ o-containing and Na+ o-free solutions.
In several ways, RD enzyme appears to behave quite similarly to wild-type Na,K -ATPase. The K + o concentration for half-maximal activation of Na,K -pump current at 0 mV was 2.41 and 0.19 mM in 148 mM Na+ o-containing and Na+ o-free solutions, respectively. These K 0 0.5 values and the ∼12-fold increase in the apparent affinity for K + o activation of Na,K -pump current in Na+ o-free solutions are similar to those values reported previously for native enzyme (Nakao and Gadsby 1989; De Weer 1992; Sagar and Rakowski 1994; Berlin and Peluffo 1997). As with wild-type Na,K -ATPase, the apparent affinity for K + o is V M-dependent, consistent with the idea that at least some of the Na+ o and K + o binding sites exist in the membrane dielectric. During K + o binding reactions with RD enzyme, over a third of the membrane electric field is dissipated. A similar fractional distance has been reported for Na,K -ATPase in rat cardiac ventricular myocytes (Berlin and Peluffo 1997), although this value is somewhat larger than that reported in other preparations (Sagar and Rakowski 1994). The V M-dependent properties of Na+ o binding in RD enzyme appear to be similar to wild-type Na,K -ATPase; however, they were not studied in detail. The kinetic and V M-dependent properties of transient charge movements with RD enzyme that occur during electroneutral Na+–Na+ exchange (Peluffo, R.D., and J.R. Berlin, unpublished observations) are also quite similar to transient charge movements observed in cardiac myocytes (Nakao and Gadsby 1986; Peluffo and Berlin 1997) and oocytes (Rakowski 1993). Consistent with RD enzyme, the V M dependence of K 0.5 for K + o activation of Na,K -pump current recorded in Xenopus oocytes is similar for ouabain-sensitive and -insensitive (Gln118Arg and Asn129Asp) forms of the Torpedo Na,K -ATPase (Vasilets et al. 1998). Thus, mutations that yield the ouabain-insensitive RD enzyme from the sheep α subunit (Gln111Arg and Asn122Asp) do not appear to significantly alter the electrogenic properties of extracellular ion binding to the Na,K -ATPase.
K + o Dependence of Na,K -pump Current in HeLa Cells Expressing Variant Na,K -ATPase Enzymes
Modifications in the side-chain structure of residues 775 and 779 produced large changes in the apparent affinity for K + o activation of Na,K -pump current when determinations were performed in Na+ o-free solutions. Mutations at Glu779 yielded values of K 0 0.5 for K + o that varied over a 25-fold range. The substitution Glu779Gln had little effect on the K 0 0.5 for K + o; however, the substitution Glu779Ala resulted in a large increase in K 0 0.5. Since the substitution Glu to Gln removes the carboxyl moiety, these results suggest that the presence of this functional group at residue 779 is not critical in determining the kinetics of K + o-dependent reactions. On the other hand, either the loss of the carbonylic oxygen or the change in side-chain bulk that occurs with Ala substitution at this residue does affect the kinetics of K + o-dependent reactions.
In considering a mechanistic interpretation of our experimental results, it could be argued that the observed changes in apparent K + o affinity are produced by alterations in reactions other than K + o binding/occlusion steps. For instance, the ATP concentration that half-maximally stimulates Na,K -ATPase activity in Glu781Ala enzyme from rat (corresponding to Glu779Ala of sheep enzyme) is approximately fivefold lower than in wild-type enzyme (Vilsen 1995; Koster et al. 1996), consistent with instability of the K +-occluded form of the enzyme. However, acceleration of K + deocclusion would be expected to produce an increase in apparent K + o affinity, opposite of the observed data. The kinetics of Na+-dependent reactions could not be responsible for the observed decrease in apparent K + o affinity. The cell interior was dialyzed against the patch electrode solution containing 115 mM Na+, a concentration high enough to saturate intracellular Na+ binding sites, even in the variant enzymes (Vilsen 1995; Feng and Lingrel 1995; Koster et al. 1996), and the use of Na+ o-free solutions removed Na+ o-dependent reaction kinetics as a consideration in the observed changes in K 0.5 for K + o. Finally, Argüello et al. 1996 showed that phosphoenzyme stability is similar with RD and Glu779Ala enzymes in the absence of Na+ and K +. Thus, the lower apparent K + o affinity in Glu779Ala enzyme cannot be attributed to changes in enzyme dephosphorylation rate. Taken together with previous data, the 25-fold range of K 0 0.5 for K + o in enzymes containing substitutions at Glu779 is most easily interpreted as a change in K + o-dependent reaction kinetics rather than a change in the kinetics of other reactions. Even so, these results do not distinguish whether the change in kinetics occurs in K + o-binding and/or occlusion reactions. However, previously published data suggest that the kinetics of both reaction steps may be affected by substitutions at Glu779 (Nielsen et al. 1998).
Activation of Na,K -ATPase activity (Feng and Lingrel 1995, Vilsen 1995; Koster et al. 1996) and Na,K -pump current by K + o (Argüello et al. 1996) with Glu779Ala enzyme has been reported to occur with an apparent affinity three to five times lower than wild-type or RD enzyme. These previous reports measured enzyme activation in the simultaneous presence of Na+ and K +, whereas the present experiments were performed with intact membranes under Na+ o-free conditions. Our own data show that substituting alanine at Glu779 has significant effects on the interaction of Na+ and K + at extracellular sites (Peluffo et al. 2000). Thus, previous reports have probably underestimated the change in K +-dependent activation of enzyme activity that occurs with this variant enzyme.
The substitution Glu779Gln has been reported to cut Tl+ occlusion in half (Nielsen et al. 1998); however, the present data give little indication that the capacity of Glu779Gln enzyme to occlude K + is different than RD enzyme. Maximal turnover rates of both RD and Glu779Gln enzymes (Argüello, J.M., unpublished data) as well as maximum Na,K -pump current densities in cells expressing these enzymes are similar, consistent with enzymes that have the same net charge transfer per transport cycle and, therefore, the same ion transport stoichiometry. Instead, an alternative explanation for the reported reduction in Tl+ occlusion is that K +-occluded Glu779Gln enzyme is less stable than wild-type enzyme. Such instability of the K +-occluded enzyme might affect ion binding determinations in a manner that would yield an apparent decrease in Tl+ binding stoichiometry. Thus, our results indicate a significant role of Glu779 in K + o binding, but they do not support a change in cation transport stoichiometry.
The serine at residue 775 of the Na,K -ATPase is thought to be involved in ion coordination. Substitution of Ala for Ser775 has been shown to produce a 30-fold decrease in the apparent affinity for K + activation of Na,K -ATPase activity (Argüello and Lingrel 1995) and K + influx (Blostein et al. 1997), and a 90-fold increase in the K 0.5 for K + displacement of bound ATP (Pedersen et al. 1998). Likewise, in the present experiments, K 0 0.5 for K + o activation of Na,K -pump current was increased more than 150-fold. These results show that K +-dependent reaction kinetics are quite sensitive to mutations at Ser775. Measurements of Tl+ binding also suggest that this residue is important for ion binding reactions (Pedersen et al. 1998). Furthermore, alterations in the kinetics of other reaction steps do not account for such a large change in the apparent affinity for K + o (Argüello and Lingrel 1995; Blostein et al. 1997), so it seems reasonable to conclude that the loss of the side-chain hydroxyl group of this amino acid greatly affects K + binding/occlusion reaction kinetics.
VM Dependence of Na,K -pump Current in Variant Enzymes
Previous work has shown that the V M dependence of Na,K -pump current is affected by substitutions Glu779Ala (Argüello et al. 1996) and Glu779Gln (Peluffo et al. 1997). In both instances, the negative slope of the I-V relationship that occurs in the presence of nonsaturating K + o concentrations with RD (Argüello et al. 1996) and wild-type (Rakowski et al. 1991) enzyme was not observed over the entire range of V M (−100 to +60 mV) tested with these enzyme variants. Mechanistic interpretation of these results, however, was complicated by the conditions in which the experiments were conducted. Na,K -pump currents (i.e., K + o-activated currents) were measured with Na+ o- and K + o-containing solutions (Argüello et al. 1996) so that changes in extracellular ion binding reaction kinetics could not be distinguished easily from changes in the V M dependence of ion binding. Glu779Ala enzyme also has the additional complication that electrogenic Na+–Na+ exchange occurs in Na+ o-containing solutions (Argüello et al. 1996; Peluffo et al. 2000). To simplify the interpretation of the data, the present experiments were conducted in Na+ o-free solutions so that the V M dependence and kinetics of Na+ o-dependent reactions would not affect K + o binding reactions. The absence of Na+ o also prevented Na+–Na+ exchange mediated by Glu779Ala enzyme. Given these conditions, a change in the V M dependence and/or kinetics of K + o-dependent reactions should have been readily apparent in the properties of Na,K -pump current.
As evidenced by the negative slopes of the I-V relationships observed at nonsaturating K + o concentrations, it is obvious that K + o-dependent reactions are V M dependent in both Glu779 enzyme variants and Ser775Ala enzyme. Thus, the apparent V M independence of Na,K -pump current observed in Na+ o-containing solutions with Glu779 enzyme variants must result from Na+ o interactions with the Na,K -ATPase. The mechanism of these interactions is the focus of the accompanying paper (Peluffo et al. 2000).
The fraction of the membrane dielectric dissipated during K + o-dependent reactions, λK , was not significantly different for Na,K -pump current mediated by RD, Ser775Ala, and Glu779Ala enzymes. Given the 150-fold variation in the apparent K + o affinity amongst these enzyme variants, this result shows that the kinetics of K +-dependent binding/occlusion reactions can be greatly altered without affecting the V M dependence of these reactions. Mutations at other residues, however, have been reported to have some effect on both kinetics and V M dependence of K + o binding reactions (Vasilets et al. 1998). Together, these results suggest that the structural features of the Na,K -ATPase protein that determine the kinetics of ion-dependent reactions are separable, at least in part, from those features that determine their V M dependence.
Membrane potential–dependent reactions during ion transport by the Na,K -ATPase appear to occur largely during ion binding; i.e., binding occurs in an ion well (Rakowski et al. 1997). This conclusion was first established for Na+ o binding by studying transient reaction kinetics and unidirectional ion flux rates during electroneutral Na+–Na+ exchange (Nakao and Gadsby 1986; Bühler et al. 1991; Fendler et al. 1993; Gadsby et al. 1993; Rakowski 1993; Heyse et al. 1994; Hilgemann 1994a; Wuddel and Apell 1995). These experiments established that release/rebinding of at least one Na+ was responsible for the highly V M-dependent step that moves the equivalent of a charge through two thirds to three quarters of the membrane dielectric. More recently, similar measurements of transient reaction kinetics during electroneutral K +–K + exchange showed that K + o binding steps are also V M dependent, but move the equivalent of a charge through approximately one third of the membrane dielectric (Peluffo and Berlin 1997).
The lack of effect of mutations Ser775Ala and Glu779Ala on the V M dependence of Na,K -pump current suggests that the K + o ion well is unaffected even as the kinetics of associated binding/occlusion reactions are altered. The question then remains as to whether any inferences concerning the structure of the K + ion well can be drawn from these data. In this regard, two general models for ion wells in the Na,K -ATPase have been developed. In one model, it is postulated that the ion well is a channel-like pore, a so-called “high field access channel” (Gadsby et al. 1993). If such a channel exists in the Na,K -ATPase, the present data suggest that functional groups involved in K + o coordination are separate from those that form the ion well. In this case, the effect of mutations would be explained as a perturbation in the structure of the K + coordination pocket that is distinct from that portion of the channel over which the membrane dielectric is dissipated. An alternative explanation would be that the opening/closing kinetics of an electroneutral occlusion gate, similar to that postulated by Forbush 1988, could be altered by these mutations. Such an explanation could account for the present data without the necessity of invoking separate structures for the ion well and ion binding pocket.
In the second model of an ion well, it is postulated that V M-dependent ion binding occurs via a chelation-type rearrangement of protein structure in which a V M-independent transitional ion binding step is followed by a rapid V M-dependent ion occlusion/deocclusion reaction (Hilgemann 1994b). To explain the present data, it would be necessary to postulate that mutations at Ser775 and Glu779 alter the kinetics of the chelation reaction without affecting the movement of extrinsic and intrinsic protein charges through the membrane dielectric. This model and the alternate channel model above would also easily fit in with previous reports that substitutions Glu779Ala (Vilsen 1995; Nielsen et al. 1998) and Ser775Ala (Blostein et al. 1997; Pedersen et al. 1998) destabilize/perturb ion occlusion by the Na,K -ATPase. In any case, more information concerning how mutations affect the V M dependence of ion binding is needed before these various structural models for the ion well can be distinguished.
In summary, the present data show that mutations to Ser775 and Glu779 can greatly alter the apparent affinity for K + o with little or no effect on the V M dependence of K +-dependent transport. Given corroborating evidence from biochemical studies, the decrease in apparent K + o affinity appears to reflect changes in intrinsic ion binding affinity or occlusion/deocclusion kinetics. Thus, these results support previous reports suggesting that these amino acids are involved in ion binding reactions by the Na,K -ATPase. The lack of effect of these mutations on the V M dependence of K + o binding shows that a functional separation between requirements for ion wells and ion binding is possible. These data do not allow us to conclude how this separation is manifested on a structural basis; however, they do place limits on how various models of ion wells in the Na,K -ATPase could relate structurally to ion binding and occlusion by the enzyme.
Acknowledgments
The authors thank Dr. Jerry B Lingrel for support and valuable discussions throughout this project and acknowledge the excellent technical assistance of Ms. Palak Raval-Nelson and Ms. Marguarita Schmid.
This work was supported by a postdoctoral fellowship from the Southeastern Pennsylvania Affiliate of the American Heart Association (AHA) (R.D. Peluffo), a Grant-in-Aid from the AHA (J.R. Berlin), and National Institutes of Health grants HL03373 (J.M. Argüello), GM57253, and HL43712 (J.R. Berlin).
Footnotes
Portions of this work were previously published in abstract form (Peluffo, R.D., J.M. Argüello, J.B Lingrel, and J.R. Berlin. 1999. Biophys. J. 76:A388).
References
- Andersen J.P., Vilsen B. Structure–function relationships of cation translocation by Ca2+ and Na+,K +-ATPases studied by site-directed mutagenesis. FEBS Lett. 1995;359:101–106. doi: 10.1016/0014-5793(95)00019-6. [DOI] [PubMed] [Google Scholar]
- Argüello J.M., Kaplan J.H. Glutamate 779, an intramembrane carboxyl, is essential for monovalent cation binding by the Na,K -ATPase. J. Biol. Chem. 1994;269:6892–6899. [PubMed] [Google Scholar]
- Argüello J.M., Lingrel J.B. Substitutions of Serine 775 in the α subunit of the Na,K -ATPase selectively disrupt K + high affinity activation without affecting Na+ interaction. J. Biol. Chem. 1995;270:22764–22771. doi: 10.1074/jbc.270.39.22764. [DOI] [PubMed] [Google Scholar]
- Argüello J.M., Peluffo R.D., Feng J., Lingrel J.B, Berlin J.R. Substitution of glutamic 779 with alanine in the Na,K -ATPase α subunit removes voltage dependence of ion transport. J. Biol. Chem. 1996;271:24610–24616. doi: 10.1074/jbc.271.40.24610. [DOI] [PubMed] [Google Scholar]
- Berlin J.R., Peluffo R.D. Mechanism of electrogenic reaction steps during K + transport by the Na,K -ATPase. Ann. NY Acad. Sci. 1997;834:251–259. doi: 10.1111/j.1749-6632.1997.tb52256.x. [DOI] [PubMed] [Google Scholar]
- Blostein R., Wilczynska A., Karlish S.J.D., Argüello J.M., Lingrel J.B. Evidence that Ser775 in the alpha subunit of the Na,K -ATPase is a residue in the cation binding pocket. J. Biol. Chem. 1997;272:24987–24993. doi: 10.1074/jbc.272.40.24987. [DOI] [PubMed] [Google Scholar]
- Bühler R., Stürmer W., Apell H.-J., Läuger P. Charge translocation by the Na,K -pumpI. K inetics of local field changes studied by time-resolved fluorescence measurements. J. Membr. Biol. 1991;121:141–161. doi: 10.1007/BF01870529. [DOI] [PubMed] [Google Scholar]
- De Weer P. Cellular sodium–potassium transport. In: Seldin D.W., Giebisch G., editors. The K idneyPhysiology and Pathophysiology. Raven Press; New York, NY: 1992. pp. 93–112. [Google Scholar]
- Fendler K., Jaruschewski S., Hobbs A., Albers W., Froehlich J.P. Pre–steady-state charge translocation in NaK -ATPase from eel electric organ. J. Gen. Physiol. 1993;102:631–666. doi: 10.1085/jgp.102.4.631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feng J., Lingrel J.B. Functional consequences of substitutions of the carboxyl residue glutamate 779 of the Na,K -ATPase. Cell. Mol. Biol. Res. 1995;41:29–37. [PubMed] [Google Scholar]
- Forbush B. The interaction of amines with the occluded state of the Na,K -pump. J. Biol. Chem. 1988;263:7979–7988. [PubMed] [Google Scholar]
- Gadsby D.C., Nakao M. Steady-state current–voltage relationship of the Na/K pump in guinea pig ventricular myocytes. J. Gen. Physiol. 1989;94:511–537. doi: 10.1085/jgp.94.3.511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gadsby D.C., Rakowski R.F., De Weer P. Extracellular access to the Na,K pumppathway similar to ion channel. Science. 1993;260:100–103. doi: 10.1126/science.7682009. [DOI] [PubMed] [Google Scholar]
- Glynn I.M. The Na+,K +-transporting adenosine triphosphatase. In: Martonosi A.N., editor. The Enzymes of Biological Membranes. 2nd ed. Plenum Publishing Corp; New York, NY: 1985. pp. 35–114. [Google Scholar]
- Hansen U.-P., Gradmann D., Sanders D., Slayman C.L. Interpretation of current–voltage relationships for “active” ion transport systems. I. Steady-state reaction-kinetic analysis of class-I mechanisms. J. Membr. Biol. 1981;63:165–190. doi: 10.1007/BF01870979. [DOI] [PubMed] [Google Scholar]
- Heyse S., Wuddel I., Apell H.-J., Stürmer W. Partial reactions of the Na,K -ATPasedetermination of rate constants. J. Gen. Physiol. 1994;104:197–240. doi: 10.1085/jgp.104.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hilgemann D.W. Channel-like function of the Na,K pump probed at microsecond resolution in giant membrane patches Science 263 1994. 1429 1432a [DOI] [PubMed] [Google Scholar]
- Hilgemann D.W. Flexibility and constraint in the interpretation of Na+/K + pump electrogenicityWhat is an access channel? Bamberg E., Schoner W. The Sodium PumpStructure, Mechanism, Hormonal Control, and its Role in Disease 1994. 507 516 Steinkopff; Darmstadt, Germany: b [Google Scholar]
- Jewell E.A., Lingrel J.B. Comparison of the substrate dependence properties of the rat Na,K -ATPase α1, α2, and α3 isoforms expressed in HeLa cells. J. Biol. Chem. 1991;266:16925–16930. [PubMed] [Google Scholar]
- Karlish S.J.D., Goldshleger R., Stein W.D. A 19-kDa C-terminal tryptic fragment of the α chain of Na/K -ATPase is essential for occlusion and transport of cations. Proc. Natl. Acad. Sci. USA. 1990;87:4566–4570. doi: 10.1073/pnas.87.12.4566. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koster J.C., Blanco G., Mills P.B., Mercer R.W. Substitutions of glutamate 781 in the Na,K -ATPase α subunit demonstrate reduced cation selectivity and an increased affinity for ATP. J. Biol. Chem. 1996;271:2413–2421. doi: 10.1074/jbc.271.5.2413. [DOI] [PubMed] [Google Scholar]
- Läuger P. Electrogenic Ion Pumps 1991. Sinauer Associates; Sunderland, MA: pp. 65–69 [Google Scholar]
- Lingrel J.B, Kuntzweiler T. Na+,K +-ATPase. J. Biol. Chem. 1994;269:19659–19662. [PubMed] [Google Scholar]
- Liu L., Askari A. Evidence for the existence of two ATP-sensitive Rb+ occlusion pockets within the transmembrane domains of Na+/K +-ATPase. J. Biol. Chem. 1997;272:14380–14386. doi: 10.1074/jbc.272.22.14380. [DOI] [PubMed] [Google Scholar]
- Lutsenko S., Anderko R., Kaplan J.H. Membrane disposition of the M5–M6 hairpin of the Na+,K +-ATPase α subunit is ligand dependent. Proc. Natl. Acad. Sci. USA. 1995;92:7936–7940. doi: 10.1073/pnas.92.17.7936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakao M., Gadsby D.C. Voltage dependence of Na translocation by the Na/K pump. Nature. 1986;323:628–630. doi: 10.1038/323628a0. [DOI] [PubMed] [Google Scholar]
- Nakao M., Gadsby D.C. and [K ] dependence of the Na/K pump current–voltage relationship in guinea pig ventricular myocytes J. Gen. Physiol. 94 1989. 539 565[Na] [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nielsen J.M., Pedersen P.A., Karlish S.J.D., Jorgensen P.L. Importance of intramembrane carboxylic acids for occlusion of K + ions at equilibrium in renal Na,K -ATPase. Biochemistry. 1998;37:1961–1968. doi: 10.1021/bi972524q. [DOI] [PubMed] [Google Scholar]
- Or E., David P., Shainskaya A., Tal D.M., Karlish S.J.D. Effects of competitive sodium-like antagonists on Na,K -ATPase suggests that cation occlusion from the cytoplasmic surface occurs in two steps. J. Biol. Chem. 1993;268:16929–16937. [PubMed] [Google Scholar]
- Pedersen P.A., Nielsen J.M., Rasmussen J.H., Jorgensen P.L. Contribution to Tl+, K +, and Na+ binding of Asn776, Ser775, Thr774, Thr772, and Tyr771 in cytoplasmic part of fifth transmembrane segment in α-subunit of renal Na,K -ATPase. Biochemistry. 1998;37:17818–17827. doi: 10.1021/bi981898w. [DOI] [PubMed] [Google Scholar]
- Peluffo R.D., Argüello J.M., Lingrel J.B, Berlin J.R. Electrogenic sodium–sodium exchange carried out by Na,K -ATPase containing the amino acid substitution Glu779Ala. J. Gen. Physiol. 2000;116:61–73. doi: 10.1085/jgp.116.1.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo R.D., Berlin J.R. Electrogenic K + transport by the Na+-K + pump in rat cardiac ventricular myocytes. J. Physiol. 1997;501:33–40. doi: 10.1111/j.1469-7793.1997.033bo.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peluffo R.D., Lingrel J.B, Argüello J.M., Berlin J.R. Changes to Na,K -ATPase α-subunit Glu779 separate the structural basis for VM and ion dependence of Na,K -pump current. Ann. NY Acad. Sci. 1997;834:339–342. doi: 10.1111/j.1749-6632.1997.tb52265.x. [DOI] [PubMed] [Google Scholar]
- Price E.M., Lingrel J.B. Structure–function relationships in the Na,K -ATPase alpha subunitsite-directed mutagenesis of glutamine-111 to arginine and asparagine-122 to aspartic acid generates a ouabain-resistant enzyme. Biochemistry. 1988;27:8400–8408. doi: 10.1021/bi00422a016. [DOI] [PubMed] [Google Scholar]
- Rakowski R.F. Charge movement by the Na/K pump in Xenopus oocytes. J. Gen. Physiol. 1993;101:117–144. doi: 10.1085/jgp.101.1.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rakowski R.F., Gadsby D.C., De Weer P. Voltage dependence of the Na/K pump. J. Membr. Biol. 1997;155:105–112. doi: 10.1007/s002329900162. [DOI] [PubMed] [Google Scholar]
- Rakowski R.F., Vasilets L.A., LaTona J., Schwarz W. A negative slope in the current–voltage relationship of the Na+/K + pump in Xenopus oocytes produced by reduction of external [K +] J. Membr. Biol. 1991;121:177–187. doi: 10.1007/BF01870531. [DOI] [PubMed] [Google Scholar]
- Sagar A., Rakowski R.F. Access channel model for the voltage dependence of the forward-running Na+/K + pump. J. Gen. Physiol. 1994;103:869–894. doi: 10.1085/jgp.103.5.869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shainskaya A., Karlish S.J.D. Evidence that the cation occlusion domain of Na/K -ATPase consists of a complex of membrane-spanning segments. Analysis of limit membrane-embedded tryptic fragments. J. Biol. Chem. 1994;269:10780–10789. [PubMed] [Google Scholar]
- Shainskaya A., Nesaty V., Karlish S.J.D. Interactions between fragments of trypsinized Na,K -ATPase detected by thermal inactivation of Rb+ occlusion and dissociation of the M5/M6 fragment. J. Biol. Chem. 1998;273:7311–7319. doi: 10.1074/jbc.273.13.7311. [DOI] [PubMed] [Google Scholar]
- Vasilets L.A., Takeda K., Kawamura M., Schwarz W. Significance of the glutamic acid residues Glu334, Glu959, and Glu960 of the α subunits of Torpedo Na+,K + pumps for transport activity and ouabain binding. Biochim. Biophys. Acta. 1998;1368:137–149. doi: 10.1016/s0005-2736(97)00195-8. [DOI] [PubMed] [Google Scholar]
- Vilsen B. Mutant Glu781→Ala of the rat kidney Na+,K +-ATPase displays low cation affinity and catayzes ATP hydrolysis at a high rate in the absence of potassium ions. Biochemistry. 1995;34:1455–1463. doi: 10.1021/bi00004a041. [DOI] [PubMed] [Google Scholar]
- Wuddel I., Apell H.-J. Electrogenicity of the sodium transport pathway in the Na,K -ATPase probed by charge-pulse experiments. Biophys. J. 1995;69:909–921. doi: 10.1016/S0006-3495(95)79965-9. [DOI] [PMC free article] [PubMed] [Google Scholar]