Abstract
Low birth weight in humans is predictive of hypertension in adult life. Although the mechanisms underlying this link remain unknown, fetal overexposure to glucocorticoids has been implicated. We previously showed that prenatal dexamethasone (DEX) exposure in the rat lowers birth weight and programmes adult hypertension. The current study aimed to further investigate the nature of this hypertension and to elucidate its origins. Unlike previous studies, we assessed offspring blood pressure (BP) with radiotelemetry, which is unaffected by stress artefacts of measurement. We show that prenatal DEX during the last week of pregnancy results in offspring of low birth weight (14% reduction) that have lower basal BP in adulthood (∼4–8 mmHg lower); with the commonly expected hypertensive phenotype only being noted when these offspring are subjected to even mild disturbance or a more severe stressor (up to 30 mmHg higher than controls). Moreover, DEX-treated offspring sustain their stress-induced hypertension for longer. Promotion of systemic catecholamine release (amphetamine) induced a significantly greater rise of BP in the DEX animals (77% increase) over that observed in the vehicle controls. Additionally, we demonstrate that the isolated mesenteric vasculature of DEX-treated offspring display greater sensitivity to noradrenaline and other vasoconstrictors. We therefore conclude that altered sympathetic responses mediate the stress-induced hypertension associated with prenatal DEX programming.
Introduction
Much evidence suggests that transient adversity in the prenatal environment exerts permanent effects on subsequent physiology and increases disease risk in adult life (Curhan et al. 1996b, Fall et al. 1998, Law et al. 2001, Seckl 2004). A plethora of epidemiological studies have shown that low birth weight, a proposed surrogate for intrauterine adversity, is associated with hypertension (Barker 1990), type 2 diabetes (McCance et al. 1994), the metabolic syndrome and deaths from ischemic heart disease in later life (Curhan et al. 1996a, Barker 1999). To explain these links, the concept of developmental ‘programming’ has been advanced, whereby a factor acting during a critical developmental window affects the ontogeny of specifically vulnerable cell targets, permanently altering tissue structure and/or function and hence the eventual risk of disease (Benediktsson et al. 1993, Barker 2003, Seckl 2004). However, such environmentally determined developmental effects are widespread in the animal kingdom and often associate with phenotypes which appear to represent a finely balanced transient advantage in a particular environment. Such developmental ‘trade-offs’ can become disadvantageous late in the lifespan.
Fetal ‘programming’ may be investigated in a controlled manner using laboratory animals that age relatively rapidly and have similar or identical genetic backgrounds. In rats and many other mammalian species, fetal exposure to excess glucocorticoids via maternal stress, inhibition of placental 11β-hydroxysteroid dehydrogenase type 2, the normal ‘barrier’ to maternal glucocorticoids, or its bypass with non-substrate synthetic glucocorticoids, such as dexamethasone (DEX), commonly used in obstetric practice, all lower birth weight. The adult offspring show permanently elevated glucose and insulin levels and hypertension (Levitt et al. 1996, Nyirenda et al. 1998, 2001, Langdown et al. 2001, Sugden et al. 2001). Although programmed increases in the expression of key hepatic gluconeogenic enzymes may later underpin hyperglycaemia (Nyirenda et al. 1998, 2001), little is known of the mechanisms by which glucocorticoids ‘programme’ higher blood pressure (BP) or whether this phenotype carries any advantage.
Prenatal DEX, specifically in the last trimester, affects the development and maturation of specific organs related to BP control and maintenance; notably the developing heart, kidney, vasculature and brain (Torres et al. 1997, Ortiz et al. 2003, Kreider et al. 2005, Roghair et al. 2005). Alterations in the activity or responsivity of the sympathetic nervous system (SNS), either systemically or in one of these specific regions, could provide a potential explanation for the manner in which prenatal glucocorticoids promote adulthood hypertension. Indeed, in humans, increased SNS activity has long been proposed as a mechanism to explain the pathogenesis of glucocorticoid-induced hypertension (Whitworth et al. 1995, Saruta 1996). Consistent with this hypothesis, elevated SNS activity, established in utero, links small size at birth with raised BP in adult life (Phillips & Barker 1997). In rats, prenatal DEX alters the development of cardiac and central sympathetic innervation and activity (Slotkin et al. 1992, Bian et al. 1993). Permanent changes in the pattern and expression of adrenergic receptors (Huff et al. 1991, Bian et al. 1992) have the potential to alter vascular responsivity to vasoconstrictors. Similar findings have been reported with DEX administration during pregnancy in sheep (Stein et al. 1994, Padbury et al. 1995, Tseng et al. 1995).
To study more closely diurnal variation and the role of the SNS in adult BP in animals prenatally treated with excess glucocorticoids, we used the DEX-exposed rat model. Radiotelemetry (Anderson et al. 1999) enabled the remote recording of BP in conscious, unrestrained rats with minimal stress effects.
Materials and Methods
Animals
Adult Wistar rats (200–250 g; Harlan UK Ltd, Bichester, UK) were maintained under conditions of controlled lighting (lights on 0700–1900 h) and temperature (22 °C) with ad libitum access to food (standard rat chow) and water. All experiments were performed according to the UK Animals (Scientific Procedures) Act, 1986.
Prenatal treatments
Virgin female rats were housed with male rats. Pregnancy was confirmed by the presence of a vaginal plug, checked every morning. Pregnant females were housed singly, and randomly assigned to one of two treatment groups (n=9–10 per group). DEX (100 μg/kg per day, dissolved in 4% ethanol in 0·9% saline, 200 μg/ml; Sigma–Aldrich) was injected (subcutaneously) daily (0900 h) on embryonic days 15–21 inclusive (Nyirenda et al. 1998); control animals received vehicle (VEH) injections.
Litters
On the day of birth (postnatal day 1), litters were weighed, sexed and culled to eight pups per litter, retaining equal numbers of male and female pups where possible. Litters were then left undisturbed till weaning (postnatal day 21), apart from routine weekly maintenance. After weaning, male and female pups from each litter were housed in single sex groups of two to four. All rats underwent a minimum of 2 weeks of daily handling prior to being utilised in experiments. Females were tested during oestrus, confirmed by vaginal cytology. Epithelial cells were aspirated from the vagina with 1 ml saline and placed on a glass slide for microscopic inspection. The oestrous cycle was then staged according to the Journal of Animal Technicians Association, 12, No. 1. Staging was independently verified by at least one other researcher/animal technician.
Radiotelemetry monitoring of offspring BP, heart rate and activity
BP (systolic BP, SBP; diastolic BP, DBP; mean arterial pressure, MAP and pulse pressure, PP), heart rate (HR), and activity were assessed by radiotelemetry (Dataquest IV, Data Sciences International, St Paul, MN, USA; Anderson et al. 1999). Randomly selected littermates (five males and five females, from eight vehicle and eight DEX litters) at 7–8 months were anaesthetised with halothane. A flexible catheter was secured in the abdominal aorta, and the telemetry transmitter was sutured to the abdominal wall. On recovery, rats were housed in individual cages, and each cage was placed over a receiver panel with output to a PC. Each cage and its receiver panel were housed in a specially designed steel cage rack, to prevent radio signal disturbance from neighbouring animals, and the rack was stored in a quiet room. After a 10-day recovery period, haemodynamic measurements were recorded for 10 s every 30 min (Khan et al. 2003).
Induction of stress by disturbance, weighing and restraint procedure
Offspring were subjected to a series of graded stressors: i) simple disturbance (a researcher entering their room); ii) being weighed and iii) restraint in a Perspex cylinder for 15 min. To evaluate the stress response to weighing, haemodynamic measurements were recorded prior to and immediately after removal of the rat from its cage and again at 5-min post-weighing, when the animal was replaced. For the restraint procedure, measurements were collected prior to the rat being placed in the cylinder, throughout the 15-min restraint period, and at 15-min post-restraint, on being returned to its cage. A rest period of 2 days was allowed between experiments.
Haemodynamic responses to alterations in catecholaminergic mechanisms
Haemodynamic responses were collected for 15 min following a low dose of i.p. d-amphetamine (0·5 mg/kg), to cause systemic catecholamine release.
Vascular responses to noradrenaline, vasopressin and potassium chloride
Pairs of rats (one VEH and one DEX) were anaesthetised with sodium pentobarbitone, and the mesenteric vasculature was catheterised and perfused with Krebs bicarbonate Ringer's solution, and isolated as previously described (McGregor 1965). Perfusion pressure with constant flow rate was measured continuously via an Elcomatic EM 720 transducer, and recorded on a MacLab Chart V3.3.5 program (ADI, Oxford, UK). On alternate days, mesenteric preparations from each treatment group were attached to alternate transducers, to exclude the possibility of any positional artefact. Following a 15-min equilibration period, a brief ‘wake-up’ bolus of 20 μM noradrenaline (NA; Sigma–Aldrich) was administered (Hadoke et al. 2006).
Twelve pairs of mesenteric vasculature preparations from both male and female offspring were tested simultaneously for periods of 3 min with increasing concentrations of agonists in the following order: NA (0·1–20 μM), vasopressin (AVP; 0·5–20 nM; Sigma–Aldrich) and potassium chloride (KCl; 25–125 mM), all agonists were prepared in Ringer's solution. Between tests, the vasculatures were infused with Ringer's solution alone, during which time the perfusion pressure returned to basal values. A recovery period of at least 30 min was allowed between each agonist.
Statistical analyses
BP, HR and activity
Basal BP parameters (SBP, DBP, MAP, PP and HR) and activities for each prenatal treatment (VEH or DEX) and gender, are shown in Table 2A (means±s.d). Data are calculated for each rat as an average over 3 days of telemetry recording. Statistical analysis of the data was by multivariate repeated measures MANOVA (with prenatal treatment, gender and light/dark period as dependent variables, and rat identification number/representative of each litter and time of day as random variables) which was carried out on the raw BP data for each rat and significance values for each dependent variable, together with interactions between variables are shown in Table 2.
Table 2.
(A) Blood pressure parameters measured under basal conditions in adult offspring of VEH- and dexamethasone (DEX)-treated dams. (B) Table of P values resulting from MANOVA of telemetry data in A. Dependent variables are prenatal treatment (VEH or DEX), gender and day or night measurements. Results are mean±s.d. (number of observations), from all data collected over 72-h period for each rat
| (A) Telemetry data | ||||
|---|---|---|---|---|
| VEH ♂ | DEX ♂ | VEH ♀ | DEX ♀ | |
| Systolic (mmHg) | ||||
| Av daily BP | 144·5±10·6 (750) | 140·2±15·2 (717) | 138·3±10·4 (450) | 129·7±12·7 (749) |
| Av BP in light phase | 141·7±9·5 (390) | 138·3±16·2 (386) | 135·2±10·2 (234) | 126·6±11·8 (390) |
| Av BP in dark phase | 147·6±11·0 (360) | 142·5±13·7 (331) | 141·6±9·5 (216) | 133·1±12·8 (359) |
| Diastolic (mmHg) | ||||
| Av daily BP | 106·1±9·8 (750) | 103·3±12·8 (717) | 101·5±8·9 (450) | 99·4±13·0 (749) |
| Av BP in light phase | 103·4±8·6 (390) | 101·6±13·4 (386) | 99·4±8·4 (234) | 96·3±12·0 (390) |
| Av BP in dark phase | 109·0±10·2 (360) | 105·3±11·4 (331) | 103·8±8·9 (216) | 102·7±13·3 (360) |
| MAP (mmHg) | ||||
| Av daily BP | 118·9±10·6 (750) | 115·6±15·5 (717) | 113·8±9·2 (450) | 109·4±4·7 (749) |
| Av BP in light phase | 116·1±8·7 (390) | 113·9±14·4 (386) | 111·3±8·9 (234) | 106·4±11·7 (390) |
| Av BP in dark phase | 121·9±10·3 (360) | 117·7±12·1 (331) | 116·4±8·9 (216) | 112·7±12·9 (359) |
| Pulse pressure (mmHg) | ||||
| Av daily PP | 38·5±3·9 (750) | 36·9±3·7 (717) | 36·8±4·0 (450) | 30·4±5·9 (749) |
| Av PP in light phase | 38·3±3·7 (390) | 36·7±3·8 (386) | 35·8±3·8 (234) | 30·3±5·1 (390) |
| Av PP in dark phase | 38·7±4·2 (360) | 37·2±3·6 (331) | 37·8±4·08 (216) | 30·4±5·9 (359) |
| Heart Rate (beats/min) | ||||
| Av daily HR | 339·3±60·4 (750) | 349·2±62·3 (717) | 366·9±64·2 (450) | 366·0±62·2 (750) |
| Av HR in light phase | 306·7±44·1 (390) | 323·1±53·3 (386) | 335·7±54·8 (234) | 332·1±48·4 (390) |
| Av HR in dark phase | 374·6±55·7 (360) | 379·6±58·4 (331) | 400·7±56·0 (216) | 398·7±56·5 (359) |
| Activity (arbitrary units) | ||||
| Av daily activity | 2·88±2·81 (745) | 2·51±2·50 (745) | 4·85±4·38 (447) | 3·50±3·74 (750) |
| Av activity in light phase | 1·41±1·73 (385) | 1·29±1·58 (385) | 2·53±2·90 (231) | 1·70±2·25 (385) |
| Av activity in dark phase | 4·46±2·88 (360) | 3·82±2·64 (360) | 7·32±4·34 (216) | 5·42±4·05 (360) |
| (B) P values resulting from MANOVA of telemetry data | |||||
|---|---|---|---|---|---|
| PNT | Day/night | Gender | Interaction PNT×gender | Interaction PNT×day/night phase | |
| Parameter | |||||
| SBP | 0·001 | 0·001 | 0·001 | 0·003 | 0·467 |
| DBP | 0·001 | 0·001 | 0·001 | 0·008 | 0·847 |
| MAP | 0·001 | 0·001 | 0·001 | 0·489 | 0·894 |
| PP | 0·001 | 0·001 | 0·001 | 0·001 | 0·017 |
| HR | 0·038 | 0·001 | 0·001 | 0·002 | 0·236 |
| Activity | 0·001 | 0·001 | 0·001 | 0·001 | 0·001 |
Bold numbers indicate significance (P<0·05) of dependent variables and interactions of these variables. SBP, systolic blood pressure; DBP, diastolic blood pressure; MAP, mean arterial pressure; PP, pulse pressure; HR, heart rate.
Values following drug treatment (amphetamine) were analysed by three-way ANOVA with prenatal treatment, amphetamine treatment and gender as variables, followed by Dunnett's post hoc test. Statistical significance was assumed at a value of P<0·05.
Vascular studies
All data are mean±s.e.m. For each mesenteric preparation, the maximal response and the concentration of agonist required for a 50% response (EC50) were determined. Differences in the mean log EC50 and maximal response values between VEH- and DEX-treated offspring mesenteric vasculatures were compared using Student's unpaired t-test. Data were further analysed by two-way ANOVA (prenatal treatment×dose of agonist); results were considered significant when P<0·05.
Results
Gestational weight gain, birth phenotype and catch-up growth
DEX administration throughout the final week of gestation resulted in a significant reduction in maternal weight gain, with no differences noted in the length of gestation, litter size, ratio of male to female pups born or pup viability (Table 1). Consistent with previous studies, DEX caused a significant reduction in the birth weight of offspring, which was observed to the same degree in both male and female pups (O'Regan et al. 2004). By weaning (postnatal day 21) and throughout adult life, the body weights of male and female offspring, treated with VEH or DEX were similar, that is, full catch-up growth had occurred (Table 1). Kidney size was not apparently altered by prenatal glucocorticoid treatment in either males (VEH: 0·005±0·0002 g/g bwt, n=7 and DEX: 0·0051±0·00014 g/g bwt, n=5) or females (VEH: 0·00535±0·00011 g/g bwt, n=5 and DEX: 0·00495±0·000177 g/g <0·001 bwt, n=6).
Table 1.
Maternal weight before treatment, maternal weight gain during the last week of pregnancy (days 15–21), gestation length, litter size, male:female ratio and group, male, and female offspring weight at birth, postnatal day 21, and at 7 months of age (i.e. adulthood). Results are mean±s.e.m.
| Birth weight (g) | Postnatal weight Day 21 | Weight at 7 months | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Maternal weight before treatment (g) | Maternal weight gain (g) | Gestation length (d) | Litter size | Male:female ratio | Group | Male | Female | Group | Male (g) | Female | Group | Male (g) | Female | |
| VEH | 365±14 (n=8) | 97±5 | 22±0 | 13±2·0 | 1·1±0·1 | 6·6±0·1 (n=99) | 6·6±0·9 (n=55) | 6·5±0·8 (n=44) | 55·7±1·1 (n=52) | 56·4±1·7 (n=26) | 57·6±1·2 (n=26) | 525±13 (n=20) | 690±12 (n=10) | 360±15 (n=10) |
| DEX | 363±6 (n=9) | 58±5* | 22±0 | 12±1·8 | 1·2±0·2 | 5·7±0·1† (n=108) | 5·6±0·8† (n=56) | 5·7±0·7† (n=52) | 52·7±1·4 (n=56) | 54±2·1 (n=29) | 55·2±1·2 (n=52) | 507±19·5 (n=20) | 653±25 (n=10) | 353±13 (n=10) |
*P<0·05, †P<0·01 compared with vehicle group.
Radiotelemetric monitoring of offspring BP, HR and activity under basal conditions
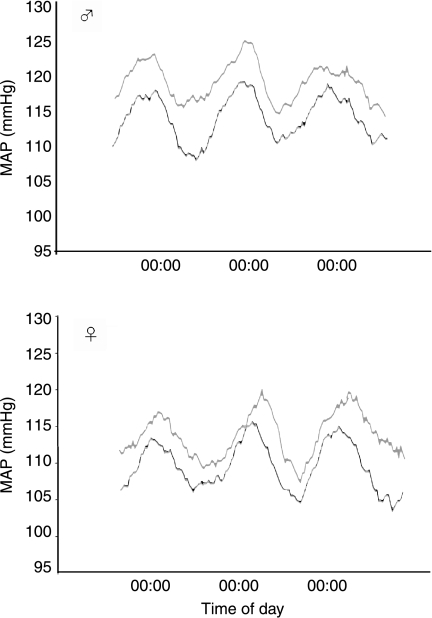
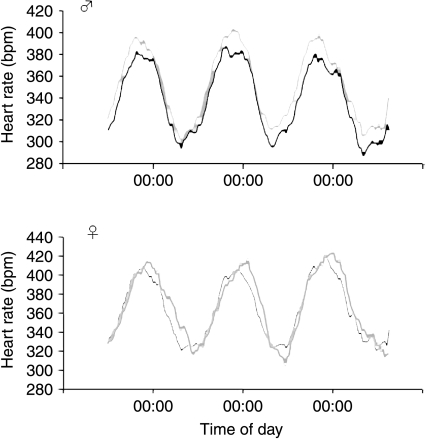
BP, HR and activity showed the expected sex variations and circadian variation in male and female offspring of both the prenatal treatment groups with the highest BP (MAP, SBP and DBP), HR and activity levels in the dark phase and lowest values during the light phase (Table 2 and Figs 1 and 2). Contrary to previous reports using indirect (tail cuff) and direct but stressful (carotid cannulation) methods to assess BP, both male and female DEX offspring showed basal hypotension with lower SBP, DBP, PP and HR compared with VEH rats (Figs 1 and 2 and Table 2), which was not dependent on whether the measurements were taken in the active, dark phase or the quiescent, light phase. However, there was a significant interaction between BP parameters and gender (Table 2), greater effects of prenatal treatment (PNT) being apparent in the males.
Figure 1.
Basal mean arterial blood pressure (MAP) in male and female offspring exposed to VEH (grey) or DEX (black) during the last week of gestation. Data are expressed as 6-h rolling averages over 3 days for each treatment group. n=4–5 per group.
Figure 2.
Basal heart rate patterns in male and female offspring exposed to VEH (grey) or DEX (black) during the last week of gestation. Data are expressed as 6-h rolling averages over 3 days. n=4–5 per group.
It was also observed that the DEX rats were less active than the VEH controls (Table 2), this effect was greater in the females compared with the males (interaction of PNT and gender, P<0·001) and was observed mostly in the active, dark phase of the 24 h (interaction of PNT and day/night phase, P<0·001). The reduced activity of the DEX rats may contribute to the observed hypotension.
Haemodynamic and activity responses to graded stress
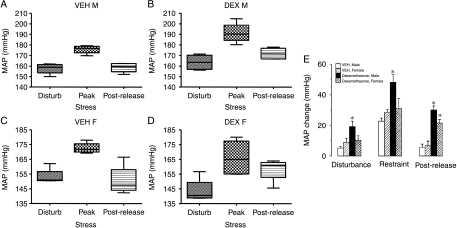
In utero exposure to DEX has been shown to cause a lifelong elevation in basal BP when measured by tail-cuff plethysmography and carotid cannulation. An exaggerated stress response to these measurement techniques could account for apparent hypertension. As shown in Fig. 3 (A, B and E), male, prenatal DEX-treated offspring were exquisitely sensitive to stress, displaying graded hypertensive responses when disturbed by a researcher entering their room and when restrained. Even weighing elicited a hypersensitive response in DEX-treated offspring (change in SBP; VEH 10±2 mmHg and DEX 19±3 mmHg; P<0·05). Moreover, DEX-treated offspring start with a reduced MAP compared with vehicle controls (Fig. 3A and B) and maintain their stress-induced hypertension for longer once released from restraint (Fig. 3E), and after weighing (change in SBP 5-min post-weighing; VEH 7±5 mmHg and DEX 23±5 mmHg; P<0·05). DEX-treated offspring further displayed significantly greater increases in HR in response to weighing (VEH 117±17 bpm and DEX 156±25 bpm; P<0·05), and restraint (VEH 86±11 bpm and DEX 136±19 bpm; P<0·05). The greater tachycardia on disturbance of prenatal DEX offspring was not associated with changes in activity (weighing: VEH 5·2±1 AU, DEX 5±1 AU and restraint: VEH 4·8±1·2 AU, DEX 4·6±1·8 AU). Although there was a significant response in BP to stress in all females, there was no significant difference between the VEH- and DEX-treated offspring in response to disturbance or restraint stress but the recovery period after stress shows DEX females take longer to return basal levels of MAP following restraint (Fig. 3C–E).
Figure 3.
Box–Whisker plots depicting MAP in response to disturbance, restraint and at 15-min post-restraint (n=4–5 per group) in (A) male offspring from VEH-treated dams, (B) male offspring from DEX-treated dams, (C) female offspring from VEH-treated dams and (E) female offspring from DEX-treated dams, (E) shows stress-induced MAP changes in prenatal VEH- and DEX-treated offspring, following disturbance, restraint and at 15-min post-restraint (mean±s.e.m., n=4–5 per group). *P<0·05 compared with vehicle group.
Prenatal DEX causes exaggerated hypertensive responses to catecholaminergic stimulation
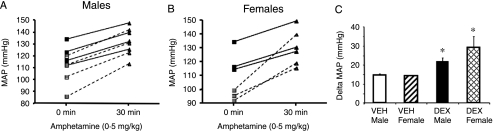
To assess the role of the SNS in mediating this stress-induced hypertension, we administered a low dose of d-amphetamine, to induce catecholamine release. While the DEX-treated offspring showed lower basal MAPs prior to amphetamine administration (Fig. 4A and B), they showed significantly greater increases in MAP and HR when treated with the drug compared with controls (amphetamine treatment F(1,20)=21·54, P=0·0002; prenatal treatment F(1,20)=13·19, P=0·0017; Fig. 4A–C). When the data were separated into male and female responses, it was demonstrated that there was no indication of a sex-specific effect (F(1,20)=0·52, P=0·48; Fig. 4C).
Figure 4.
Line plots indicating MAP from individual rats 0 and 30 min following amphetamine (0·5 mg/kg) in (A) male and (B) female offspring from either VEH (black symbols with solid line) or DEX (grey symbols with dotted line) dams. (C) Δ-MAP response to amphetamine (0·5 mg/kg; i.p.), in male and female offspring of VEH- and DEX-treated dams. Results are mean±s.e.m., three-way ANOVA (prenatal treatment×gender×postnatal drug), n=7 (4 ♂ and 3 ♀); *P<0·05 compared with vehicle group.
Responsiveness of mesenteric vasculature to NA, AVP and KCL
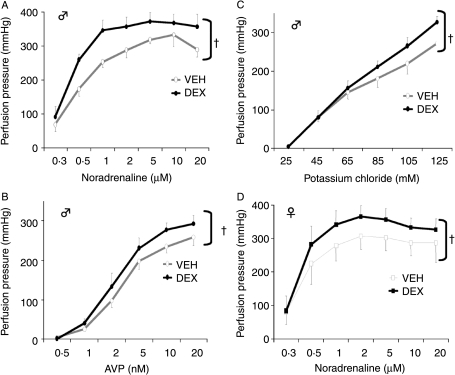
To examine further the basis for increased responsivity to catecholaminergic stimulation in DEX-exposed offspring, resistance (mesenteric) vessels were perfused ex vivo (Fig. 5A–C). Basal perfusion pressures were similar in the two groups of rats. Infusion of all agonists caused a concentration-dependent increase in perfusion pressures, with plateaux reached in both groups at 5 μM for NA and 10 nM for AVP. However, tissues from DEX-treated male offspring were significantly more responsive (the curves from DEX-treated offspring were significantly different from VEH controls; Fig. 5) and the Vmax (indicative of number of receptors) was higher in the DEX group in response to NA in both sexes (male VEH: 305±13 mmHg; male DEX: 360±4 mmHg; P=0·004; t-test. Female VEH: 292±5 mmHg; female DEX: 345±7 mmHg; P=0·001).
Figure 5.
Male offspring dose–response curves to (A) noradrenaline, (B) vasopressin, (C) potassium chloride and (D) female offspring dose–response curve to noradrenaline, in the isolated mesenteric vasculature from VEH- and DEX-treated rats. Results are mean±s.e.m., n=6 per group. †P<0·01 compared with vehicle group.
Female offspring
Several reports suggest that prenatal ‘programming’ phenotypes are sexually dimorphic, so the perfusion studies were repeated in females. Similar basal perfusion pressures were noted in the two prenatal treatment groups. NA caused a concentration-dependent increase in perfusion pressures, with plateaux reached at 2 μM in both treatment groups (Fig. 5D). However, the mesenteric vasculatures from DEX-treated offspring were significantly more responsive to NA, (as analysed by two-way ANOVA), even though the maximal contraction (VEH 344±40 mmHg versus DEX 373±30 mmHg; P=0·16) and EC50 (vehicle 0·43±0·02 μM versus DEX 0·39±0·03 μM; P=0·16) values were similar for both treatment groups. DEX treatment did not affect the pattern of responses to either AVP or KCl. Maximal responses in both groups were achieved at 5 nM AVP and 125 mM KCl (data not shown).
Discussion
The striking novel finding of this study is that prenatal DEX results in lower basal BP in the male and female adult offspring; with the commonly expected hypertensive phenotype only being noted when these offspring are subjected to a stressor, after which they exhibit a prolonged and excessive hypertensive response. These exaggerated hypertensive responses are mediated by alterations in the responsivity of the SNS, being further exaggerated by promotion of catecholamine release. Additionally, DEX-treated offspring display greater sensitivity to catecholamines, and only in males other vasoconstrictors, in resistance (mesenteric) vasculature.
Unexpectedly, over many days of recording undisturbed rats in their home cages, prenatal DEX-exposed adult rats showed lower BP than controls. This contrasts with both tail cuff plethysmography and direct carotid cannulation data in this model (Levitt et al. 1996) and in other models of ‘fetal programming’ (Woodall et al. 1996, Langley-Evans 1997, Woods 2006) most of which have generally employed techniques which involve stressful preheating of the tail and/or physical restraint or short-term recovery from anaesthesia. Indeed, several studies have documented by telemetry the stress-induced hypertension observed following restraint or using the tail-cuff protocol (Gross & Luft 2003, Whitesall et al. 2004, D'Angelo et al. 2005, Kurtz et al. 2005), confirming the telemetry technique as the ‘gold standard’ for BP recording. In our study, radiotelemetric measurements were recorded 1 week after implantation of intra-arterial cannulae to allow complete recovery from established effects of surgery. Previous studies have used telemetry to determine BP in other models of fetal programming following maternal undernutrition (Tonkiss et al. 1998, Khan et al. 2003, Fernandez-Twinn et al. 2006) or uterine artery ligation (Jansson & Lambert 1999). BP differences by telemetry have generally been slight, with normotension or at most mild hypertension, compared with more overt hypertension using indirect techniques. The quite clear-cut lower basal BP seen here in males and females in both active and quiescent phases may therefore reflect specific effects of prenatal glucocorticoid excess compared with other manipulations, even though it is acknowledged that glucocorticoid programming is combined with an effect on food intake and maternal weight (present data; Levitt et al. 1996, Welberg et al. 2001, Woods 2006). However, short-term measurements of BP, even over a few hours, failed to attribute statistical significance between the prenatally treated groups in this study, emphasizing the statistical power of the telemetric BP measurements, but highlighting potential differences between studies and analysis of the data.
The hypotension observed in the DEX rats may in part be attributable to decreased activity in DEX rats compared with VEH. However, activity differences are greater in the female DEX rats (compared with female VEH rats), while BP parameters of hypotension are greater in the male DEX rats indicating that several parameters may be altered to mediate the BP changes and that these mechanisms may be sex specific.
Merely entering the room in which the rats were housed was sufficient to drive hypertension selectively in male, prenatal DEX-exposed offspring. When further subjected to a moderate stressor (weighing) and the more severe restraint stress the hypertensive responses of the male DEX-treated offspring were significantly augmented, suggesting increased sensitivity to environmental stress. An increased reactivity to stressful environmental stimuli may lead to established hypertension through frequent transient BP elevations (Folkow et al. 1978). Whether or not the prenatal DEX-exposed offspring will eventually become permanently hypertensive warrants further study, as true life consists of a constant series of ‘stressful situations’ that have to be dealt with, and this is not mimicked in undisturbed laboratory animals. Indeed, the DEX-treated offspring maintained their hypertension long after the removal of each stressor. Interestingly, offspring of malnourished dams display a greater hypertensive response to stress (ammonia odour) echoing the clear stress-responsive hypertension shown here in prenatal glucocorticoid programming (Tonkiss et al. 1998). It is therefore tempting to speculate that prenatal glucocorticoid programming of exaggerated cardiovascular responses to stress may be the fundamental change in these models, with permanent hypertension a secondary phenomenon dependent upon the postnatal environment and its challenges. This would parallel the emerging concepts of amplification of the low birth weight baby metabolic phenotype by postnatal caloric excess (Ozanne & Nicholas Hales 2005). Moreover, lower basal BP but enhanced responses to stress align perhaps better with concepts of adaptive advantages to the predicted (stressful) postnatal environment of a stressed pregnancy than merely constant hypertension, which has few apparent benefits.
DEX-treated offspring had higher HR with stress, a marker, although indirect, of increased sympathetic drive. The cardiodynamic responses to catecholamine release with amphetamine (or catecholamine depletion with reserpine, O'Regan unpublished results) strongly support this notion of a more responsive SNS in DEX-programmed adult rats. Amphetamine caused an exaggerated increase in BP in DEX-treated offspring compared with controls and reserpine abolished BP differences between DEX and VEH groups basally and in response to stress. The SNS participates in several stress responses, even in fetal life (McMillen et al. 2001) and therefore prenatal exposure to various stressors might affect the development of sympathetic innervation or its regulation. Epidemiological evidence of HR variability in infants demonstrates a positive correlation of this index of sympathetic activity with low birth weight, suggesting that decreased fetal growth is associated with a dysfunctional autonomic nervous system which may play a role in programming of BP (Massin et al. 2001). Indeed in rats, the offspring of prenatal DEX-treated dams have increased NA turnover in central brain regions concerned with BP control (Slotkin et al. 1992), however, cardiac β-adrenergic responses are blunted in low protein diet-induced-programmed rats (Fernandez-Twinn et al. 2006). In distinct prenatal rat models, gestational nicotine impairs the maturation of both central and peripheral catecholaminergic pathways (Navarro et al. 1990) and hypoxia alters development of sympathetic centres involved in BP regulation (Peyronnet et al. 2002), effects which persist into adulthood. Similarly, ligation of the umbilical artery alters SNS activity in newborn sheep (Oyama et al. 1992) and adult female rat offspring (Jansson & Lambert 1999). Taken together, these data indicate that diverse challenges in utero alter the development and function of the adult SNS. A recent study of low birth weight adult humans proposed that their lower resting muscle sympathetic nerve activities were a function of altered SNS development (Weitz et al. 2003).
Sex effects on BP regulation in prenatal-programming models have been shown in a few studies (Kind et al. 2002, O'Regan et al. 2004), but not others (Langley-Evans et al. 1996, Woods & Weeks 2005), although the hypertension may be generated by sex-specific mechanisms (McMullen & Langley-Evans 2005a,b). Indeed in humans, females have been shown to have more efficient regulation of SNS pathways, making them less susceptible to hypertension (Hinojosa-Laborde et al. 1999). However, in this study, we show similar effects on BP in males and females, but the magnitude of hypotension and a greater stress-induced hypertension is observed in males. The response to catecholamine release was similar in both sexes. Therefore, further studies will be required to tease out the mechanisms underpinning the sex-specific responsivity in this programming model.
Whatever the changes in SNS drive, DEX-treated offspring showed greater in vitro mesenteric pressor responses to NA in both male and females suggesting increased tissue sensitivity. There were also greater pressor responses to AVP and a depolarizing potassium solution in males only. Similar results were obtained in a study looking at female offspring from mothers treated with DEX throughout pregnancy (Hadoke et al. 2006). The in vitro perfusion preparation used in these studies was separated from other in vivo systems (e.g. circulating angiotensin II, endothelin), suggesting that a change in receptor binding or alterations in the production of local vasorelaxants/constrictors underlies the increased vascular sensitivity. Glucocorticoids may regulate the synthesis of vasoactive compounds, such as prostaglandins or nitric oxide, which in turn modulate peripheral vascular reactivity. Indeed, inhibition of nitric oxide synthesis (Wallerath et al. 1999) and decreased levels of both prostaglandin and its mediator adenylate cyclase (Handa et al. 1984) have been demonstrated in rats with adult DEX-induced hypertension. Similarly, increased mesenteric vasculature sensitivity to NA has been observed in rats treated as adults with low doses of DEX (Russo et al. 1990). Whether the effects are due to the known modest elevations of corticosterone in DEX-programmed rats (Levitt et al. 1996, Welberg et al. 2001) or reflect primary developmental effects on the mesenteric vasculature that persist into adult life remains uncertain. The gender differences in mesenteric vasculature responses of DEX-treated offspring to both AVP and KCl are intriguing, and could possibly reflect changes in vascular reactivity during the reproductive cycle (Dalle Lucca et al. 2000), and the effect of sex steroids on vascular smooth muscle sensitivity (Garcia-Villalon et al. 1996). Interestingly, gender has previously been demonstrated to have no effect on the sensitivity to NA in both the rat tail artery and aorta (Li & Duckles 1994, Fulton & Stallone 2002).
The present study provides novel and exciting information regarding the nature and mechanisms of glucocorticoid-induced alterations in postnatal BP. As the prenatal glucocorticoid exposure models extend our understanding of the fetal origins phenomena, it is clear that there is a need for replication and extended studies in humans. Only then, will we be better equipped to understand both the immediate and long-term consequences of antenatal glucocorticoid treatment.
Acknowledgements
Expert animal assistance was provided by Gillian Brooker for the surgical implantation of telemetric devices, and by Willy Mungall. This study was supported by a Wellcome Trust CVRI studentship (D O'R), the Wellcome Trust (J R S and M C H) and the Medical Research Council (C J K). The authors declare that there is no conflict of interest that would prejudice the impartiality of this scientific work.
References
- Anderson NH, Devlin AM, Graham D, Morton JJ, Hamilton CA, Reid JL, Schork NJ, Dominiczak AF. Telemetry for cardiovascular monitoring in a pharmacological study: new approaches to data analysis. Hypertension. 1999;33:248–255. doi: 10.1161/01.hyp.33.1.248. [DOI] [PubMed] [Google Scholar]
- Barker DJ. The fetal and infant origins of adult disease. British Medical Journal. 1990;301:1111. doi: 10.1136/bmj.301.6761.1111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barker DJ. Fetal origins of cardiovascular disease. Annals of Medicine. 1999;31(Suppl 1):3–6. [PubMed] [Google Scholar]
- Barker DJ. The developmental origins of adult disease. European Journal of Epidemiology. 2003;18:733–736. doi: 10.1023/a:1025388901248. [DOI] [PubMed] [Google Scholar]
- Benediktsson R, Lindsay RS, Noble J, Seckl JR, Edwards CR. Glucocorticoid exposure in utero: new model for adult hypertension. Lancet. 1993;341:339–341. doi: 10.1016/0140-6736(93)90138-7. [DOI] [PubMed] [Google Scholar]
- Bian XP, Seidler FJ, Slotkin TA. Promotional role for glucocorticoids in the development of intracellular signalling: enhanced cardiac and renal adenylate cyclase reactivity to beta-adrenergic and non-adrenergic stimuli after low-dose fetal dexamethasone exposure. Journal of Developmental Physiology. 1992;17:289–297. [PubMed] [Google Scholar]
- Bian X, Seidler FJ, Slotkin TA. Fetal dexamethasone exposure interferes with establishment of cardiac noradrenergic innervation and sympathetic activity. Teratology. 1993;47:109–117. doi: 10.1002/tera.1420470203. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Willett WC, Rimm EB, Spiegelman D, Ascherio AL, Stampfer MJ. Birth weight and adult hypertension, diabetes mellitus, and obesity in US men. Circulation. 1996a;94:3246–3250. doi: 10.1161/01.cir.94.12.3246. [DOI] [PubMed] [Google Scholar]
- Curhan GC, Chertow GM, Willett WC, Spiegelman D, Colditz GA, Manson JE, Speizer FE, Stampfer MJ. Birth weight and adult hypertension and obesity in women. Circulation. 1996b;94:1310–1315. doi: 10.1161/01.cir.94.6.1310. [DOI] [PubMed] [Google Scholar]
- Dalle Lucca JJ, Adeagbo AS, Alsip NL. Influence of oestrous cycle and pregnancy on the reactivity of the rat mesenteric vascular bed. Human Reproduction. 2000;15:961–968. doi: 10.1093/humrep/15.4.961. [DOI] [PubMed] [Google Scholar]
- D'Angelo G, Elmarakby AA, Pollock DM, Stepp DW. Fructose feeding increases insulin resistance but not blood pressure in Sprague–Dawley rats. Hypertension. 2005;46:806–811. doi: 10.1161/01.HYP.0000182697.39687.34. [DOI] [PubMed] [Google Scholar]
- Fall CH, Stein CE, Kumaran K, Cox V, Osmond C, Barker DJ, Hales CN. Size at birth, maternal weight, and type 2 diabetes in South India. Diabetic Medicine. 1998;15:220–227. doi: 10.1002/(SICI)1096-9136(199803)15:3<220::AID-DIA544>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- Fernandez-Twinn DS, Ekizoglou S, Wayman A, Petry CJ, Ozanne SE. Maternal low protein diet programs cardiac beta-adrenergic response and signalling in 3 month old male offspring. American Journal of Physiology. 2006;291:R429–R436. doi: 10.1152/ajpregu.00608.2005. [DOI] [PubMed] [Google Scholar]
- Folkow B, Hallback MIL, Genest J, Koiw E, Kuchel O. Physiopathology of spontaneous hypertension in rats. In: Genest J, Koiw E, Kuchel O, editors. Hypertension: Pathophysiology and Treatment. MacGraw Hill; New York: 1978. pp. 507–529. [Google Scholar]
- Fulton CT, Stallone JN. Sexual dimorphism in prostanoid-potentiated vascular contraction: roles of endothelium and ovarian steroids. American Journal of Physiology. 2002;283:H2062–H2073. doi: 10.1152/ajpheart.00099.2002. [DOI] [PubMed] [Google Scholar]
- Garcia-Villalon AL, Buchholz JN, Krause DN, Duckles SP. Sex differences in the effects of 17 beta-estradiol on vascular adrenergic responses. European Journal of Pharmacology. 1996;314:339–345. doi: 10.1016/s0014-2999(96)00565-1. [DOI] [PubMed] [Google Scholar]
- Gross V, Luft FC. Exercising restraint in measuring blood pressure in conscious mice. Hypertension. 2003;41:879–881. doi: 10.1161/01.HYP.0000060866.69947.D1. [DOI] [PubMed] [Google Scholar]
- Hadoke PW, Lindsay RS, Seckl JR, Walker BR, Kenyon CJ. Altered vascular contractility in adult female rats with hypertension programmed by prenatal glucocorticoid exposure. Journal of Endocrinology. 2006;188:435–442. doi: 10.1677/joe.1.06506. [DOI] [PubMed] [Google Scholar]
- Handa M, Kondo K, Suzuki H, Saruta T. Dexamethasone hypertension in rats: role of prostaglandins and pressor sensitivity to norepinephrine. Hypertension. 1984;6:236–241. [PubMed] [Google Scholar]
- Hinojosa-Laborde C, Chapa I, Lange D, Haywood JR. Gender differences in sympathetic nervous system regulation. Clinical and Experimental Pharmacology and Physiology. 1999;26:122–126. doi: 10.1046/j.1440-1681.1999.02995.x. [DOI] [PubMed] [Google Scholar]
- Huff RA, Seidler FJ, Slotkin TA. Glucocorticoids regulate the ontogenetic transition of adrenergic receptor subtypes in rat liver. Life Sciences. 1991;48:1059–1065. doi: 10.1016/0024-3205(91)90507-8. [DOI] [PubMed] [Google Scholar]
- Jansson T, Lambert GW. Effect of intrauterine growth restriction on blood pressure, glucose tolerance and sympathetic nervous system activity in the rat at 3–4 months of age. Journal of Hypertension. 1999;17:1239–1248. doi: 10.1097/00004872-199917090-00002. [DOI] [PubMed] [Google Scholar]
- Khan IY, Taylor PD, Dekou V, Seed PT, Lakasing L, Graham D, Dominiczak AF, Hanson MA, Poston L. Gender-linked hypertension in offspring of lard-fed pregnant rats. Hypertension. 2003;41:168–175. doi: 10.1161/01.hyp.0000047511.97879.fc. [DOI] [PubMed] [Google Scholar]
- Kind KL, Simonetta G, Clifton PM, Robinson JS, Owens JA. Effect of maternal feed restriction on blood pressure in the adult guinea pig. Experimental Physiology. 2002;87:469–477. doi: 10.1111/j.1469-445x.2002.tb00060.x. [DOI] [PubMed] [Google Scholar]
- Kreider ML, Aldridge JE, Cousins MM, Oliver CA, Seidler FJ, Slotkin TA. Disruption of rat forebrain development by glucocorticoids: critical perinatal periods for effects on neural cell acquisition and on cell signaling cascades mediating noradrenergic and cholinergic neurotransmitter/neurotrophic responses. Neuropsychopharmacology. 2005;30:1841–1855. doi: 10.1038/sj.npp.1300743. [DOI] [PubMed] [Google Scholar]
- Kurtz TW, Griffin KA, Bidani AK, Davisson RL, Hall JE. Recommendations for blood pressure measurement in humans and experimental animals: part 2: blood pressure measurement in experimental animals: a statement for professionals from the Subcommittee of Professional and Public Education of the American Heart Association Council on High Blood Pressure Research. Arteriosclerosis, Thrombosis, and Vascular Biology. 2005;25:E22–E33. doi: 10.1161/01.ATV.0000158419.98675.d7. [DOI] [PubMed] [Google Scholar]
- Langdown ML, Smith ND, Sugden MC, Holness MJ. Excessive glucocorticoid exposure during late intrauterine development modulates the expression of cardiac uncoupling proteins in adult hypertensive male offspring. Pflugers Archiv. 2001;442:248–255. doi: 10.1007/s004240100519. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC. Hypertension induced by foetal exposure to a maternal low-protein diet, in the rat, is prevented by pharmacological blockade of maternal glucocorticoid synthesis. Journal of Hypertension. 1997;15:537–544. doi: 10.1097/00004872-199715050-00010. [DOI] [PubMed] [Google Scholar]
- Langley-Evans SC, Phillips GJ, Benediktsson R, Gardner DS, Edwards CR, Jackson AA, Seckl JR. Protein intake in pregnancy, placental glucocorticoid metabolism and the programming of hypertension in the rat. Placenta. 1996;17:169–172. doi: 10.1016/s0143-4004(96)80010-5. [DOI] [PubMed] [Google Scholar]
- Law CM, Egger P, Dada O, Delgado H, Kylberg E, Lavin P, Tang GH, von Hertzen H, Shiell AW, Barker DJ. Body size at birth and blood pressure among children in developing countries. International Journal of Epidemiology. 2001;30:52–57. doi: 10.1093/ije/30.1.52. [DOI] [PubMed] [Google Scholar]
- Levitt NS, Lindsay RS, Holmes MC, Seckl JR. Dexamethasone in the last week of pregnancy attenuates hippocampal glucocorticoid receptor gene expression and elevates blood pressure in the adult offspring in the rat. Neuroendocrinology. 1996;64:412–418. doi: 10.1159/000127146. [DOI] [PubMed] [Google Scholar]
- Li Z, Duckles SP. Influence of gender on vascular reactivity in the rat. Journal of Pharmacology and Experimental Therapeutics. 1994;268:1426–1431. [PubMed] [Google Scholar]
- Massin MM, Withofs N, Maeyns K, Ravet F. The influence of fetal and postnatal growth on heart rate variability in young infants. Cardiology. 2001;95:80–83. doi: 10.1159/000047350. [DOI] [PubMed] [Google Scholar]
- McCance DR, Pettitt DJ, Hanson RL, Jacobsson LT, Knowler WC, Bennett PH. Birth weight and non-insulin dependent diabetes: thrifty genotype, thrifty phenotype, or surviving small baby genotype? British Medical Journal. 1994;308:942–945. doi: 10.1136/bmj.308.6934.942. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McGregor DD. The effect of sympathetic nerve stimulation on vasoconstrictor responses in perfused mesenteric blood vessel of the rat. Journal of Physiology. 1965;177:21–30. doi: 10.1113/jphysiol.1965.sp007572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McMillen IC, Adams MB, Ross JT, Coulter CL, Simonetta G, Owens JA, Robinson JS, Edwards LJ. Fetal growth restriction: adaptations and consequences. Reproduction. 2001;122:195–204. doi: 10.1530/rep.0.1220195. [DOI] [PubMed] [Google Scholar]
- McMullen S, Langley-Evans SC. Maternal low-protein diet in rat pregnancy programs blood pressure through sex-specific mechanisms. American Journal of Physiology. 2005a;288:R85–R90. doi: 10.1152/ajpregu.00435.2004. [DOI] [PubMed] [Google Scholar]
- McMullen S, Langley-Evans SC. Sex-specific effects of prenatal low-protein and carbenoxolone exposure on renal angiotensin receptor expression in rats. Hypertension. 2005b;46:1374–1380. doi: 10.1161/01.HYP.0000188702.96256.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro HA, Mills E, Seidler FJ, Baker FE, Lappi SE, Tayyeb MI, Spencer JR, Slotkin TA. Prenatal nicotine exposure impairs beta-adrenergic function: persistent chronotropic subsensitivity despite recovery from deficits in receptor binding. Brain Research Bulletin. 1990;25:233–237. doi: 10.1016/0361-9230(90)90066-9. [DOI] [PubMed] [Google Scholar]
- Nyirenda MJ, Lindsay RS, Kenyon CJ, Burchell A, Seckl JR. Glucocorticoid exposure in late gestation permanently programs rat hepatic phosphoenolpyruvate carboxykinase and glucocorticoid receptor expression and causes glucose intolerance in adult offspring. Journal of Clinical Investigation. 1998;101:2174–2181. doi: 10.1172/JCI1567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyirenda MJ, Welberg LA, Seckl JR. Programming hyperglycaemia in the rat through prenatal exposure to glucocorticoids-fetal effect or maternal influence? Journal of Endocrinology. 2001;170:653–660. doi: 10.1677/joe.0.1700653. [DOI] [PubMed] [Google Scholar]
- O'Regan D, Kenyon CJ, Seckl JR, Holmes MC. Glucocorticoid exposure in late gestation in the rat permanently programs gender-specific differences in adult cardiovascular and metabolic physiology. American Journal of Physiology. 2004;287:E863–E870. doi: 10.1152/ajpendo.00137.2004. [DOI] [PubMed] [Google Scholar]
- Ortiz LA, Quan A, Zarzar F, Weinberg A, Baum M. Prenatal dexamethasone programs hypertension and renal injury in the rat. Hypertension. 2003;41:328–334. doi: 10.1161/01.hyp.0000049763.51269.51. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oyama K, Padbury J, Martinez A, Chappell B, Stein H, Humme J. Effects of fetal growth retardation on the development of central and peripheral catecholaminergic pathways in the sheep. Journal of Developmental Physiology. 1992;18:217–222. [PubMed] [Google Scholar]
- Ozanne SE, Nicholas Hales C. Poor fetal growth followed by rapid postnatal catch-up growth leads to premature death. Mechanisms of Ageing and Development. 2005;126:852–854. doi: 10.1016/j.mad.2005.03.005. [DOI] [PubMed] [Google Scholar]
- Padbury JF, Polk DH, Ervin MG, Berry LM, Ikegami M, Jobe AH. Postnatal cardiovascular and metabolic responses to a single intramuscular dose of betamethasone in fetal sheep born prematurely by cesarean section. Pediatric Research. 1995;38:709–715. doi: 10.1203/00006450-199511000-00013. [DOI] [PubMed] [Google Scholar]
- Peyronnet J, Dalmaz Y, Ehrstrom M, Mamet J, Roux JC, Pequignot JM, Thoren HP, Lagercrantz H. Long-lasting adverse effects of prenatal hypoxia on developing autonomic nervous system and cardiovascular parameters in rats. Pflugers Archiv. 2002;443:858–865. doi: 10.1007/s00424-001-0766-9. [DOI] [PubMed] [Google Scholar]
- Phillips DI, Barker DJ. Association between low birthweight and high resting pulse in adult life: is the sympathetic nervous system involved in programming the insulin resistance syndrome? Diabetic Medicine. 1997;14:673–677. doi: 10.1002/(SICI)1096-9136(199708)14:8<673::AID-DIA458>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Roghair RD, Lamb FS, Miller FJ, Jr, Scholz TD, Segar JL. Early gestation dexamethasone programs enhanced postnatal ovine coronary artery vascular reactivity. American Journal of Physiology. 2005;288:R46–R53. doi: 10.1152/ajpregu.00165.2004. [DOI] [PubMed] [Google Scholar]
- Russo D, Fraser R, Kenyon CJ. Increased sensitivity to noradrenaline in glucocorticoid-treated rats: the effects of indomethacin and desipramine. Journal of Hypertension. 1990;8:827–833. doi: 10.1097/00004872-199009000-00006. [DOI] [PubMed] [Google Scholar]
- Saruta T. Mechanism of glucocorticoid-induced hypertension. Hypertension Research. 1996;19:1–8. doi: 10.1291/hypres.19.1. [DOI] [PubMed] [Google Scholar]
- Seckl JR. Prenatal glucocorticoids and long-term programming. European Journal of Endocrinology. 2004;151(Suppl 3):U49–U62. doi: 10.1530/eje.0.151u049. [DOI] [PubMed] [Google Scholar]
- Slotkin TA, Lappi SE, McCook EC, Tayyeb MI, Eylers JP, Seidler FJ. Glucocorticoids and the development of neuronal function: effects of prenatal dexamethasone exposure on central noradrenergic activity. Biology of the Neonate. 1992;61:326–336. doi: 10.1159/000243761. [DOI] [PubMed] [Google Scholar]
- Stein HM, Martinez A, Blount L, Oyama K, Padbury JF. The effects of corticosteroids and thyrotropin-releasing hormone on newborn adaptation and sympathoadrenal mechanisms in preterm sheep. American Journal of Obstetrics and Gynecology. 1994;171:17–24. doi: 10.1016/s0002-9378(94)70071-0. [DOI] [PubMed] [Google Scholar]
- Sugden MC, Langdown ML, Munns MJ, Holness MJ. Maternal glucocorticoid treatment modulates placental leptin and leptin receptor expression and materno–fetal leptin physiology during late pregnancy, and elicits hypertension associated with hyperleptinaemia in the early-growth-retarded adult offspring. European Journal of Endocrinology. 2001;145:529–539. doi: 10.1530/eje.0.1450529. [DOI] [PubMed] [Google Scholar]
- Tonkiss J, Trzcinska M, Galler JR, Ruiz-Opazo N, Herrera VL. Prenatal malnutrition-induced changes in blood pressure: dissociation of stress and nonstress responses using radiotelemetry. Hypertension. 1998;32:108–114. doi: 10.1161/01.hyp.32.1.108. [DOI] [PubMed] [Google Scholar]
- Torres A, Belser WW, III, Umeda PK, Tucker D. Indicators of delayed maturation of rat heart treated prenatally with dexamethasone. Pediatric Research. 1997;42:139–144. doi: 10.1203/00006450-199708000-00001. [DOI] [PubMed] [Google Scholar]
- Tseng YT, Tucker MA, Kashiwai KT, Waschek JA, Padbury JF. Regulation of beta 1-adrenoceptors by glucocorticoids and thyroid hormones in fetal sheep. European Journal of Pharmacology. 1995;289:353–359. doi: 10.1016/0922-4106(95)90113-2. [DOI] [PubMed] [Google Scholar]
- Wallerath T, Witte K, Schafer SC, Schwarz PM, Prellwitz W, Wohlfart P, Kleinert H, Lehr HA, Lemmer B, Forstermann U. Down-regulation of the expression of endothelial NO synthase is likely to contribute to glucocorticoid-mediated hypertension. PNAS. 1999;96:13357–13362. doi: 10.1073/pnas.96.23.13357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weitz G, Deckert P, Heindl S, Struck J, Perras B, Dodt C. Evidence for lower sympathetic nerve activity in young adults with low birth weight. Journal of Hypertension. 2003;21:943–950. doi: 10.1097/00004872-200305000-00019. [DOI] [PubMed] [Google Scholar]
- Welberg LA, Seckl JR, Holmes MC. Prenatal glucocorticoid programming of brain corticosteroid receptors and corticotrophin-releasing hormone: possible implications for behaviour. Neuroscience. 2001;104:71–79. doi: 10.1016/s0306-4522(01)00065-3. [DOI] [PubMed] [Google Scholar]
- Whitesall SE, Hoff JB, Vollmer AP, D'Alecy LG. Comparison of simultaneous measurement of mouse systolic arterial blood pressure by radiotelemetry and tail-cuff methods. American Journal of Physiology. 2004;286:H2408–H2415. doi: 10.1152/ajpheart.01089.2003. [DOI] [PubMed] [Google Scholar]
- Whitworth JA, Brown MA, Kelly JJ, Williamson PM. Experimental studies on cortisol-induced hypertension in humans. Steroids. 1995;9:395–480. [PubMed] [Google Scholar]
- Woodall SM, Johnston BM, Breier BH, Gluckman PD. Chronic maternal undernutrition in the rat leads to delayed postnatal growth and elevated blood pressure of offspring. Pediatric Research. 1996;40:438–443. doi: 10.1203/00006450-199609000-00012. [DOI] [PubMed] [Google Scholar]
- Woods LL. Maternal glucocorticoids and prenatal programming of hypertension. American Journal of Physiology. 2006;291:R1069–R1075. doi: 10.1152/ajpregu.00753.2005. [DOI] [PubMed] [Google Scholar]
- Woods LL, Weeks DA. Prenatal programming of adult blood pressure: role of maternal corticosteroids. American Journal of Physiology. 2005:R955–R962. doi: 10.1152/ajpregu.00455.2004. [DOI] [PubMed] [Google Scholar]