Abstract
Vascular changes after acute spinal cord trauma are important factors that predispose quadriplegia, in most cases irreversible. Repair of the spinal blood flow helps the spinal cord recovery. The average time to arrive and perform surgery is 3 h in most cases. It is important to determine the critical ischemia time in order to offer better functional prognosis. A spinal cord section and vascular clamping of the spinal anterior artery at C5–C6 model was used to determine critical ischemia time. The objective was to establish a critical ischemia time in a model of acute spinal cord section. Four groups of dogs were used, anterior approach and vascular clamp of spinal anterior artery with 1, 2, 3, and 4 h of ischemia and posterior hemisection of spinal cord at C5–C6 was performed. Clinical evaluation was made during 12 weeks and morphological evaluation at the end of this period. We obtained a maximal neurological coordination at 23 days average. Two cases showed sequels of right upper limb paresis at 1 and 3 ischemia hours. There was nerve conduction delay of 56% at 3 h of ischemia. Morphological examination showed 25% of damaged area. The VIII and IX Rexed’s laminae were the most affected. The critical ischemia time was 3 h. Dogs with 4 h did not exhibit any recovery.
Keywords: Spinal cord injury, Anterior spinal artery, Critical ischemia time
Introduction
In most countries, there are 20–40 acute spinal cord injuries/1,000,000 in adults per year. The main causes of spinal cord trauma are automobile accidents, sports and recreational activities, diving injuries, and home accidents. Nearly half of the patients present a complete damage of the spinal cord with motor and sensory dysfunction below the level of lesion; in almost two of three patients the lesion is at a cervical level [1]. In the United States there are 10,000 new cases per year with great cost due to its attention and strategies for decreasing the temporary or permanent disabilities that can have significant effects [2].
A posttraumatic quadriplegia is a paralysis of four limbs which occurs as a consequence of severe cervical lesion and the sequelae are generally irreversible. The present day treatment is focusing on preventing acute and permanent disabilities, which consist of early stabilization, handling of neurogenic bladder, early rehabilitation, tendinous transpositions, and electric peripheral muscle stimulation. In 1671, Stensen made the first experiment on the blood supply of the spinal cord when, in a dogfish, he showed that ligation of the aorta caused tail paralysis which recovered when the ligature was loosened [3].
The pathological changes after cervical lesion in dogs were described in the following manner: Allen in 1911, was the first to describe this: “At 15 min a petechial hemorrhage of gray matter and white matter edema was observed, at 2 h the gray matter hemorrhage increased, and at 4 h there were axis-cylinders “numerous swollen axis cylinders” [1]. Ducker in 1971, shows changes of severe necrosis at 6 days [1]. Dohrmann referred a distention of muscular layer of small veins by accumulation of red blood cells without axonal changes at 5 min. At 15–30 min small hemorrhages with red blood cells extravasation and discrete axonal changes were observed and at 4 h there is a disruption of myelin sheaths, axonal degeneration, and ischemic endothelial lesion.
Nemecek in 1978 defined the severe necrosis as “self-destruction” which occurs after 6 days of acute lesion. Griffiths and McCulloch showed from the first few days progressive axonal changes and necrotic areas [1]. Bresnahan and Balentine, by means of electronic microscopy, found that the trauma induces granular dissolution of the axoplasm and vesicular disruption of myelin, especially in the white matter. With this support, the treatment is focusing on decreasing the inflammation using drugs such as methilprednisolone and nimodipine [1]. The main obstacle in obtaining a good prognosis is the delay between the moment in which the trauma occurs and the arrival to the emergency services. It is important to point out that in cervical trauma additionally to spinal cord lesion; there is an area of vascular damage.
In previous works [4], we experimentally reproduced the quadriplegia in dogs with spinal cord section and spinal anterior artery section at the cervical level and observed that the section of only spinal cord with preservation of vascular supply did not cause irreversible neurological lesion in spite of the 1st day after the trauma, observing signs of quadriplegia, however, the animals recovered totally. We concluded that the spinal cord vascular supply at the motor area of the spinal cord had a principal role in the damage reversion. In our experimental groups of dogs, the microsurgical repair of vessels sectioned permit the neurological recovery in a maximum of 8 weeks [4].
The repair of the spinal blood flow helps the spinal cord recovery, however, it is unknown if the revascularization had good results after 3 h of spinal section, which in most cases is the average time to arrive and perform surgery. For these reasons it is important to know the critical ischemia time with the purpose of offering a better functional prognosis.
Vascular supply of cervical spinal cord
The vascular supply in the motor area of the spinal cord depends on the anterior spinal artery, this is a branch of vertebral arteries, arising from the brachiocephalic trunk and the subclavian artery [5–7].
The centrifugal artery system of sulcal arteries supplies most of the gray and white matter of ventral and lateral spinal cord, each sulcal artery supplies one half of the spinal cord. The centripetal artery system arising at the posterior spinal arteries supplies the posterior white and gray matter. In contrast with classic concepts there is not any pial arterial plexus in the ventral and ventro-lateral surfaces, except by infrequent transversal branches of anterior spinal artery. In the posterior columns there are two systems of large veins: the posteromedial septal vein and the posterior oblique vein that drains the posterior columns, of the poster medial gray matter and the posterior gray commissure.
The remainder of gray and white matter is drained by radial and sulcal veins [8]. It has been postulated that the vascular mechanisms are important in the physiopathology of the acute damage of spinal cord and the progressive necrosis. The vascular flow reduction had a relationship with the severity of the damage. The mechanism that causes the posttraumatic ischemia is unknown, the spasm of the sulcal arteries reduces the blood supply at the side of the lesion; the intramedullary branches of the posterior spinal arteries at the level, showed distal occlusions with the resulting gray and white posterior matter ischemia. It is probable that capillary occlusion and disruption occur at the lesion level as a result of the direct mechanic trauma [9].
Materials and methods
A hemi-section of 50% of the right half at the level of C5–C6 was performed. It was determined that with a section greater than 50% at this level, the animal in the model did not recuperate respiratory automatism or died in the attempt.
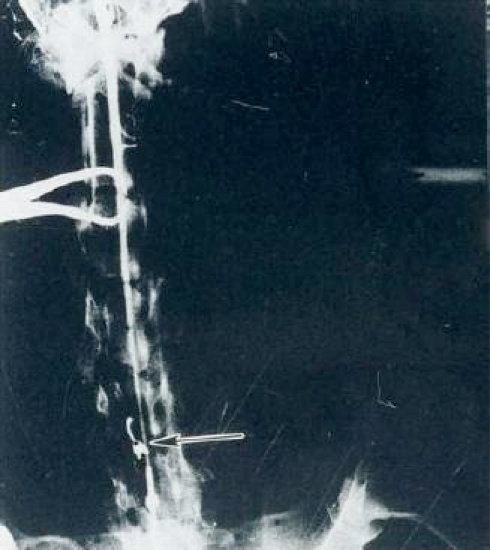
Simultaneously with the spinal cord section a vascular mini bulldog clamp angled 45° (20–25 g) was placed to cause ischemia at the level of the anterior spinal artery, separating the animals into four groups with 1, 2, 3, and 4 h of ischemia; clinically noticing the changes from the time immediately after surgery in the stage of quadriplegia, by daily clinical analysis, until the time of final evaluation of each case. Angiographic controls were taken (Fig. 1).
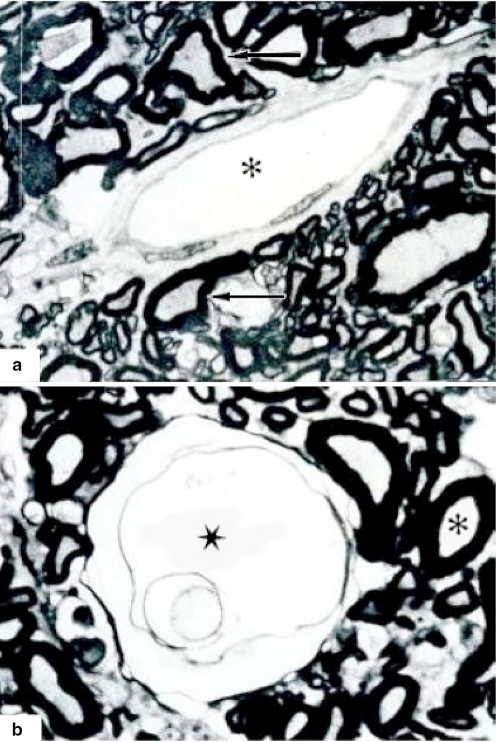
Fig. 1.
Vascular clamp (arrow) on the anterior spinal artery (C5–C6)
The purpose of applying ischemia was to determine the time limit until which clinical recuperation was obtained. The clinical evaluations were done at the end of the research. The handling of animals was in accordance with the Mexican official Norm (NOM-0062-ZOO-1999) technical specifications for the reproduction, care and use of laboratory animals, and in accordance to the National Institutes of Health guidelines for the care and use of laboratory animals (NIH Publications No. 82-23. Revised 1978).
Four groups of half-blooded dogs with a weight of 15–22 kg of both sexes were used in this study (n = 2 for each one). International rules for handling experimental animals in all stages of research were applied. In all groups a basal Seldinger’s arteriography was performed with the purpose of observing the vascular permeability of anterior spinal artery. Briefly, a catheter unit, 16GA-12 in with needle 14 GA. DESERET, Medical, Inc. Parke Davis Co., Sandy, UT, USA, was implanted at the trans-femoral artery and through the left subclavian and left vertebral artery to flow through the anterior spinal artery. Through it a Conray Tc-43 Mallinckrodt, St. Louis, MO, USA, solution at doses of 1.0–2.0 ml/kg was perfused as contrast medium.
Each group was put under a temporary obstruction of blood flow with the purpose of having several different times of ischemia using a vascular clamp on the anterior spinal artery at the level of C5–C6. Each group had 1, 2, 3, and 4 h of ischemia time, respectively. To check the obstruction of the spinal anterior artery and seeing its permeability after clamping at the end of temporary ischemia, a trans-femoral arteriography was done as previously described. The dogs were operated in a surgical room and under anesthesia by IV injection of sodium pentobarbital (28 mg/kg) in a supine decubitus position with tracheal intubation, controlled ventilatory support and intravenous line, shaving of the anterior surface of the neck, with asepsis and aseptic operative fields.
The approach was performed vertically by left ventrolateral route with dissection by planes until the bone plane at the level of C5–C6. Discectomy, resection of distal half of the C5 vertebral body and resection of proximal half of C6 vertebral body, meninges incision in the form of an Ι, dissection of the anterior spinal artery that lies on the anterior furrow of the spinal cord. With the purpose of having temporary different ischemia times, a vascular clamp was placed on the anterior spinal artery for variable times from 1 to 4 h sparing its integrity (Fig. 1). Then a transversal scalpel cutting the right half of the cord (cutting 50% of the spinal cord) was practiced, sectioning its motor and sensory areas at the levels of C5–C6, protecting the anterior spinal artery. To make the cut, a measurement of the diameter of the spinal cord at C5–C6 was performed using a surgical strip. Direct visual confirmation of the cutting percentage was made, using the surgical strip, and a microscopic analysis confirming the percentage during the histological evaluation was performed also. The level of vertebral excision and level of ischemia and recovery of the blood flow altering clamping were checked using a fluoroscopic control and Seldinger’s trans-femoral arteriography; the vascular clamp was removed after each period of time (Fig. 2). The surgical field was closed by layers and the ventilatory support was removed when the dog awoke.
Fig. 2.
One hour of ischemia. A (100×) Motor neurons are in different degeneration phases and death (turbid degeneration, chromatolysis) (arrowheads). Peripheral nucleus, broken and disrupted chromatin which forms clusters in the periphery of nucleus showing clear halo (arrows). The cytoplasm is duplicated in size with a turbid appearance surrounding the degenerated or dead cells. Big arrow shows a normal motor neuron. B (400×) Normal structure is lost with dissolution of cords and fascicles, a white zone of fibrosis and diffuse gliosis (asterisk). Nerve fibers are disrupted with degeneration of axons (arrows). C Vessels with a complete detachment of its endothelium (arrows). Asterisk shows fibrosis and diffused gliosis. Hematoxylin and eosin staining
Clinical evaluation
From the moment of conscious recovery, we recorded daily the following clinical variables: muscular contractions, upper and lower limb motor activity, tail mobility, response to painful stimuli, sphincter control function, and possible sequel and using Daniels motor scale and the American Spinal Injury Association (ASIA) Impairment Scale [10–12].
Neurophysiological evaluation
The electrical activity was evaluated using somatosensory evoked potential (SSEP) (Nicolett, mod. Viking IV) at 30 Hz of low filters frequency and high filters frequency of 3 kHz, with a sweeping speed of 10 ms, 2 μV of amplitude per division, using stimulus of 2.3 Hz corresponding to 2.3 stimulus per second, with 12 mA of intensity and 0.2 ms of duration. After anesthesia, the electrodes were implanted by a trench of the right sciatic nerve. The stimulus was captured at the brain in a point at the union of the two lines, the first one being the union between the two mastoid portions and the second being the union of the nasal base and the occipital protuberance. The electrical ground was applied at the frontal level on the dog’s head. This evaluation was performed before and after the surgery and then again 3 months later.
Histopathological evaluation
All animals were killed at 12 weeks by anesthetic overdose. Tissue samples were taken of the spinal cord 1 cm proximal and distal to the cord section.
The percentage of section was measured using standard cross section of tissue embedded in paraffin and stained with hematoxylin–eosin, Masson and Cluber-Barrera methods. We studied the morphology of the spinal cord and with semifine cross sections embedded in epoxy resin (poly/bed) and stained with toluidine blue method, the percentage of normal and altered axons, blood vessels and endothelial characteristics were evaluated in all the groups. The thickness of the myelin sheaths was determined in histological sections by using Carl Zeiss Image Analyzer (Zeiss image 3 = at 400×).
Statistical analysis
The statistical analysis (SPSS for Windows) was done by X2 nonparametrical variables and Student’s t test for parametrical variables and correlation analysis between variables: width, latency and percentage of the delay of SSEP, and ischemia time.
Results
Clinical evaluation
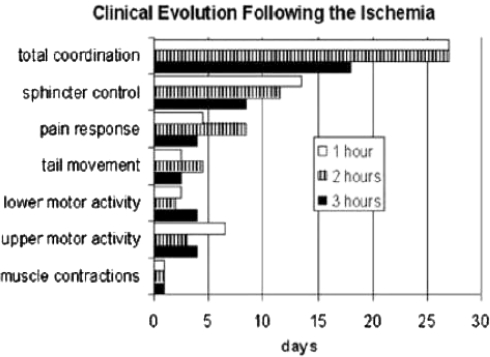
The postsurgical clinical variables observed after 1 h of ischemia were as follows: On the 1st day there are spontaneous respiratory movements, and muscular contractions are present. On the 2nd and/or 3rd day tail movements and lower limbs motor activity were observed. After 4th and 5th day answer to pain stimuli at distal level to spinal cord section occurred. On the 21st day there was a recovery of sphincter control. Maximal neurological coordination was observed on the 28th day (Graph 1). The only sequel observed was a right upper limb paresis.
After 2 h of ischemia, the animals showed on the 1st day muscular contractions, lower limbs motor activity was observed on the 2nd postsurgical day. The control of sphincter was observed on the 11th day and maximal neurological coordination on the 28th day. (Graph 1) ASIA E and Daniels 5.
After 3 h of ischemia, the animals presented spontaneous respiratory movements at the end of the surgery and muscular contractions on the 1st day. Motor activity of upper and lower limbs, tail movements, and answer to pain stimuli were observed on the 2nd to 6th day postsurgical event. The sphincter control was present from the 5th to 12th day and only one animal presented paresis of the right upper limb as sequel (Graph 1). ASIA E and Daniels 5, except in one case with right thoracic extremity, ASIA C and Daniels 3. In the experimental group corresponding to 4 h, all animals died between the 3rd and 4th hour of postsurgery. Animal’s death in this group was an occurrence not previewed by this study, another seven dogs were operated in order to obtain survivors without success. No clinical nor histopathological evaluation was performed due to the fact of being considered nonrelevant at that time.
Neurophysiological evaluation
With 1 h of ischemia, a delay of the nervous impulse up to 65% was observed whereas, with 2 h of ischemia the delay was 9% and with 3 h of ischemia the delay was up to 56%. Latency time was increased proportionally to a longer ischemia time, with slower electrical impulse (r = 0.525 ns), and reduction of its amplitude (r = 0.179 ns).
Histopathological evaluation
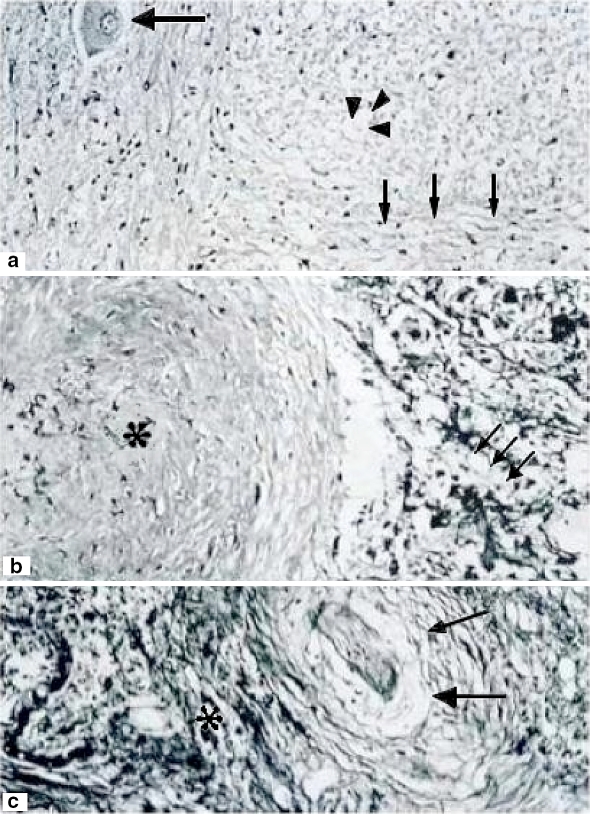
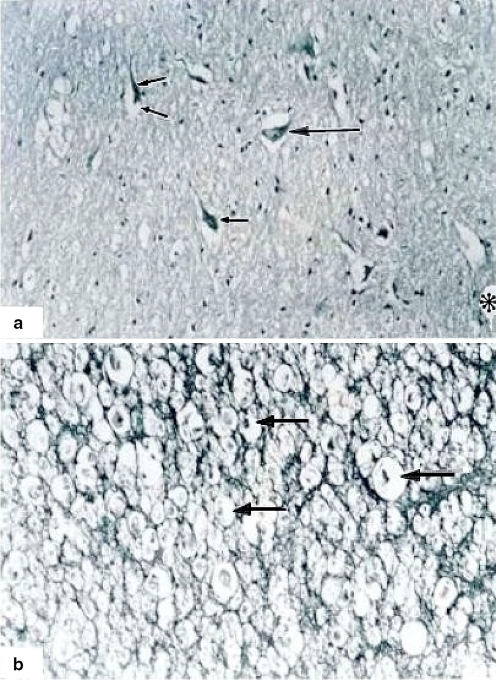
In a coronal section no apparent alterations were observed in one case after 1 h of ischemia. The white matter showed nerve fibers with well-defined cords and fascicles. However, with semifine cross sections and stained with toluidine blue method on a proximal coronal section of spinal cord lesion some scarce degenerated axons and vessels having a normal appearance were observed (Fig. 5). On a distal coronal section of spinal cord to the level of lesion many axons with dissociation and elongation of myelin as vacuolization in different axons were observed. In another case, motor neurons in different degeneration phases, death, and important gliosis were observed. (Fig. 2). After 2 h of ischemia time, an apparent lesion of 0.6 mm of length with fibrosis surrounding it was observed. In the gray matter of ventral horns, neurons were observed in different degeneration phases and death. Neurons with the cytoplasm enlargement without Nissl’s bodies, total lysis with clear and thin halo surrounding the plasmalemma with additional diffused gliosis was observed as well as an extensive fibrosis (Fig. 3). At a distal section fasciculation alterations with abundant cellular and axonal remainders were observed (Fig. 5).
Fig. 4.
Three hours of ischemia with extensive chromatolysis. A (100×) Neuronal degeneration and chromatolysis (small arrows) and not completely damaged (just a clear halo surrounding it) neurons (big arrow). B (400×) White matter with altered cords. Some of the nerves show necrosis and severe dissolution (arrowheads). However, in other areas nerves are intact (big arrows). Asterisk shows a small normal vessel. C Scare zone with fibrosis and general severe damage and total loss of cytoarchitecture. There is also a degenerated neuron (small arrow) and a damaged vessel (arrowheads). Hematoxylin and eosin staining
Graph 1.
Critical evolution following ischemia
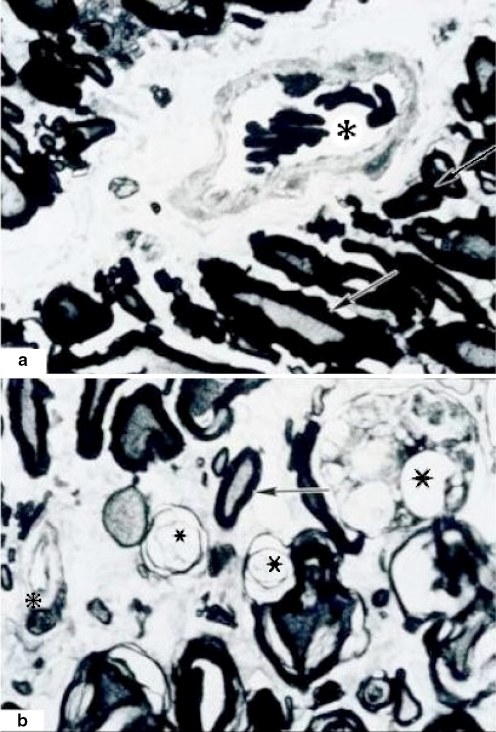
At 3 h of ischemia time, a damaged zone of 10–15% of extension involving white and gray matter was observed, neuronal degeneration and death (Figs. 3, 4). In another section the white matter is observed with pathological and normal cells (Figs. 3, 4, 5, 6). In some areas vascular and axonal remainders were observed and a vessel with discrete increase of endothelium thickness.
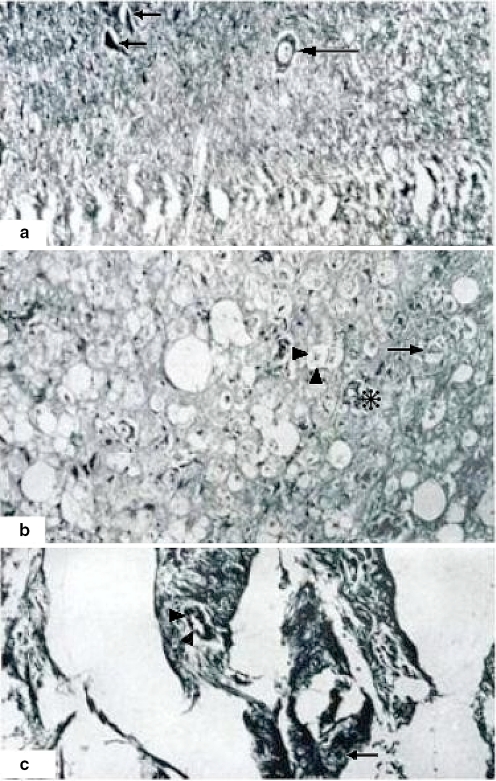
Fig. 6.
Semifine coronal sections. Myelin dissociation at 3 h of ischemia. A (800×) Proximal section with a segment of preserved fascicules. Some axons with discrete myelin dissociation (arrows) are observed. There is a normal vessel (asterisk). B (800×) Distal section. Almost total degeneration of axons with elongation and dissociation of myelin and multiple axonal remainders (stars). A vessel with hypertrophic endothelium is showed (small asterisk). There is a normal axon (arrow) surrounded by a necrotic area. Tissue samples in semifine cross sections were embedded in epoxy resin (poly/bed) 1 μm in width. Toluidine blue method staining
Fig. 3.
Three hours of ischemia. A (100×) Neuronal degeneration and death (small arrows), neurons and vessels with clear halos surrounding the nucleus (big arrow). Asterisk shows a damaged vessel. B (400×) White matter with neuronal degeneration and chromatolysis (arrows). Hematoxylin and eosin staining
Fig. 5.
A Semifine coronal sections, 1 h of ischemia. (800×) Proximal section showing normal and abnormal (dissociated myelin) axons and fascicles (arrows). A normal vessel is visible at the center (asterisk). B In semifine distal coronal sections, 2 h of ischemia axons show dissociation and total liquefaction (star). Asterisk shows an axon with myelin thickness. Tissue samples in semifine cross sections were embedded in epoxy resin (poly/bed) 1 μm in width. Toluidine blue method staining
The scar zone with fibrosis was observed with a general severe damage, total loss of the cytoarchitecture, general damage is observed in neurons, vessels and the gliosis are diffused with endothelium hypertrophy (Figs. 4, 6).
Extension of axonal degeneration and myelin alteration
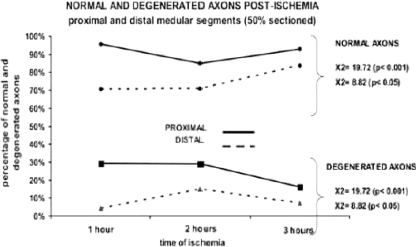
There is a major proportion of degenerated and normal axons in distal segments of the spinal cord. Using a Student’s t test we observe a significant difference of normal axons between proximal and distal segments with 1 and 2 h of ischemia (P < 0.05) while degenerated axons were only significant with 2 h of ischemia (P < 0.001) (Graph 2).
Graph 2.
Linear representation of normal and degenerated axons postischemia
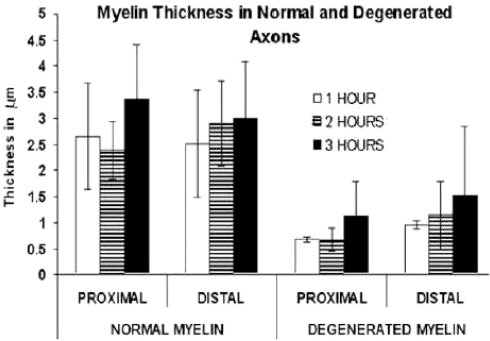
With respect to thickness of myelin sheath using a Student’s t test, we observed a significant difference between proximal and distal segments (Graph 3) with normal and degenerate myelin with only 2 h of ischemia (P < 0.05); always the thickness of the myelin sheaths was larger in the distal segment. Comparing normal myelin against degenerated myelin, in the proximal segment a significant difference was observed with 1 h (P < 0.001), 2 h (P < 0.05), and 3 h (P < 0.001) of ischemia, the thickness of normal myelin was larger. In the distal segment the thickness of normal myelin was always larger with a significant difference at 1 h (P < 0.001), 2 h (P < 0.001), and 3 h (P < 0.01) of ischemia.
Graph 3.
Myelin thickness in normal and degenerated axons
Morphological findings with blood vessels
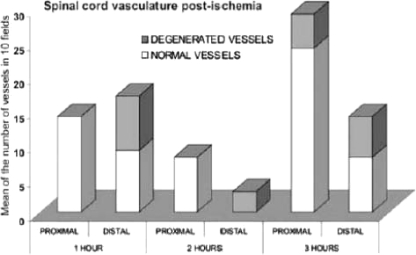
In group 1, 1 h of ischemia, we did not observe any apparent alterations. In group 2, 2 h of ischemia, a 10–15% of the vessels showed distal alterations with breaking or dissolution of its endothelium and detachment of the intimate tunic of the middle layer of the vessels. In group 3, 3 h of ischemia, we found alterations in 10–15% of the vessel with light detachment of intimate middle layers, endothelial cells with many vesicles, and some vessels with complete alteration of all layers. In another case of 3 h of ischemia, it showed damage of distal vessels, with rupture and dissolution of the layers, the endothelium was observed with multiple vesicles, elongation of muscular layer with wide spaces between them. The basal layer was detached in some areas (Graph 4).
Graph 4.
Spinal cord vasculature postischemia
Discussion
The term “spinal shock” applies to all anatomic and physiological phenomena that surround traumatic section of the spinal cord, resulting in a temporal loss or depression of all or most of spinal reflexes below the lesion level.
The mechanism of spinal lesion usually is traumatic and occurs immediately, although in some cases a progression in several hours is described [13]. The acute phase of spinal cord lesion comprises a primary and secondary pathologic model. The primary model comprises the effects of contusion, laceration, and elongation of nervous tissue and direct vascular trauma; these changes are irreversible [14] due to the permanent vascular lesion as is proven in the present work. The secondary model comprises the posttraumatic ischemic changes, loss of energetic metabolism, edema, free radicals, electrolytic changes as intracellular calcium increase. These secondary changes are reversible and determine the therapeutic strategies [14–17].
In other experimental models different evaluations have been made as to free radicals scavengers, upload receptor antagonist, calcium channel blocking, plasma expansors, osmotic diuretics, hypothermia, cyclooxygenase inhibitors, serotonin antagonists, and N-methyl d-aspartate (NMDA) receptor antagonists. We propose that an alternative handling in order to break the feedback mechanism and interrupt many aspects of secondary lesion cascade should include pharmacological and surgical strategies [14].
In the experimental groups of this work a correlation does not exist after surgery between the clinical changes, nerve conduction velocity, and histopathological findings with the different ischemia times, because it is in the 1, 2, and 3 h of ischemia that the dogs have a good evolution with sphincter control and voluntary motor activity. Only two cases, of the groups 1 and 3, the paresis of the right upper limbs was present with the SSEP. We observed a delay in the nerve conduction up to 65% in 1 h of ischemia, 9% in 2 h of ischemia, and up 56% in 3 h of ischemia. The histopathological findings did not show any correlation with the clinical findings, although it did show a partial correlation with the SSEP findings. In group 1, there was 25% of section, and the Rexed’s area [18] VII, VIII, and IX were affected, group 2 and 3 showed up to 15% of section. In group 2, the IX Rexed’s area was involved and in group 3 VIII and IX areas were found with damage.
Four hours after the clamping and cutting the recovery was not possible, the animals died before reaching that time. We repeated the experiment in this group, always with the same result (a total of 9). In the first, second, and third groups, the animals were under experimental control conditions for 3 months, with almost a total clinical recovery, that did not show correlation with the SSEP and histopathological findings. In a previous work of the author a similar discordance was observed, the clinical evolution did not correspond with the pathological evolution. However, we consider that there are vascular and neuronal regeneration changes [4].
The pathological analysis at the level of segment with the lesion determined an axonic index that indicates neuronal regeneration and this was larger at 3 h of ischemia as evidence of recovery. The thickness of the myelin sheaths increased at 3 h of ischemia, but the normal myelin thickness was always larger than the degenerated myelin in proximal and distal segments, the degenerated myelin thickness is larger in a distal segment in comparison with the proximal segment and increase with a longer evolution time. These changes were evident from 1 h of ischemia with distal degeneration of axonal cytoskeleton and myelin sheaths, and were evident in all the experimental groups. In the proximal segment only with 3 h of ischemia abnormal vessels were observed, while in the distal segment abnormal vessels were always evident. At 1 and 3 h half of the vessels were abnormal, indicating a regeneration process. With 2 h a few abnormal vessels were evident, confirming the statistical difference of abnormal vessels between proximal and distal segments of the section. The analysis of cases with recovery (n = 5) a total of nine cases with 4 h of ischemia (n = 9) without any recuperation. The X2 with Yate’s correlation was 9.98 with P = 0.0015, and the Fisher’s test showed P = 0.00049, both statistically significant. The SSEP showed a larger latency time and amplitude reduction with larger ischemia time with delay in nerve conduction velocity, thus reducing the nervous signal.
We do not know enough concerning molecular composition of synapsis of the central nervous system. An important point being how much of the union material is energetic and how much is a particular type of synapsis. The knowledge of this composition will permit the classification and understanding of them. Not enough is known yet as to the synapsis joining, especially what proportion of the molecules are building as individual molecules locally and which are preformed as a group. The answer to this question will help to define the nature of the classification process.
The complete change of functional molecules at synapses is unknown, including the same synaptic structure and if these molecules are substitute to individual molecules or in a group of related molecules [19].
There are several hypothesis: (1) a structural change at synapses (increased or decreased in a particular kind of synapses, new synapses, removal of synapses, or configuration changes at synapses); (2) molecular composition changes in existing synapses; synthesis of new molecules or new molecular combinations; (3) functional or state changes in the existing molecules or synapses (phosphorylation, or posttranscriptional changes) [19].
We are working with the present model and the following experiments will give the answers of points A and B, in order to complete the basic and clinical analysis of the present model.
Conclusions
The repair of the spinal blood flow helps the spinal cord to recovery. In this work we concluded that there is a maximum 3-h period, which we called “critical ischemia time”, for the spinal recovery to be possible. We have less than 4 h to offer a possible neurological benefit, by means of a spinal cord revascularization in cases of acute spinal cord injury with spinal cord section without any added treatment.
References
- 1.Tator ChH, Fehling MG. Review of the secondary injury theory of acute spinal cord trauma with emphasis on vascular mechanisms. J Neurosurg. 1991;5:15–26. doi: 10.3171/jns.1991.75.1.0015. [DOI] [PubMed] [Google Scholar]
- 2.Rhoney DH, Luer MS, Hughes M, Hatton J. New pharmacological approaches to acute spinal cord injury. . Pharmacotherapy. 1996;16(3):382–392. [PubMed] [Google Scholar]
- 3.Hughes JT. Historical review of paraplegia before 1918. Paraplegia. 1987;25:168–171. doi: 10.1038/sc.1987.30. [DOI] [PubMed] [Google Scholar]
- 4.Bitar-Alatorre WE, Jiménez RM, Ortiz GG, Delgado R, Sanmiguel S, Sánchez-Corona J, Feria-Velazco A.Vascular microsurgery of acute spinal cord injury in dogs Arch Med Res 1992234235–236.1340302 [Google Scholar]
- 5.Netter FH (1987) Nervous system anatomy and physiology. Brain areteriography. Salvat, pp 50–51
- 6.Getty R (1982) Anatomy of domestic animals, vol II, Salvat, pp 1750–1752, 1766–1771, 1832–1847
- 7.Miller ME (1964) Anatomy of the dog. Saunder W.B., pp 315–317, 533–543
- 8.Koyanagi I, Tator ChH, Lea PJ. Three dimensional analysis of the vascular system in the rat spinal cord with scanning electron microscopy of vascular corrosion casts. Part 1: normal spinal cord. Neurosurgery. 1993;33:277–284. [PubMed] [Google Scholar]
- 9.Koyanagi I, Tator ChH, Lea PJ. Three dimensional analysis of the vascular system in the rat spinal cord with scanning electron microscopy of vascular corrosion casts. Part 2: acute spinal cord injury. Neurosurgery. 1993;33(2):285–292. doi: 10.1227/00006123-199308000-00016. [DOI] [PubMed] [Google Scholar]
- 10.Frankel HL, Hancock DO, Hyslop G. The value of postural reduction in the initial management of closed injuries of the spine with paraplegia and tetraplegia. Part I. Paraplegia. 1969;7:179–192. doi: 10.1038/sc.1969.30. [DOI] [PubMed] [Google Scholar]
- 11.Palapa García LR, Anaya Vallejo S, Ramírez Gutiérrez R, Seanchez Flores L. Lesiones por flexodistracción de la columna cervical tratadas con placa por vía posterior. Rev Mex Ortop Traum. 1997;11(3):163–169. [Google Scholar]
- 12.American Spinal Injury Association: International Standards for Neurological Classifications of Spinal Cord Injury (revised) (2000) American Spinal Injury Association, Chicago, pp 1–23
- 13.Atkinson PP, Atkinson JL. Spinal shock. Clin Proc. 1996;71(4):384–389. doi: 10.4065/71.4.384. [DOI] [PubMed] [Google Scholar]
- 14.Harat M, Radek A, Kochanowski J. The physiopathology of acute spinal cord injury and a hope for a successful. Neurol Neurochir Pol. 1996;30(1):123–135. [PubMed] [Google Scholar]
- 15.Lee M, Lee E, Kim Y, Choi B, Park S, Park H, et al. Ischemic injury-specific gene expression in the rat spinal cord injury model using hypoxia-inducible system. Spine. 2005;30(24):2729–2734. doi: 10.1097/01.brs.0000190395.43772.f3. [DOI] [PubMed] [Google Scholar]
- 16.Park E, Velumian AA, Fehlings MG. The role of excitotoxicity in secondary mechanisms of spinal cord injury: a review with an emphasis on the implications for white matter degeneration. J Neurotrauma. 2004;21:754–774. doi: 10.1089/0897715041269641. [DOI] [PubMed] [Google Scholar]
- 17.Lu K, Liang CL, Chen HJ, et al. Injury severity and cell death mechanisms: effects of concomitant hypovolemic hypotension on spinal cord ischemia reperfusion in rats. Exp Neurol. 2004;185:120–132. doi: 10.1016/j.expneurol.2003.09.011. [DOI] [PubMed] [Google Scholar]
- 18.López-Antunez (1983) Functional anatomy of nervous system. Esquema de Rexed, Limusa, México, pp 140–142, 667–670, 677–678
- 19.Baudry M, Thompson R, Davis J. Synaptic plasticity, molecular, cellular and functional aspects. A Bradford book. Cambridge: MIT Press; 1983. pp. 13–39. [Google Scholar]