Abstract
Previous studies have shown that zoledronic acid administration can increase mineral content and strength in distraction osteogenesis. Of the few studies that have examined the use of bisphosphonates in spinal arthrodesis, none have assessed the effect of single dose treatment. The objective of this study was to evaluate the feasibility of enhancing spinal fusion rate using single dose zoledronic acid (ZA) to increase fusion-mass size and mineral density. Forty-eight New Zealand white rabbits underwent an L6–L7 intertransverse process fusion. The L6–L7 model is more challenging than the more commonly used level of L5–L6. Animals were randomly allocated to one of three groups, one received iliac crest bone graft alone, one group received iliac crest bone graft with locally administered zoledronic acid, 20 μg, and one group received iliac crest bone graft with a single dose of systemically administered zoledronic acid, 0.1 mg/kg. ZA doses were administered at the time of surgery. Twenty-four rabbits were culled at 6 weeks and 24 rabbits were culled at 12 weeks. Success of spinal fusion was determined by manual palpation. Specimens were evaluated radiographically, underwent quantitative computerised tomography analysis and were tested biomechanically in flexion and extension. In the six-week group, only five of the 24 spines fused with no noticeable trend with respect to treatment. In the 12-week group there was a trend toward increased fusion in the systemically administered ZA group (63%) versus the other two groups (25%) but was not statistically significant (p = 0.15). Radiographically, the local ZA treatment group showed a delay in remodelling with the presence of unremodelled bone chips. The 12-week systemic ZA group exhibited an 86% increase in BMC, a 31% increase in vBMD and a 41% increase in the volume of the fusion-mass (p < 0.05). The 12-week local ZA group also showed significant increases in BMC (69%), vBMD (31%) and total fusion-mass volume (29%) (p < 0.05). Biomechanical testing showed that the range of motion in flexion decreased to 4.5 (±2.5) degrees and 4.8 (±4.7) degrees for the local and systemic groups respectively compared to 9.6 (±4.9) degrees for the control group (p < 0.05). This study has shown that zoledronic acid increased fusion-mass size and bone mineral content. Systemic ZA led to an increased fusion rate; however the fusion rate remained below 100%. We suggest that bisphosphonate treatment may require an anabolic conjunctive therapy to ensure enhanced successful fusion.
Keywords: Posterolateral fusion, Rabbit, Zoledronic acid, Animal model, Pseudarthrosis
Introduction
Lumbar spine fusion is a common surgical procedure, the classic method being an intertransverse process fusion with iliac crest bone graft. Current reported non-union rates of the lumbar spine vary from 0 to 56%, i.e. up to one-half of operations fail to produce spinal fusion [8]. This is more of a concern in some paediatric conditions such as neurofibromatosis and spondyloepiphyseal dysplasia, where spinal deformity occurs commonly and attempts at fusion are frequently unsuccessful.
Bisphosphonates are anti-catabolic drugs with regard to their effect on bone [14]. Studies using bolus dosing of nitrogen-containing bisphosphonates (N-BP) in distraction osteogenesis have shown significant increases in callus volume, mineral content and strength in treated animals, even in the presence of stress-shielding [10,12]. We therefore investigated the effect of single-dose zoledronic acid (a third generation N-BP) in rabbit lumbar spinal fusion.
In this experiment, a New Zealand white rabbit model of L6–L7 intertransverse process spinal fusion was used to evaluate the effect of a single dose of zoledronic acid (ZA) on autograft fusion-mass. We hypothesised that single dose ZA will increase fusion-mass size and bone mineral content. Further, we hypothesised that these increases will lead to an increase in the fusion rate.
Methods
Experimental design
Ethics approval was obtained from the local Animal Ethics committee. Forty-eight New Zealand white rabbits were allowed one-week acclimatisation and housed in individual cages. The rabbits had a mean weight of 2.4 kg and were 20–24 weeks old at the time of surgery. These rabbits were considered to have just reached skeletal maturity. The rabbits underwent an L6–L7 intertransverse process spinal fusion using iliac crest bone graft. The L6–L7 intertransverse process fusion was utilized as it was expected to have a much lower fusion rate than the usual rabbit model at L5–L6, due to smaller transverse process and proximity to sacrum. The experimental design consisted of three treatment groups; a control group, a local zoledronic acid group (ZA mixed with bone graft) and a single dose systemic zoledronic acid group (ZA administered intravenously at the time of surgery). Rabbits from the control and local groups received a saline infusion of the same volume as administered in the systemic ZA group. Two time points were investigated; six and 12 weeks containing equal amount of rabbits.
Surgical procedure
After shaving and preparing the surgical site, the rabbits were placed prone on the operating table. A midline incision was made extending from L5 to the proximal sacrum. A subcutaneous dissection was carried out exposing the lumbar musculature and the iliac crests. Two paramedian fascial incisions were then developed between the multifidus and longissimus bilaterally exposing the transverse process of L6 and L7. The posterior iliac crests were exposed bilaterally allowing one gram of autograft to be harvested from each side. The L6 and L7 transverse processes were decorticated prior to addition of bone graft. Closure of the fascia and skin were then performed. Systemic ZA was administered as a single IV infusion over 20 min at surgery at 0.1 mg/kg, a standard dose from previous experiments, approximating a clinical dose. Local ZA was applied mixed with the iliac crest at a dose of 20 μg. The local dose was based on an approximation that 10% of the systemic dose would reach the target area of the spine, based on pilot biodistribution data. Rabbits were killed at the 6 and 12-week marks. The spines were harvested from L5 to the sacrum and denuded of soft tissues.
Manual palpation
At the time of harvest individual spines underwent manual palpation to determine whether they were fused. Each motion segment was graded as fused (no apparent motion) or not fused (motion present).
Radiographic analysis
Posteroanterior radiographs were taken and remodelling blindly graded on a three-point scale. Grade 1: many bone chips still clearly visible in the fusion-mass; Grade 2: some bone chips visible in the fusion-mass along with new bone; Grade 3: mostly new bone in fusion-mass with only very occasional residual bone chips.
Quantitative computerised tomography
Thirteen 2 mm slices were made through each spine, centred between L6 and L7, using a pQCT scanner and analysis software (Stratec XCT-960A, Stratec Medizintechnik Gmbh, Pforzeim, Germany). The fusion-mass was isolated as the region of interest for analysis. Bone mineral content (BMC, g), fusion volume (cm3) and volumetric bone mineral density (vBMD, g/cm3) were determined for the total bilateral fusion-mass.
Biomechanical testing
The L5 vertebral body and the proximal sacrum were potted in aluminium rings (φ50 mm and 20 mm height) with dental stone (Argi-Rock, Argibond, Victoria, Australia), leaving the L6–L7 fused joint exposed. An Instron 8874 materials tester was used to test the spine in flexion and extension using a custom made jig. A maximum non-destructive load of 0.27 Nm was applied at a loading rate of 3.8°/s [7]. A total of six load/unload cycles were performed for each flexion and extension and parameters were calculated from the final loading cycle. Load–displacement curves where used to determine the neutral zone (NZ, degrees), the range of motion (ROM, degrees) and the stiffness (Nm/degrees) of the fused joint in flexion and in extension.
Statistics
The means and standard deviations (SD) were derived for each treatment group. Due to the small sample size per group (n = 8) the data was assumed to be non-parametric. A Kruskal–Wallis test was used to identify statistical difference between treatment groups. A Mann–Whitney test was applied post hoc to assess the difference between two treatment groups. p ≤ 0.05 was considered statistically significant.
Results
A total of 10 rabbits were excluded. Five rabbits died due to anaesthetic complications: one control, one local and three systemic. These rabbits died under anaesthetic at the time of surgery and independent of treatment. Four rabbits were excluded due to deep wound infections: three systemic and one local. These infections were at the site of incision and not considered an effect of the treatment. One rabbit was excluded due to wrong level surgery. Excluded animals were replaced such that there were a total of 24 rabbits at each time point.
Manual palpation
At the six-week-time point, only five of the 24 spines fused with no noticeable trend with respect to treatment: 1/8 in the control and 2/8 for the local and 2/8 for the systemic groups. At the 12-week-time point there was a trend toward increased fusion in the systemic ZA group (5/8, 63%) versus the other two groups (2/8, 25%) but was not statistically significant (p = 0.15).
Radiographic analysis
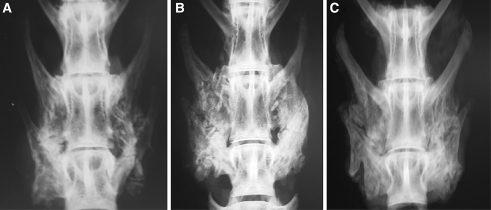
There was a difference in assessment of radiographic features of remodelling between the treatment groups (Table 1). The local ZA group displayed a severe delay in remodelling even at 12 weeks, with the majority of spines still containing unremodelled bone chips (Fig. 1). The systemic ZA group had improved remodelling scores between six and 12 weeks, while remodelling was most advanced in the control group.
Table 1.
Radiographic analysis results for three point grading scale
| 6 weeks | 12 weeks | |||||
|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 1 | Grade 2 | Grade 3 | |
| Saline | 2 | 4 | 2 | 0 | 3 | 5 |
| Local ZA | 4 | 4 | 0 | 5 | 3 | 0 |
| Systemic ZA | 5 | 3 | 0 | 1 | 5 | 2 |
Grade 1 many bone chips still clearly visible in the fusion-mass; Grade 2 some bone chips visible in the fusion-mass along with new bone; Grade 3 mostly new bone in fusion-mass with only very occasional residual bone chips
Fig. 1.
Representative posteroanterior radiographs of the rabbit spines at 12 weeks for (a) saline, (b) local ZA and (c) systemic ZA groups
Quantitative computerised tomography
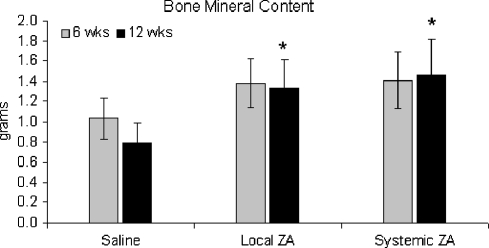
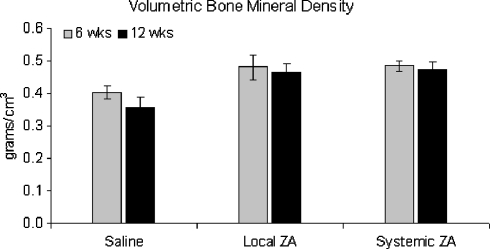
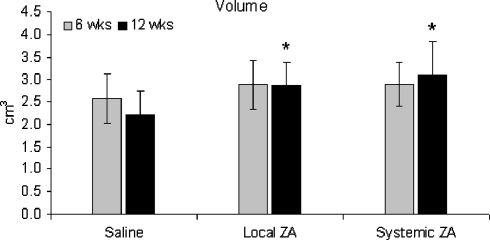
Both BMC and vBMD of the fusion-mass was increased in the ZA treated groups at six weeks (Figs. 2, 3). These values were maintained over time, whereas the BMC and vBMD in the control group fell over time. At 12 weeks, BMC was 68 and 86% greater than controls for the local and systemic ZA groups respectively (Fig. 2). There was a non-significant increase in fusion-mass volume at six weeks for the treatment groups (Fig. 4). At 12 weeks maintenance in fusion-mass volume in treated groups compared to a reduction in the control group led to a 29% increase in local ZA and 41% increase in systemic ZA over controls (p < 0.05).
Fig. 2.
Bone mineral content (g) of the fusion-mass at 6 and 12 weeks. *p < 0.05 versus saline
Fig. 3.
Volumetric bone mineral density (g/cm3) of the fusion-mass at 6 and 12 weeks
Fig. 4.
Volume (cm3) of the fusion-mass as six and 12 weeks. *p < 0.05 versus saline
Biomechanical testing
There was no change in the flexion range of motion (ROM) at 6 weeks for either treatment group. At 12 weeks, however, the ROM in flexion increased in the controls and decreased by 53% (p = 0.015) and 50% (p = 0.04) for the local and systemic treatment groups, respectively. The stiffness in flexion increased at 12 weeks by 56 and 40% for local and systemic treatment groups respectively (NS, p = 0.131) (see Table 2).
Table 2.
Biomechanical testing results
| n | ROM flexion (SD) | ROM extension (SD) | Neutral zone (SD) | Stiffness flexion (SD) | Stiffness extension (SD) | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 6 weeks | 12 weeks | 6 weeks | 12 weeks | 6 weeks | 12 weeks | 6 weeks | 12 weeks | 6 weeks | 12 weeks | ||
| Saline | 8 | 6.5 (4.1) | 9.6 (4.9) | 2.5 (1.0) | 3.1 (1.1) | 2.9 (1.3) | 3.8 (2.0) | 0.031 (0.013) | 0.025 (0.011) | 0.058 (0.025) | 0.047 (0.027) |
| Local ZA | 8 | 6.7 (6.1) | 4.5 (2.5)* | 2.7 (2.1) | 2.0 (1.1) | 3.4 (2.8) | 2.1 (0.8) | 0.037 (0.018) | 0.039 (0.018) | 0.069 (0.032) | 0.080 (0.027) |
| Systemic ZA | 7/8a | 7.0 (5.0) | 4.8 (4.7)* | 2.6 (0.8) | 2.2 (1.0) | 3.0 (1.4) | 2.5 (1.5) | 0.027 (0.017) | 0.035 (0.013) | 0.061 (0.034) | 0.072 (0.038) |
Range of motion (ROM) and neutral zone (NZ) in degrees. Stiffness in Nm/degree
a One sample from the six-week-systemic group was damaged prior to biomechanical testing and thus excluded from biomechanical analysis. This reduced the sample size from 8 to 7
* p < 0.05 versus control
Discussion
Non-union rates for lumbar spine fusion have been reported to vary from 0 to 56% [8]. This complication is the most frequent for spinal fusion and much research has focused on developing treatments that ensure union. Bisphosphonates have been used clinically for a number of decades to treat a variety of bone disorders characterised by increased bone turnover. Bisphosphonates are classed as an anti-catabolic drug [14] and it is this classification that makes them suitable to treat bone conditions that are a result of catabolism such as stress-shielding or disuse osteoporosis. There are various causes that lead to non-union in bone repair; one of them being increased catabolism that occurs before sufficient bone has formed for union to occur successfully. We have shown in a number of studies that single dose N-BP treatment transiently inhibits catabolism and allows callus size to increase and unite in distraction osteogenesis and open fracture models [1,10,12].
Few studies have assessed bisphosphonate therapy in spinal fusion, and those papers have mainly looked at the effect of continued treatment. Lehman et al. assessed the effect of daily alendronate sodium (0.05 mg/kg) on L5–L6 intertransverse process spinal fusion in rabbits [9]. The authors concluded that alendronate delays bone fusion in a rabbit model, as determined by the histological Emery Grading Scale [5]. They found an inhibition of bone maturation and a decrease in fusion rate. Bae et al. found a dose-dependent affect with alendronate in the same rabbit spine fusion model [3]. They documented significant increases in volume of fusion-mass in both high and low-dose-treatment groups as measured by axial computed tomography. However, they found that a high dose led to a lower fusion rate than the low dose and control groups. The differences in fusion rates were not statistically significant. A recent paper by Babat et al. suggested delayed fusion with pamidronate dosing, but the total dose was high (around 8 mg/kg) and the drug was given on a continuous basis [2].
Our purpose in using bisphosphonates in spinal fusion is to prevent premature remodelling, not to delay it indefinitely as must occur with continuous treatment. Zoledronic acid is a potent drug being trialled for once a year IV administration for osteoporosis, lending itself to bolus single dose administration [13]. We also chose to assess local administration, and though the conclusions must be limited as we only used one regimen (20 μg), it seems that this acted similarly to continuous dosing with a remodelling delay. A single IV dose wore off over time allowing remodelling but still giving the potential benefit of an increased fusion-mass.
We chose to pursue L6–L7 intertransverse process fusion model rather than the usual L5–L6 rabbit model due to the much lower fusion rate as a result of the smaller transverse processes and proximity to the sacrum. In prior studies made at L5–L6 in rabbits, the fusion rates with autologous graft alone have varied from 33 to 73% [4, 7]. This relatively high fusion rate makes it difficult to power a study to show a statistical difference based on fusion rates. We chose L6–L7 as a model we predicted would have a lower baseline fusion rate. The fusion rate was indeed low at 13% at 6 weeks and 25% at 12 weeks, and as such this study provides a useful more challenging model for future evaluations. A post-hoc power analysis tells us that with n = 8 at each time point and a baseline fusion rate of 25%, the power was acceptable (0.8) to detect a difference only if the treated group fusion rate was 100%.
Manual palpation of the fusion mass is commonly reported but can also lead to error. In this study the fusion masses were graded by one of two surgeons performing the procedure. It was not possible for us to analyse inter-observer error.
The present study confirmed our hypothesis that ZA administration would increase fusion-mass mineral content, density and size, and that this increase would be maintained for a longer period of time. We had further hypothesized that by maintaining a high fusion-mass density and size, the spine would be more likely to fuse. The results suggest there is a trend for increased fusion rates, with the systemic ZA group showing a 63% fusion rate at 12 weeks compared to the 25% fusion rate for controls. However, further optimisation is required to achieve 100% fusion in this challenging model.
We can state that our hypothesis can be rejected for the local ZA group. Despite maintaining an increase in fusion-mass volume, manual palpation showed no trend in increased fusion for the local group. However, according to the biomechanical testing results, there was a trend for a decrease in the ROM in flexion for both ZA groups, in addition to an increase in fusion stiffness. These findings contradict the results for the manual palpation. Clinically, manual palpation is the “gold standard” for assessing the success of spinal fusion and therefore is the obvious method experimentally. It is however an inexact science and previous studies have documented discrepancies between manual palpation results and biomechanical analysis [4, 9]. We acknowledge that solid conclusions are difficult to make due to the differences, and therefore would require further work to elucidate the results.
It is of note that in the saline control group, fusion-mass reduced over time even though fusion had not occurred in the majority of cases. This is the exact problem that we were trying to address. We believe that substantial remodelling and resorption of bone (catabolism) prior to bony union is inappropriate. The problem can occur due to a combination of local biological and mechanical factors. One of these factors may be stress-shielding from immobilisation, which would favour resorption [6]. With bolus systemic ZA administration we were able to transiently delay remodelling. The results suggest however, that this may not be sufficient to lead to fusion if the anabolic input is not high. A conjunctive anabolic stimulus may be required to ensure fusion. We have found that systemic ZA in conjunction with OP-1 significantly increased callus BMC, volume and strength in a critical size defect model in rats compared to OP-1 alone [11], and this combination will be evaluated in future work.
This study demonstrated that a single systemic dose of ZA increases bone mineral content and size of the fusion-mass in an L6–L7 intertransverse rabbit model. Although fusion rates increased in the systemic ZA group, this still fell short of optimal. Adding further anabolic stimulus to this model as well as controlling premature fusion-mass catabolism without gross remodelling delay will be the goal of future research.
Acknowledgements
The authors would like to thank Dr Richard Appleyard and Ronald Ho from SpineMed for their assistance in use of mechanical testing equipment.
References
- 1.Amanat N, Brown R, Bilston LE, Little DG. A single systemic dose of pamidronate improves bone mineral content and accelerates restoration of strength in a rat model of fracture repair. J Orthop Res. 2005;23:1029–1034. doi: 10.1016/j.orthres.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 2.Babat LB, McLain R, Milks R, Ferrara L, Sohn MJ. The effects of the antiresorptive agents calcitonin and pamidronate on spine fusion in a rabbit model. Spine J. 2005;5:542–547. doi: 10.1016/j.spinee.2005.01.008. [DOI] [PubMed] [Google Scholar]
- 3.Bae H, Yee A, Friess D, Yoo J, Johnstone B. Alendronate influences bone volume in rabbit posterolateral spine fusion. Spine J. 2002;2:98–99. doi: 10.1016/S1529-9430(02)00256-5. [DOI] [Google Scholar]
- 4.Boden S, Schimandle J, Hutton W. An experimental lumbar intertransverse process spinal fusion model. Spine. 1995;20:412–420. doi: 10.1097/00007632-199502001-00003. [DOI] [PubMed] [Google Scholar]
- 5.Emery SE, Brazinski MS, Koka A, Bensusan JS, Stevenson S. The biological and biomechanical effects of irradiation on anterior spinal bone grafts in a canine model. J Bone Joint Surg Am. 1994;76:540–548. doi: 10.2106/00004623-199404000-00008. [DOI] [PubMed] [Google Scholar]
- 6.Frost H. Bone’s mechanostat: a 2003 update. Anat Rec A Discov Mol Cell Evol Biol. 2003;275A:1081–1101. doi: 10.1002/ar.a.10119. [DOI] [PubMed] [Google Scholar]
- 7.Grauer JN, Patel TC, Erulkar JS, Troiano NW, Panjabi MM, Friedlaender GE. 2000 young investigator research award winner. Evaluation of OP-1 as a graft substitute for intertransverse process lumbar fusion. Spine. 2001;26:127–133. doi: 10.1097/00007632-200101150-00004. [DOI] [PubMed] [Google Scholar]
- 8.Lee C, Dorcil J, Radomisli T. Nonunion of the spine: a review. Clin Orthop. 2004;419:71–75. doi: 10.1097/00003086-200402000-00012. [DOI] [PubMed] [Google Scholar]
- 9.Lehman R, Kuklo T, Freedman B, Cowart J, Mense M, Riew K. The effect of alendronate sodium on spinal fusion: a rabbit model. Spine J. 2004;4:36–43. doi: 10.1016/S1529-9430(03)00427-3. [DOI] [PubMed] [Google Scholar]
- 10.Little DG, Cornell MS, Hile MS, Briody J, Cowell CT, Bilston L (2001) Effect of pamidronate on distraction osteogenesis and fixator-related osteoporosis. Injury 32:SD-14–SD-20 [DOI] [PubMed]
- 11.Little DG, McDonald M, Bransford R, Godfrey CB, Amanat N. Manipulation of the anabolic and catabolic responses with OP-1 and zoledronic acid in a rat critical defect model. J Bone Miner Res. 2005;20:2044–2052. doi: 10.1359/JBMR.050712. [DOI] [PubMed] [Google Scholar]
- 12.Little DG, Smith NC, Williams PR, Briody JN, Bilston LE, Smith EJ, Gardiner EM, Cowell CT. Zoledronic acid prevents osteopenia and increases bone strength in a rabbit model of distraction osteogenesis. J Bone Miner Res. 2003;18:1300–1307. doi: 10.1359/jbmr.2003.18.7.1300. [DOI] [PubMed] [Google Scholar]
- 13.Reid I, Brown J, Burckhardt P, Horowitz Z, Richardson P, Trechsel U, Widmer A, Devogelaer J, Kauffman J, Jaeger P, Body J, Meunier P. Intravenous zoledronic acid in postmenopausal women with low bone mineral density. N Engl J Med. 2002;346:653–661. doi: 10.1056/NEJMoa011807. [DOI] [PubMed] [Google Scholar]
- 14.Riggs B, Parfitt A. Drugs used to treat osteoporosis: the critical need for a uniform nomenclature based on their action in bone remodeling. J Bone Miner Res. 2005;20:177–184. doi: 10.1359/JBMR.041114. [DOI] [PubMed] [Google Scholar]