Abstract
Recent advances in molecular biology, cell biology and material sciences have opened a new emerging field of techniques for the treatment of musculoskeletal disorders. These new treatment modalities aim for biological repair of the affected tissues by introducing cell-based tissue replacements, genetic modifications of resident cells or a combination thereof. So far, these techniques have been successfully applied to various tissues such as bone and cartilage. However, application of these treatment modalities to cure intervertebral disc degeneration is in its very early stages and mostly limited to experimental studies in vitro or in animal studies. We will discuss the potential and possible shortcomings of current approaches to biologically cure disc degeneration by gene therapy or tissue engineering. Despite the increasing number of studies examining the therapeutic potential of biological treatment strategies, a practicable solution to routinely cure disc degeneration might not be available in the near future. However, knowledge gained from these attempts might be applied in a foreseeable future to cure the low back pain that often accompanies disc degeneration and therefore be beneficial for the patient.
Keywords: Intervertebral disc, Degeneration, Disc repair, Gene therapy, Tissue engineering, Mesenchymal stem cells
Introduction
The term tissue engineering has been first used in 1989 at a NIH National Science Foundation workshop and describes the development of a, often cell-based, biological replacement to restore, maintain, and improve the function of damaged tissues and organs. Recent developments in molecular biology, material sciences and improved knowledge of the human genome contributed that tissue engineering became a proliferating field of research with widespread clinical applications [181]. Combination of tissue engineering with gene therapeutic approaches to modify and tailor the properties of the used cells even multiplied the power of tissue engineering. Advanced knowledge about molecular changes in various diseases and the advances in tissue engineering laid the base for biological and specifically aimed approaches to cure musculoskeletal diseases.
Recently, orthopedic applications of gene therapy/tissue engineering approaches have been successfully tested in animals or even in clinical trials (reviewed in [88]). For example gene therapy for rheumatoid arthritis [48], orthopedic bone and soft tissue tumors [158], Duchenne’s/Becker’s muscular dystrophy [168], haemophilia [73, 98, 128] and osteogenesis imperfecta [78] have been tested in phase I clinical trials. However, there are yet no human gene therapy trials in tissue repair of cartilage-like tissue as articular cartilage, meniscus, ligaments and tendons [88]. The same is true for the intervertebral disc.
Like no other musculoskeletal tissue, the lumbar intervertebral disc undergoes very extensive destructive changes with age and degeneration, respectively [26] (Fig. 1). The overall degree of this tissue destruction is closely linked to age but the extent of alterations varied notably between the different components of the disc [33]. Substantial individual differences were observed resulting in young individuals exhibiting the disc of an elderly person and vice versa [25]. Furthermore, there is no close link between degeneration and pain. Several MRI studies have revealed that the rate of asymptomatic disc degeneration can range from 7–72% in individuals who did never experience relevant low back pain (LBP) [23, 156, 215]. We concur with Vernon-Roberts [204, 205] that differentiating normal ageing from pathological degeneration is very difficult. We therefore use the term “disc degeneration” to indicate the aforementioned changes without implying that disc degeneration is synonymous with a painful disc. Nevertheless, many clinicians and researchers believe that the intervertebral disc is a predominant source of LBP because of the extensive destructive changes that ultimately lead to an ankylosed motion segment [204, 205].
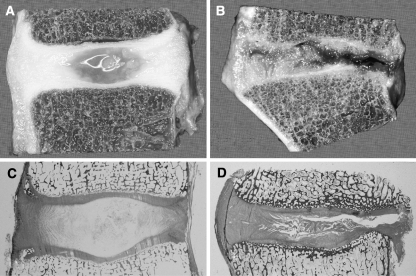
Fig. 1.
Degenerative changes of the intervertebral disc. Comparison of young and healthy (a, c) and severely degenerated (b, d) discs illustrates that alterations are observed in all anatomical regions of the disc and are obvious on macroscopical (a, b) and histological (c, d) level
In this review, we will discuss recent findings regarding molecular mechanisms that are involved in causing or propagating degenerative alterations of the intervertebral disc. We will also relate these findings to recent developments of biological strategies to therapy disc degeneration.
Morphological hallmarks of disc degeneration
Intervertebral discs (IVD) provide flexibility to the spine (bending and rotation) and transmit loads from body weight and muscle activity. The discs consist of three highly specialized structures, the endplates, the anulus fibrosus and the nucleus pulposus.
The two cartilaginous endplates form the inferior and superior interface between the disc and the adjacent vertebrae, therefore enclosing the disc axially. The anulus fibrosus is made up of several lamellae consisting of parallel collagen fibers interspersed by elastin fibers [129, 221]. Surrounded by the anulus fibrosus is the nucleus pulposus, the gelatinous core, which consists of randomly organized collagen fibers, radially arranged elastin fibers and a highly hydrated aggrecan-containing gel [82, 222]. The highly hydrated proteoglycans in the nucleus pulposus are essential to maintain the osmotic pressure and therefore have a major effect on the load bearing properties of the disc [124]. Anulus fibrosus and nucleus pulposus also differ in the morphology of their cellular components. The cells of the anulus fibrosus show a fibroblast-like morphology in the outer anulus and are rather chondrocyte-like in the inner region, whereas the cells of the nucleus pulposus are usually chondrocyte-like with single cells located in matrix-surrounded lacunae [30, 166, 210]. Besides the outermost part of the anulus fibrosus, the healthy adult intervertebral disc is accepted to be avascular [36, 49]. The blood vessels found in the outer anulus fibrosus seem to originate from the adjacent longitudinal filament [24].
With increasing age, as growth and skeletal maturation proceed, degenerative processes begin to change the morphology and therefore the function of the disc. This is also reflected by changes in the biosynthesis and denaturation of the extracellular matrix during progressing age [11, 12]. The nucleus pulposus of degenerated discs is characterized by a decreased water and proteoglycan content leading to the loss of its gel-like appearance and hydrostatic properties [26, 124]. Degenerative changes of the anulus fibrosus are less obvious, but result in irregular lamellae with the collagen and elastin networks becoming more disorganized.
Replacing the gel-like structure of the nucleus pulposus with fibro-cartilaginous tissue results in decreased flexibility and therefore often in cleft formation with fissures. Cell proliferation is often observed in the nucleus leading to formation of lacunae containing multi-cell clusters [68, 85]. Despite the observed signs of cell proliferation, up to 50% of the cells show signs of necrosis and some of them reveal signs of apoptosis, potentially resulting in a cell loss from the disc [62, 159, 199].
Although there is broad consensus about these hallmarks of degeneration, the question if neo-vascularization and/or neo-innervation of the inner parts of the disc occur during degeneration is still discussed [51, 86, 115]. Several studies describe neo-vascularization of the inner portions of the disc, possibly accompanied by nerve fibers, however, it is not completely clear at which stage of degeneration neo-vascularization occurs [29, 43, 67, 86, 150, 151, 161, 188]. Clarification of this question is of special importance since the interplay between neo-vascularization and neo-innervation might be of crucial importance for the pain sensation caused by degenerated discs.
Etiology of disc degeneration
Although the etiology of disc degeneration is far from being understood, there is consensus that not a single factor can be hold responsible for the complex phenomenon of disc degeneration. Rather a multitude of exogenous and endogenous factors, each contributing individually, might influence the progress of degenerative changes of the discs. These factors can be divided into three main groups: mechanical load, genetic predisposition, and nutritional effects.
Effects of mechanical load
Originally, injuries due to abnormal loads were thought to be the main cause for structural alterations that initiate the process of degeneration and finally lead to back pain [7]. Since then a multitude of studies, involving humans and experimental animals have been undertaken to investigate the correlation between mechanical load and disc degeneration.Although it is not clearly shown that the disc itself is directly suffering from abnormal loads [174], it seems possible that mechanical injury of adjacent vertebral bodies contributes to the initiation of disc degeneration [3]. However, several studies attempting to link disc degeneration to mechanical factors, such as heavy physical work, lifting, etc., failed to provide direct evidence for a causal relationship between back pain and mechanical load-induced disc degeneration [41, 70]. There is the possibility that these results are obstructed by additional factors like occupation, psychosocial factors and environment that might be involved in the patient’s perception of discogenic back pain [46, 47]. To investigate the influence of mechanical stress on disc degeneration and exclude any additional factors, animal experiments have been carried out. Application of dynamic, compressive load forces to the IVD of various animals resulted in changes indicating the onset of degeneration when the discs were analyzed morphologically or histologically [31, 80, 106, 121, 122]. Besides the effects of compressive forces also animals subjected to vibrational forces revealed signs of degenerative changes [217]. However, even in well-controlled animal experiments the results were contradictory. At least two studies reported that animals subjected to long-term compressive load or long-term intense exercise did not exhibit adverse effects on the respective IVD [79, 157].
Taken together, the results from clinical studies and animal experiments are not completely conclusive. The majority of the animal studies might suggest that certain forms of mechanical loads suffice to induce disc degeneration. However, several studies in humans did not provide a strong causal link between occupational exposures and disc degeneration, suggesting a more complex etiology of disc degeneration [206].
Genetic predisposition
Besides the investigations on mechanical influences on disc degeneration, several studies have demonstrated a strong familial predisposition for disc degeneration [22, 69, 120, 134, 178, 203, 206]. These findings were confirmed by twin studies that revealed an overall heritability between 52 and 74% for disc disease at the lumbar spine [17, 125, 173], with the genetic effects becoming more evident as the individuals grow older [71].
Recent technological advances to analyze the human genome confirmed the importance of genetic predisposition by identifying polymorphisms correlated with the occurrence of disc degeneration. The majority of polymorphisms identified so far affect genes encoding for proteins of the extracellular matrix. A polymorphism located in the gene encoding for aggrecan correlated with the presence of disc degeneration and led to a higher risk for multilevel degeneration at an early age [42, 96]Polymorphisms affecting collagens have mostly been found in collagen type I, II and IX genes [83, 93, 94, 144, 155, 185]. The mutations in the collagen type IX genes resulted in the replacement of an original amino acid with tryptophan, probably affecting integrity and stability of the matrix interactions [6]. Among the non-collagenous matrix proteins, polymorphisms in the gene encoding the cartilage intermediate layer protein (CILP) have been recently associated with susceptibility to lumbar disc disease [179]. The authors suggest that CILP is involved in the regulation of TGF-β signaling and that this regulation might have a crucial role in the etiology and pathogenesis of disc degeneration [179].
But not only polymorphisms in genes encoding for matrix proteins have been associated with disc degeneration. Recently, mutations in the gene encoding for the pro-inflammatory cytokine interleukin-1 (IL-1) have been associated with an increased risk of disc bulges and degenerative changes in the disc [183, 184]. However, a recent study involving a collective of patients suffering from intervertebral disc disease-related sciatica, found genetic variations in IL6, encoding for IL-6, to play a role in discogenic pain, whereas no association was found for genetic variations in IL1 [145]. The reason for the discrepancies between these two studies is not yet clear.
Besides genes encoding for matrix proteins and signaling factors genetic variations have also been identified in matrix degrading enzymes. Polymorphisms in the promoter responsible for the expression of the matrix-degrading enzyme matrix metalloproteinase-3 (MMP-3) were found to accelerate degenerative changes in the lumbar discs in the elderly [193]. In addition, polymorphisms were found in the gene encoding the vitamin D receptor [87, 95, 207, 208]. However, the mechanism by which vitamin D receptor is influencing disc degeneration is unknown.
Most polymorphisms discussed herein affect genes that are involved in the integrity or functionality of the disc matrix. This is obvious for the genes encoding aggrecan or collagen. MMP-3 is a matrix degrading enzyme and therefore mutations are disturbing the balance in matrix proteins synthesis and degradation. IL-1 was found to be involved in the up-regulation of inflammatory reactions and also MMP-1 and MMP-3 expression, which would finally lead to degradation of the disc matrix [84]. CILP, on the other hand inhibits TGF-β-dependent signaling, a growth factor that has been shown to be important for maintenance of the disc matrix [15, 152]. These findings suggest that the genetic background is responsible for the integrity of a healthy disc. If mutations in these genes occur, normally innocuous conditions or forces might lead to accelerated or enhanced degenerative changes, suggesting that disc degeneration may be explained primarily by genetic influences and that environmental factors have only modest effects [17]. However, it is important to keep in mind that despite the dominating role of genetic predisposition, Adams et al. stressed that injuries can occur when normal forces are applied to abnormally weak tissues, or when abnormally high forces are applied to normal tissues [2].
Nutritional effects
Insufficient nutritional supply of the disc cells is thought to be a major problem contributing to disc degeneration. Since the intervertebral disc is the largest avascular tissue in the human body, its cells are facing the precarious situation of having to maintain a huge extracellular matrix with a “fragile” supply of nutrients that is easily disturbed (Fig. 2). Whereas the cells in the outer anulus fibrosus may be supplied with nutrients from blood vessels in the adjacent longitudinal ligaments, the supply of the nucleus pulposus cells is almost completely dependent on the capillary network in the vertebral bodies [37, 49, 131, 202]. Due to the size of the intervertebral disc, the nutrients need to diffuse from the capillaries through the endplate and the disc matrix to the cells in the nucleus of the disc. With the originally cartilaginous endplates becoming calcified when degeneration progresses, the supply with nutrients will become even more restricted [167]. Not only the supply with nutrients like glucose and oxygen is restricted due to diffusion distances, also the removal of metabolic waste, i.e. lactic acid, becomes critical [75]. Measurements demonstrated that oxygen concentrations were very low in the nucleus and increased towards the disc surface, the lactic acid concentration showed the reverse profile. Since lactic acid is not only the major waste of disc cells but also an acid,its accumulation results in a lowered pH inside the disc [16]. In vitro experiments have shown that low oxygen concentrations and acidic pH significantly affect the synthetic activity and especially proteoglycan synthesis rates of disc cells, which might lead to a fall in proteoglycan content and therefore to disc degeneration in vivo [147].
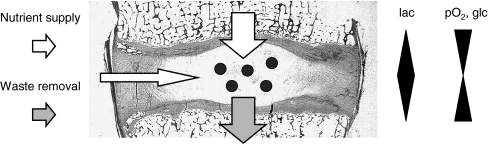
Fig. 2.
Limitations of the nutrient supply to cells of the nucleus pulposus. Due to the avascularity of the intervertebral disc nutrient supply and waste removal is entirely relying on diffusion through the cartilaginous endplates. This results in a gradient through the disc with a minimum of nutrients (pO2, glc glucose) and a maximum of waste products (lac lactate) in the middle of the disc
Taken together, these adverse conditions may lead to increased cell death and therefore reduced cell numbers in the disc [21, 76]. As a consequence, the very few remaining cells are confronted with the task to maintain an extensive matrix. It is conceivable that the progress of matrix degeneration becomes irreversible once the cell density falls below a minimal threshold. This would directly link the restriction of nutrients with the final destruction of the discal matrix.
Molecular mechanisms of degeneration
Induction of the degeneration process requires the translation of the adverse impacts discussed above, i.e. nutritional restrictions and mechanical stress into molecular mechanisms. It is thought that the disc cells serve as integrators that translate the various impacts into molecular mechanisms, such as expression of an array of matrix degrading enzymes, changes of the disc cell phenotype or initiation of signal transduction cascades. In this review, we will focus on gene products that are suspected to be involved in disc degeneration or whose expression levels have been found to change dependent on the degeneration grade of the disc and are therefore of special interest for the design of biological treatment strategies.
Matrix degrading enzymes
Disorganization of the disc matrix is one of the prevalent features of disc degeneration. According to the major constituents of the matrix, collagens, proteoglycans and fibronectin, several classes of enzymes are produced by disc cells that specifically break down these components (summarized in Table 1). The most dominant and best-characterized group of degrading enzymes is the matrix metalloproteinases (MMPs). MMPs are responsible for degradation of the various types of collagens found in many tissues and are classified according to their specificity [60]. Activation of MMPs during degenerative disc disease might be of ambiguous benefit. Whereas MMP activation in intact discs might contribute to the degeneration of matrix molecules and therefore be unfavorable, it might be beneficial in herniation by supporting the resorption of the prolapsed/extruded tissue. Herein, we will focus on MMPs involved in degeneration and therefore mainly discuss MMP expression in degenerated but contained discs.
Table 1.
Matrix degrading enzymes playing a role in intervertebral disc degeneration and its inhibitors (references given refer to studies discussing the role of the respective enzyme/inhibitor in disc degeneration)
| Enzyme | Synonym | Substrate | References |
|---|---|---|---|
| Degrading enzymes | |||
| MMP-1 | Collagenase I | Collagen I, II, III, VII, X | [10, 44, 165, 201, 214] |
| MMP-2 | Gelatinase A | Gelatin I | [165, 214] |
| MMP-3 | Stromelysin 1 | Gelatin I, III, IV Collagen III, IV, X Fibronectin Proteoglycans | [139, 165, 214] |
| MMP-9 | Gelatinase B | Gelatin I, V Collagen IV, V | [10, 165, 214] |
| MMP-13 | Collagenase III | Collagen I | [10] |
| ADAMTS4 | Aggrecanase-1 | Aggrecan | [111] |
| Cathepsin D | Collagen I, II Aggrecan, Link protein | [13] | |
| Cathepsin G | Collagens | [103] | |
| Cathepsin K | Collagenolytic activity | [13] | |
| Cathepsin L | Collagen I, II, IX, XI Aggrecan Link protein | [13] | |
| Inhibitors | |||
| TIMP-1 | MMP-inhibitor | [111, 143] | |
| TIMP-2 | MMP-inhibitor | [111] | |
| TIMP-3 | Aggrecanase-inhibitor | [111] | |
MMP matrix metalloproteinase, ADAMTS a disintegrin and metalloproteinase with thrombospondin motifs, TIMP tissue-specific inhibitor for metalloproteinases
The presence of MMPs in correlation with intervertebral disc degeneration has been investigated in various studies using cadaveric and surgical disc specimens [34, 90, 111, 165, 214]. All these studies indicated that MMPs are produced by disc cells in increasing amounts with increasing degeneration of the disc matrix. MMPs from all three matrix degrading classes (MMP-1, -2, -3, -9) displayed a very similar expression pattern and were also found to correlate with the occurrence of clefts and tears in the intervertebral disc matrix.
In additional studies, the use of herniated disc material from non-contained discs might result in false high results due to influences of extra-discal inflammatory cells infiltrating the herniated material and inducing MMP expression [44]. The knowledge gained from studies applying human specimens were furthermore confirmed by animal experiments. Anderson et al. showed that in a rabbit laceration model, macroscopic injury to the disc led to a significant increase in the expression of MMP-1, -9, -13 [10].
These in vivo studies have been supplemented by in vitro experiments to study aspects of the regulation of MMP expression in disc cells. It has been shown that disc cells isolated from degenerated discs of various animals and also from humans synthesize MMPs in vitro either spontaneously [91, 92, 139], under hydrostatic pressure [66] or after stimulation by interleukin-1 [84, 112, 180].
Besides regulation of its expression, MMP activity is regulated at two additional levels: on the post-translational level by proteolytic activation of the zymogen and the activated enzyme is furthermore modulated by its binding to tissue inhibitors of matrix metalloproteinases (TIMPs) determining the amount of free, active proteinases [189]. So far, three TIMPs have been detected in various tissues; TIMP-1, -2, and –3. Expression of TIMP-1 and -2 was found to increase in degenerated discs, whereas TIMP-3 expression did not significantly increase with increasing degeneration [111, 143]. These findings might suggest that the increased expression of MMPs may be counteracted by the increased TIMP levels [111]. However, Kanemoto et al. could not detect TIMP-1 in most of the analyzed surgical specimens that were also positive for MMP-3, suggesting an imbalance between MMP-3 and TIMP-1 as a cause for disc degeneration [90].
Besides collagens, aggrecan, a large proteoglycan, is also degraded during degeneration. Although MMP-generated and aggrecanase-generated fragments of aggrecan have been localized in IVD, role and identity of the respective aggrecanases during degeneration has yet to be clarified [165, 191]. One candidate is the so-called aggrecanase-1 or ADAMTS4 (adisintegrin and metalloproteinase with thrombospondin motifs 4) that has been found to increase with increasing degeneration [111]. Interestingly, the increase in ADAMTS4 expression was not paralleled by a rise of its inhibitor TIMP-3, suggesting that ADAMTS4 might play an important role in tissue degradation during disc degeneration [111].
Besides the well-characterized MMPs, cathepsins are another group of dominant proteinases able to degrade collagen and proteoglycans. Although cathepsins potentially contribute to degradation of the disc matrix, only few studies investigated a role of cathepsins in degenerative disc disease. Immunohistochemical analysis of cathepsins demonstrated that cathepsins D, L, K and G were found in increasing amounts in degenerated disc tissue and were associated with endplate separation and disorganization of the anulus fibrosus during degenerative disc disease [13, 103]. Although the exact mechanisms of cathepsin up-regulation are unknown, pro-inflammatory cytokines and growth factors have been reported to be involved in regulation of cathepsins [40, 45, 57, 58, 200, 211].
Since the substrate range of cathepsins and MMPs significantly overlap, the distinctive role of the two classes of proteinases during disc degeneration is not completely clear. However, it has been shown, that MMPs prefer neutral pH, whereas cathepsins show maximal activity in an acidic environment [138]. This preference of cathepsins for acidic pH might suggest a role for this class of matrix degrading enzymes later during degeneration when accumulation of lactic acid already led to a lowered pH in the discal matrix.
Pro-inflammatory mediators
Disc cells do not only produce matrix-degrading enzymes but also have the ability to initiate or propagate signal transduction cascades to manipulate their own environment. Production and secretion of cytokines, growth factors and the respective receptors by disc cells has been investigated extensively (summarized in Tables 2, 3). However, the knowledge gained so far originates from very heterogeneous experimental setups including studies on herniated discs (protruded, extruded or sequestrated discs), in vitro experiments or the use of animal models. Therefore it is often very difficult to undoubtedly identify the origin of cytokines and growth factors found in disc tissue.
Table 2.
Pro-inflammatory mediators expressed in degenerating intervertebral discs (references given refer to studies discussing the role of the respective mediator in disc degeneration)
| Mediator | Function | References |
|---|---|---|
| IL-1α | Pro-inflammatory cytokine MMP-induction | [4, 192] |
| IL-1β | Pro-inflammatory cytokine MMP-induction | [84, 135, 192] |
| IL-6 | Pro-inflammatory cytokine anti-apoptotic | [28, 84, 91, 116, 187, 192] |
| IL-8 | Pro-inflammatory cytokine chemokine | [4, 28, 116] |
| IL-10 | Inhibition of pro-inflammatory cytokines downregulation of MMPs | [4] |
| TNF-α | Pro-inflammatory cytokine pro-apoptotic | [4, 135, 192, 213] |
| GM-CSF | Pro-inflammatory cytokine | [192] |
| RANTES | Chemokine | [4] |
| PGE2 | Tissue degradation Inflammation angiogenesis | [27, 91, 192] |
| PLA2 | Biosynthesis of prostaglandins | [169] |
| COX2 | Biosynthesis of prostaglandins | [84, 135] |
IL interleukin, TNF tumor necrosis factor, GM-CSF granulocyte macrophage-colony stimulating factor, RANTES regulated on activation, normal T-cell expressed and secreted, PGE2 prostaglandin E2, PLA2 phospholipase A2COX2 cyclooxygenase-2
Table 3.
Growth factors expressed in degenerating intervertebral discs (references given refer to studies discussing the role of the respective growth factor in disc degeneration)
| Growth factor | Function (induction of) | References |
|---|---|---|
| TGF-β | Cellular proliferationProteoglycan synthesis | [104, 187, 196, 198] |
| IGF-1 | Proteoglycan synthesis (minor effect) | [148, 149, 187] |
| bFGF | Proteoglycan synthesis (minor effect) | [196, 197] |
| NGF | Formation of nerve fibres | [52, 59] |
TGF-β transforming growth factor-β, IGF-1 insulin-like growth factor-1, BFGF basic fibroblast growth factor, NGF nerve growth factor
However, analyses of disc tissue and isolated disc cells revealed that disc cells do have the ability to produce a wide array of signaling molecules potentially involved in pro-inflammatory and related pathways. Among these mediators are the (pro-inflammatory) signaling molecules IL-1α, IL-1β, IL-6, TNF-α GM-CSF (granulocyte-macrophage colony stimulating factor), IL-8, RANTES (regulated on activation, normal T-cell expressed and secreted), and IL-10 [4, 91, 102, 116, 135, 187, 192, 213]. In addition, inflammatory mediators such as leukotriene B4, thromboxane B2 and prostaglandin E2 and the respective enzymes involved in the same signaling pathway (phospholipase A2 and COX-2) were found in contained and non-contained herniations [91, 102, 135, 146, 169, 192]. Although these results summarize the abilities of disc cells to synthesize signaling mediators, the respective set of factors expressed in a certain condition are often contradictory and will need further investigations. Despite the observed contradictions, one can assume that disc cells do have the potential to produce the inflammatory cytokines necessary to mediate and propagate an inflammatory reaction. In addition, the expression of cytokine receptors suggests that these cells are not only able to initiate signal transduction cascades but also endue the requirements to react to pro-inflammatory mediators [187]. Several studies provide strong evidence, that the chondrocyte-like cells of the nucleus pulposus are the origin of the observed mediators in contained, degenerated discs. However, studies that are more detailed are needed to analyze the contribution of resident cells and potentially infiltrating immune cells to the observed levels of pro-inflammatory cytokines.
Only few studies focused on a possible effect of cytokine production on the induction of discogenic pain are therefore of special interest for treatment strategies of discogenic pain. Burke et al. [28] found significantly higher levels of IL-6 and IL-8 in discs from low back-pain patients compared to discs from sciatica patients, independently on the presence of an intact anulus fibrosus or nuclear extrusions. Surprisingly, the levels of prostaglandin E2 were not significantly different between sciatica and LBP patients. The same authors also demonstrated that nucleus pulposus cells from degenerate discs were more sensitive to pro-inflammatory stimuli in vitro compared to cells from scoliotic control discs [27]. From these studies one can conclude that cytokines may not only be involved in the degeneration of the human intervertebral disc but seem to also play a major role in the pain induction in the degeneration associated low back-pain symptom.
Growth factors
Growth factors are usually low molecular weight proteins that have the potential to increase mitogenesis, cytodifferentiation and also matrix synthesis. This combination of proliferative and biosynthetic effects on target cells and tissues originally motivated a closer investigation of growth factors and their effects on cartilage (recently reviewed in [38, 72] and summarized in Table 3).
The best-characterized growth factors are the members of the TGF-β family. Application of TGF-β on disc cells resulted in an anabolic response, characterized by increased proteoglycan synthesis and reduced tissue resorption by decreased MMP-2 secretion [61, 152]. In addition a transient proliferative effect has been observed on cultivated disc cells [61].
Human disc cells do not only respond to exogenous TGF-β but are also able to produce this growth factor. However, the available data are contradictory. Some studies report TGF-β and its cognate receptor, TGF-β receptor type II, to be generally found in tissue specimens from human herniated discs, mainly associated with disc cells or capillaries [187, 198]. Whereas others demonstrated that TGF-β expression was found only in a minority of the herniated disc tissue samples and TGF-β receptor type II could not be detected in any of the herniated discs [104]. In degenerated, non-herniated IVD there is consensus that TGF-β1 and TGF-β2 are expressed in all examined tissue samples with TGF-β1-positive cells preferentially located near tissue clefts [140, 196].
Besides the major growth factor TGF-β, Insulin-like growth factor-1 (IGF-1) has also been studied in detail. Similarly to TGF-β, IGF-1 has also been described to increase proteoglycan synthesis to reduce tissue resorption in discs by lowering the level of active MMP-2 [152, 195]. In human herniated discs the increased expression of IGF-1 has been interpreted as an attempt to repair the extruded disc material [187]. Besides these TGF-β-like functions, IGF-1 also seems to contribute to the viability of disc cells by its anti-apoptotic effect [65].
Expression of IGF-1 was found to be produced by disc cells from many species including humans [148, 149, 187]. Interestingly, animal experiments demonstrated that IGF-1 production decreased with increasing age suggesting a possible role of IGF-1 in the loss of proteoglycan synthesis observed with ageing [148, 149].
Besides these two well-investigated growth factors, less is known about other growth factors in the disc. Basic fibroblast growth factor (bFGF) is usually produced by tissues with high angiogenic activity, but is also found in the majority of samples from herniated discs and in all examined degenerated disc samples [196, 197]. The presence of bFGF in small blood vessels in herniated disc material raises the possibility of angiogenesis as an additional response by the disc cells to disc injury.
Recently, an atypical growth factor has been found inside the human IVD. Nerve growth factor (NGF) differs from the growth factors discussed so far by not exhibiting any known proliferative or anabolic functions on disc cells but has been shown to exert neurotrophic properties. Therefore, NGF might not be important for repair or maintenance of the intervertebral disc, but might play an important role in pain induction. This hypothesis was supported by NGF being exclusively present in pain level discs; whereas non-pain level discs or normal discs were entirely negative. NGF-positive cells were either found in microvascular blood vessels accompanying ingrowing nerve fibers or in chondrocyte-like cells of anulus fibrosus [52, 59]. These findings raise the possibility that not only neo-vascularization might take place in degenerated discs but also neo-innervation. Since these new nerve fibers are only found in pain-level discs strongly indicates them to be involved in, if not even responsible for, the difference between degenerated pain-level and non-pain-level discs.
Therefore, growth factor-targeted interventions to treat disc diseases would need to either supply the disc with the lacking anabolic growth factor (such as TGF-β, IGF-1, etc.) or would target the resulting pain by inhibiting the actions of NGF.
Biological treatment strategies
Biological therapies to treat degenerated IVD have mainly been used to support interbody fusions after discectomy, resulting in a loss of function for the affected motion segment (recently summarized in [123]), but scarcely to regenerate or cure the degenerated, painful disc itself and therefore restore biological function. This discrepancy is primarily caused by the unique properties of the intervertebral disc and the severe alterations that the disc is undergoing during degeneration that significantly limit the range of suitable approaches. In addition, the limited knowledge and often contradictory findings regarding disc cell biology as discussed above further complicate biological approaches. In the following, we will discuss the various techniques that have been developed and tested to overcome these difficulties and to biologically treat degenerative IVD. Common to these techniques is the aim for sustained delivery of biologically active factors to the disc that should drive regeneration or conserve the status quo of the affected tissue. The nature of the respective active factor is hereby defined by our knowledge of the molecular mechanisms active in the disc during the various stages of degeneration.
So far, four general approaches have been applied:
Delivery of the ‘naked’ factor by intradiscal injection (Fig. 3)
Gene therapy approaches that modify gene expression of resident disc cells in vivo (direct gene therapy) (Fig. 4)
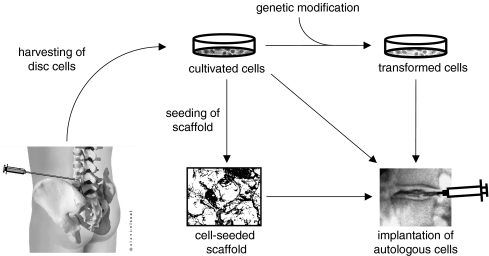
Autologous implantation of cells that have been antecedently removed, cultivated and often modified in vitro (Fig. 5)
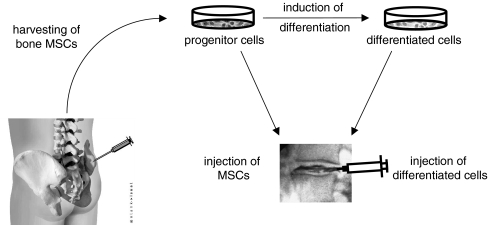
Implantation of mesenchymal stem cells (Fig. 6).
The applicability of the various approaches is largely depending on our current knowledge of disc cell biology, the state of degeneration of the intervertebral disc and also safety concerns.
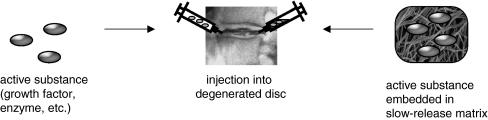
Fig. 3.
Direct injection of an active substance into the intervertebral disc matrix. Active substances, such as growth factors or anabolic enzymes can be injected directly into the disc matrix or can be injected embedded in a matrix that allows slow release of the substance into the environment
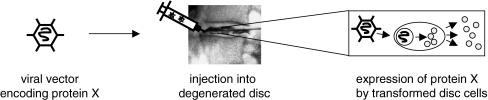
Fig. 4.
Direct gene therapy. Resident cells are genetically modified in situ in order to express beneficial genes. Modification is achieved using a carrier (for example gene engineered viral vectors). After transformation, the resident disc cells show sustained expression of the beneficial protein (protein X)
Fig. 5.
Augmentation of degenerated intervertebral discs by implantation of autologous cells. Cultivated cells could be implanted directly, genetically modified in vitro prior to implantation (indirect gene therapy), or seeded into a scaffold (tissue engineering)
Fig. 6.
Augmentation of degenerated intervertebral discs by implantation of bone-derived mesenchymal stem cells (MSC). Harvested MSC can be cultivated as progenitor cells and injected directly into the disc matrix. Alternatively, the progenitor cells can be differentiated in vitro to a disc cell-like phenotype and then injected into the disc matrix
Direct injection of an active substance
The most straightforward technique to deliver an active substance to the disc cells would be its direct injection into the disc (Fig. 3). Although direct application of beneficial factors, mostly proteins like growth factors, cytokines or anabolic enzymes, has been used frequently in vitro, only few studies have been published attempting this approach in vivo. Promising results have been reported after injecting rabbit lumbar IVD in vivo with osteogenic protein-1 (OP-1), a growth factor belonging to the TGF-β superfamily of growth factors. Direct injection of this growth factor resulted in significantly increased proteoglycan synthesis and also restoration of disc height that was found to be stable up to eight weeks after injection [9, 194]. Additional studies demonstrated that OP-1 injection even inhibited pain-related behavior in a rat disc degeneration model [8, 97]. Although these studies demonstrate the feasibility of direct injection, this technique might be limited to the presence of disc cells that are still healthy, numerous and able to respond appropriately and to a short term stimulus. In the used rodent model systems this situation is much more probably to be encountered compared to other animal models (and humans) that do differ in the composition of the disc cell population and weight bearing properties. Considering the decreasing viability and synthetic activity of human disc cells during progressing degeneration, direct injection approaches are most probably limited to only mildly degenerated discs. Since mildly degenerated discs will be primarily found in younger individuals, an only transient regeneration of the disc might not countervail the, even though minimally invasive, intervention. In this context, the rather short follow-up time common to the current studies (up to 8 weeks) is further limiting conclusions regarding the applicability of this technique to humans. Further studies will need to include animal models with IVD more comparable to the human situation, especially regarding cell population, cell density and mechanical loading.
A different approach has been chosen in a clinical pilot study recently published by Klein et al. [101]. Instead of injecting a biologically active enzyme or growth factor, the authors injected a mixture of matrix components and aiding components, the so-called ‘disc solution’, directly into the disc. After treatment and an average duration of follow-up of 13 months the patients responded well regarding reduction in a disability score and a visual analogue pain score. The authors suggested that the good outcome might be due to the combination of several components resulting in a parallel replenishing of the matrix by increased proteoglycan synthesis and induction of disc repair by simultaneous induction of multiple growth factors. Although controlled comparative studies with increased follow-ups will be needed to further validate this treatment, this approach might prove superior to the injection of a single bioactive factor. It is conceivable that the injected matrix components are able to modulate and improve the intradiscal environment enabling the disc cells, even in a severely degenerated disc, to react to the resulting secretion of growth factors and continue with the maintenance of the matrix. However, from this pilot study it is not clear if the injected components will be contained inside the disc in heavily degenerated discs and therefore be able to ensure a prolonged beneficial effect on the disc cells.
Common to these ‘injection techniques’ is the usually short-term effect that might cease when the originally injected material has been consumed or is lost to the disc cells by diffusion. In order to provide the disc with a continuing supply of beneficial factors, it would be desirable to continuously produce the factor of choice or include the substances in a pharmacological slow-release system as suggested for the use of growth factors [133]. Considering these data, it is conceivable that a combination of growth factor injection and matrix replenishment by a ‘disc solution’ might be the way to obtain a more sustained improvement of degenerated discs and might also expand the limitation regarding its application to various degeneration grades.
Gene therapy approaches
Prolonged supply of the discal matrix with a beneficial agent could be achieved by genetically modifying disc cells in order to produce a desired gene product. Due to advances in molecular genetics, techniques are readily available to insert genetic elements (DNA) into almost any type of target cell. These genetic elements usually consist of the gene encoding the desired product and a control element modulating the expression of the respective gene. Typically, two strategies can be used to achieve expression of the desired gene at the targeted site. In vivo, or direct gene therapy requires the direct introduction of the gene of interest into resident cells in situ (Fig. 4), whereas indirect, or ex vivo gene therapy requires the removal of target cells, introduction of the gene of interest in vitro and implantation of the transformed cells (Fig. 5) [141]. Since the uptake of pure, ‘naked’ DNA into host cells is usually not very efficient [216], uptake of the desired genetic material can be improved by application of a carrier, also called vector.
Two classes of vectors are currently used: non-viral vectors and viral vectors. Non-viral transporters are either molecular complexes of the DNA and a ligand [118], liposomes containing DNA [126], or particle-mediated gene transfer (‘gene gun’) [218]. Generally, the disadvantages of non-viral transporters are the rather poor expression of the transferred DNA and the limited efficiency, resulting in a restricted number of transformed cells. In addition, the introduced gene usually remains episomal, i.e. it will not be integrated into the genome of the target cell, and is therefore prone to be lost from the cell. Although, these non-viral systems have been rarely used in approaches to the treatment of degenerative disc disease, recent developments have improved the efficiency and in the future non-viral vectors might become interesting for treating disc disorders [117].
Viral vectors are very efficient transporters of genetic material; they are able to enter mammalian cells, taking over DNA replication and the protein expression machinery. For the purposes of gene therapy, several engineered viruses are available that have the original viral genome removed or inactivated and in addition are modified to not replicate nor exhibit their pathogenicity. Properties of currently used viral vectors have been recently described by Gardlik et al. [56]. In summary, these viruses vary in their ability to integrate the transferred DNA into the host cell genome, their ability to invade dividing or non-dividing cells and their infection efficiency. Due to the properties of the intervertebral disc and its cells, the virus of choice needs to efficiently infect non-dividing, quiescent cells. In addition, the low cell density inside the disc might hamper efficient infection of a sufficient fraction of the disc cells. On the other hand, the encapsulated and avascular tissue would provide an advantageous environment to achieve high concentrations of the injected viral vector, leading to higher efficiency of the infection process and also lessens the danger of an immune response against viral proteins.
During 1997, the first successful virus-mediated transfers of potentially therapeutic genes into disc cells have been published [160, 212]. Isolated disc cells from bovine or rat discs were successfully transformed by a retroviral construct containing the gene for the human interleukin-1 receptor antagonist (IL-1RA), or a bacterial β-galactosidase as a control [160, 212]. Following these initial experiments several studies have been published applying adenoviral vector constructs. These studies include the use of in vivo injections of the respective adenoviral construct in healthy or artificially degenerated rabbit IVD [106, 142] as well as in vitro transfections of human disc derived cells [136]. Taken together, these studies have demonstrated that adenoviral vectors are able to efficiently transform disc cells from various species. Importantly, no evidence has been reported that the efficiency of the gene transfer might depend on the degeneration state of the donor tissue, a prerequisite for a broad application of this technique. However, these studies do not sufficiently address concerns about safety of these vectors in clinical applications. The main disadvantage of adenoviral vectors lies in the activation of innate and adaptive parts of the patient’s immune system when the vector is applied in vivo. The resulting inflammatory responses induced by these vectors can be very strong and even fatal [164, 186]. To overcome this drawback, Lattermann et al. [109] recently tested an adeno-associated viral vector (AAV) for its applicability to disc degeneration treatment. The authors demonstrated that AAV was able to efficiently transduce human disc cells in vitro and rabbit disc cells in vivo. Although AAV caused a humoral immune response, no significant cellular immune response, as seen with adenoviral vectors, was observed. Interestingly, despite the observed humoral immune response, significant transgene expression was observed in the pre-exposed animals [109]. These findings suggest that AAV might offer a valuable and safer alternative to adenoviral vectors in the future.
Although the above studies suggest the feasibility of direct gene therapy using viral vectors to target disc cells, the question of the delivered gene and therefore expressed gene product remains open. Among the potentially beneficial genes tested so far are the anabolic factors transforming growth factor-β1 (TGF-β1), LMP-1 (LIM (LIM domain named after the three first described homeodomain proteins Lin-11, Isl-1, and Mec-3) mineralization protein-1)and Sox9 (SRY (sex-determining region Y)-box 9), and the anti-catabolic factor TIMP-1.
The first study to deliver an exogenous therapeutic gene in vivo in was performed by Nishida et al. using an adenoviral vector carrying the gene for TGF-β1 to modify cells of rabbit IVD [142]. The authors found significant increases of TGF-β1 and also proteoglycan production in the injected disc, suggesting the feasibility of direct gene therapy to treat intervertebral disc diseases [142].
An alternative potentially beneficial factor, LMP-1, has been shown to positively affect the degenerated disc matrix by increasing the disc cell production of BMPs and proteoglycans in vitro [219]. Intradiscal injection of rabbit discs in vivo resulted in increased expression of the anabolic cytokines BMP-2 and BMP-7 mRNA and also led to increased production of aggrecan mRNA [219]. These data suggest that LMP-1 is also a beneficial factor that could be applied to gene therapeutically treat disc degeneration. In contrast to the proteins discussed so far, Sox9 does not influence the proteoglycan content in the disc matrix. As a transcription factor responsible for the synthesis of type II collagen, its transfer into cells from degenerated human discs resulted in increased levels of type II collagen [153]. Injection of a Sox9 carrying adenoviral vector into stabbed rabbit discs resulted in preservation of the histologic appearance seen in healthy discs, whereas the stabbed control discs displayed degeneration-typical alterations [153]. Therefore, not only increased proteoglycan production but also collagen type II production seems to be able to prevent disc degeneration in vivo and thereby offering an attractive alternative therapeutic approach. Although the promising responses of disc cells to transformation with activating factors, it is conceivable that the increased production of a single growth factor or transcription factor might results in sub-optimal responses. A combination of related or, better, synergetic factors might be more physiological and therefore might lead to better results. In a recent study the combined transfer of TGF-β1, IGF-1 and BMP-2 revealed not only an additive effect of these factors on protein synthesis but also a synergistic amplification of protein synthesis [137].
Besides the use of anabolic factors to induce increased production of matrix components by disc cells, application of anti-catabolic factors might provide an interesting alternative. Catabolic inhibition would allow maintaining or increasing the content of the respective matrix component by slowing down its degradation without the need to force the disc cells to higher synthesis rates. Using an adenoviral vector, the gene encoding for TIMP-1 was introduced in vitro into disc cells isolated from degenerated human IVD. Gene delivery of TIMP-1 increased the proteoglycan content in the disc cell cultures, suggesting the anti-catabolic approach to be a new and promising strategy for gene therapy of degenerated discs [209].
Although these results sound very promising, the application to human IVD will be complicated by the adverse microenvironment inside a severely degenerated disc. Considering the compromised disc cell physiology due to deprivation of nutrients like glucose or oxygen and also the lowered pH due to accumulation of lactic acid, it is questionable if these cells will be able to produce reasonable amounts of growth factors over a significant period of time. Even if the production of the respective gene product could be achieved, it can be doubted that the surrounding, starving cells are able to properly respond and produce an improved matrix. Despite originating from degenerated discs, the cells used during in vitro studies have been brought to and analyzed in a completely different environment that barely resembles the in vivo situation. Therefore, extrapolations from these in vitro experiments to the abilities of transformed cells in vivo have to be very cautious. In this context it will be interesting to see how the anti-catabolic approach proves in the in vivo situation compared to the use of anabolic factors. The unavailability of a suitable animal model that would include the deprivation of nutrients from the disc cells is further complicating predictions about the applicability of direct gene therapy to degenerative disc diseases in humans.
Supplementation with autologous cells
Alternatively to genetically modifying resident disc cells, degenerated discs could be treated by supplementation of the deserted matrix with in vitro cultivated cells. To avoid immunological complications, these cells should preferentially originate from the patients body (autologous cells). Therefore, cells that are compatible with the disc tissue have to be harvested, expanded in vitro and subsequently implanted into the affected tissue. Once the cells have been removed and cultivated in vitro, this approach allows for genetic modifications of the withdrawn cells (= indirect gene therapy) and/or seeding of the cells in supporting biomaterials (= tissue engineering) prior to implantation into the degenerated disc (Fig. 5). The combination of these techniques potentially improves the efficiency of the original approach by improving cell survival or enhancing the biosynthetic activity of the implanted cells. Since the genetic modification of cultivated cells in vitro is technically very similar to the approaches discussed above, we will focus here on the cultivation of disc cells and generation of suitable implants.
Compared to similar approaches in articular cartilage, it is substantially more difficult to obtain suitable and sufficient numbers of target cells from intervertebral disc tissue. Removal of nucleus pulposus cells, the obvious target cells, would require opening of the anulus fibrosus to gain access. On the other side, removal of anulus fibrosus cells would necessarily cause damage to the anulus. In addition to the very restricted accessibility, the very low cell density in degenerated discs will further complicate the acquisition of ample cells for successful in vitro cultivation. Therefore, only a limited number of scenarios are conceivable that would allow the withdrawal of sufficient cells from the disc to perform a disc cell-based approach without further damaging the already affected disc or accelerating its degeneration. Withdrawal of herniated disc material might be one scenario that would facilitate the removal of sufficient disc cells for in vitro cultivation. However, the introduction of cells/implants after a surgical intervention on the same disc is disputed since the outcome of minimally invasive nucleotomy has been shown satisfactory for most patients [130]. Another conceivable scenario would be the use of cell-based approaches to prevent the accelerated degeneration of discs adjacent to an interbody fusion level [32, 100, 114]. The disc material removed during the fusion procedure could be used as source of cells to treat the adjacent disc. However, this would imply an intervention at a non-degenerated disc that only has the potential to degenerate later on and is therefore rather questionable. Taken together, with our current techniques, autologous disc cell transplantation is limited to few scenarios but has the potential to prove as a powerful approach within these limitations.
The most straightforward approach to augment a degenerated disc by autologous cells would be injecting a suspension of ex vivo proliferated disc cells [54, 55]. This approach is also the only one applied in clinical trials so far [55]. In these studies, the outcome was mostly satisfying with the patients significantly improving. However, the scientific data published on this approach are rather sparse. Information about cell survival, phenotype of the implanted cells and the biosynthetic abilities of these cells is not available so far. In addition, animal experiments have raised the issue that injection of a cell suspension into rabbit IVDs led to extensive leakage of the cells through the injection site [18]. So far it is not clear, why this leakage was not observed during the clinical studies or if the leakage probably was delayed and not recognized. In the animal model the leakage was significantly reduced by suspending the cells in a fibrinogen–thrombin solution (i.e. matrix-assisted cell transfer). However, this study also suggested that preceding nucleotomy and supplementation of the cells with nutrients is necessary for injection and also matrix-assisted transfer approaches in order to permit survival of the injected cells [18]. Although data from animal experiments and human clinical studies cannot be directly compared, the observations from the animal experiments raise the question if and how the cells injected into the herniated human disc survive. Further investigation on the fate of the implanted cells in vivo is therefore definitely needed.
An approach to prepare autologous disc cells for subsequent transplantation into the degenerated disc is the cultivation of the disc cells in three-dimensional cultivation systems. This approach is based on early observations during routine monolayer cultivation of disc cells that led to dedifferentiation of the cells with concurrent morphological changes [14, 107, 127]. Maldonado et al. then demonstrated that cultivation of disc cells in three-dimensional constructs conserved the native phenotype, as shown by the synthesis of matrix components similar to those observed in native discs [127]. Since the initial experiments a wide variety of techniques has been recently applied to supply disc cells with the desired three-dimensional arrangements (reviewed in [63]). Herein, we will describe experimental strategies whose feasibility has been tested in vivo, i.e. in animal experiments, and discuss these approaches.
In a study using a Sand Rat-based model system, Gruber et al. applied autologous disc cells, expanded in routine monolayer cultures and seeded into a three-dimensional scaffold, to a hollowed cavity created in the acceptor intervertebral disc [64]. After up to 33 weeks, no giant cell response was observed and the cells showed an appropriate morphology. In addition, no abnormality in the cell-surrounding matrix was observed, suggesting appropriate survival of the implanted cells during the analyzed time period. From their data the authors conclude that autologous disc cell implantation can be successful, although technically challenging. However, due to the immediate implantation after the seeding of the scaffold, the disc cells do not have the time to synthesize appropriate amounts of matrix before encountering the microenvironmental conditions inside the disc. Although this technique was successful in the given animal model, the cells might not have survived implantation into the hostile environment inside a degenerated human disc.
Cultivation of the disc cells in a three-dimensional system prior to implantation might therefore improve the chances for survival in the hostile environment. This improved approach was tested by Sato et al. [175–177] using a scaffold based on the non-immunogenic collagen derivative atelocollagen that has previously been tested in vitro and has been already applied to a rabbit model system. Rabbit anulus fibrosus cells seeded and cultivated for up to 3 weeks in this scaffold showed an increased ability to express type II collagen mRNA and deposited more type II collagen and proteoglycan compared to cells grown in monolayers. Atelocollagen scaffolds seeded with anulus fibrosus cells have been allografted into the lacunae of recipient rabbits after laser discectomy of the nucleus pulposus [175]. Implantation resulted in a significant prevention of the narrowing of the intervertebral disc space up to 12 weeks postoperative compared to the nucleotomized control animals. Histological analysis also showed that the allografted cells were viable, proliferated and produced a hyaline-like matrix in the disc tissue of the recipients. Although the rabbit model does not appropriately simulate the mechanical forces experienced by implanted disc cell in human discs, it is conceivable that cells surrounded by their own matrix might withstand mechanical forces with more success. However, experimental data are lacking to prove this point. In addition, the application of the seeded scaffold required a 16-gauge needle (approx. 1.65 mm outside diameter) that would significantly damaged the anulus fibrosus during implantation. It would therefore be necessary to somehow close the gap in the anulus fibrosus to avoid loss of the implanted construct upon axial load.
Taken together the presented approaches suggest that disc repair attempts with autologous disc cells require a delicate balance between support of the implanted cells and the feasibility of implantation without detrimental damage of discal structures. Another problem not addressed by the presented studies is the acute shortage of nutrients experienced by the cells after implantation into the degenerated disc. Considering that the nutrient supply is hardly sufficient for the original disc cells, it is questionable if the additional cells will survive for a prolonged time span in order to provide a sustained improvement of the disc.
Stem cell-based gene therapies
A recent development in tissue engineering techniques might bear the potential to circumvent at least some problems encountered with the standard tissue engineering/gene therapy approaches (Fig. 6).
Adult mesenchymal stem cells are uncommitted pluripotent stem cells that are found in several tissues like skeletal muscle, bone marrow, synovial membranes and dermis [19, 20, 39, 81, 154, 220]. Mesenchymal stem cells are of high plasticity and have a high capacity of multilineage differentiation. In addition, they are accessible and comparably easy to manipulate, making them natural candidates for orthopedic gene therapy studies [53]. In order to engineer the isolated stem cells several vector system have already been tested on adult mesenchymal stem cells and high transduction and expression rates have been achieved [89, 108, 119]. However, transforming of isolated mesenchymal stem cells is only one step on the way to its use for cell-based therapy. Being undifferentiated the stem cells’ differentiation has to be directed to yield chondrocytes or chondrocyte-like cells. Members of the BMP family of growth factors have been used so far to induce differentiation of mesenchymal stem cells into chondrocytes [1, 105, 132]. However, since BMP are not exclusively inducing cartilage differentiation, its expression needs to be carefully timed and modulated to avoid the sequent generation of bone structures. To overcome this problem, signal transduction factors that exclusively induced cartilage differentiation, have been tested. Members of the Sox family of transcription factors and a novel transcription factor, Brachyury, are potential candidates and have been tested with encouraging results [5, 50, 74]. Recently, it has been found that already co-cultivation of MSCs with disc cells might be sufficient to induce a disc cell-like phenotype in MSCs [99, 113, 162, 182]. Although these data origin from in vitro experiments, it might be conceivable that MSCs would also differentiate when brought into contact with disc cells in vivo, i.e. after injection into the disc. Besides the co-cultivation with disc cells, cultivating of mesenchymal stem cells in three-dimensional cultivating systems appears to be sufficient to induce a nucleus pulposus-like phenotype [163]. Implantation of collagen-gel-embedded mesenchymal stem cells into artificially degenerated rabbit discs resulted in preserved nuclear and anular structures, prevention of proteoglycan decrease from the nucleus pulposus and increased disc height [170–172]. The implanted cells were shown to survive and express genetic markers typical for nucleus pulposus cells. Similar results were also found after injection of a bone-derived MSC suspension into rabbit discs and injection of gel embedded MSCs into rat coccygeal discs [35, 223].
The use of mesenchymal stem cells provides a new and exciting approach to biologically treat disc degeneration with so far encouraging results. The comparably easy access to autologous mesenchymal stem cells allows overcoming one of the major culprits of conventional approaches. However, there are many open questions before the real potential of this technique can be judged. Since the exact phenotype of nucleus pulposus cells and their origin are still not undoubtedly defined it is nearly impossible to find a definitive answer if the phenotype of the differentiated MSCs is identical or only similar to the original nucleus pulposus cells. Although recent data indicate a very good accordance between differentiated MSCs and native disc cells [190], it is not yet clear if the newly adopted phenotype is stable over a prolonged time and during the severely changing environmental conditions as disc degeneration progresses. Another unclarity that also affects autologous cell transplantation approaches is the quality of the newly synthesized matrix regarding biomechanical properties. Is the new disc matrix, despite its biochemical similarity to the original matrix, able to bear the mechanical load experienced during the daily life of the individual? These questions are of outmost importance and might put the stem cell approach into perspective. However, the high expandability of MSCs together with the relative easiness to harvest the cells makes this approach highly attractive. In addition, recent studies have shown that MSCs lack MHC class I receptors [110] and that human recipients receiving MHCs from sibling donors do not exhibit an immunogenic response [77] suggesting that allogenic MSCs could be used, which would further encourage the use of MSC for disc repair approaches. However, more and extended studies are required to analyze the long-term properties of the newly formed tissue and to prove its value under mechanical load in the spine of bipedal vertebrate.
Future directions
The intervertebral disc has been shown to be a very unique, highly specialized tissue that undergoes massive alterations during degeneration. The functions of the disc require a mechanically stable structure with a highly specialized matrix to confer the needed flexibility and physical strength to the spine. Especially the bipedal gait of human beings causes severe mechanical stress to the intervertebral disc. To withstand these forces the intervertebral disc is necessarily ‘designed’ as a self-contained structure. However, self-containment has not only beneficial effects for the disc. The observed avascularity of the adult disc restricts nutrient supply to diffusion and therefore poses a major challenge for the prolonged maintenance of the discal matrix by the disc cells. Mechanical stress and nutrient restriction finally create a hostile environment, resulting in progressing death of the matrix maintaining cells and, concomitant, to an extensive decay of the matrix.
These properties and its alterations during degeneration limit and define the techniques applicable to biologically repair degenerated IVD. Although the self-contained and avascular structure would favor direct gene therapy, the very limited numbers of cells and the compact matrix in the degenerated disc outweigh the immunological advantages. Improved carrier systems to efficiently deliver genetic material to the disc cells might overcome this major disadvantage, but until then direct gene therapy might be an inferior strategy.
By autologous implantation of in vitro cultivated disc cells, disc cell implantation has the potential to refresh the number of viable cells, and therefore the matrix, inside the degenerated disc. However, the degenerative decay of the discal matrix leaves an exhausted desert that will inescapable become the host of the in vitro generated tissue, and therefore putting huge demands on the implanted cells. Despite the numerous studies that have broadened our understanding of the biochemistry and the capability of disc cells, it is questionable if the cells can be prepared in vitro for prolonged survival and proper functioning under such adverse conditions.
Because of the band-width of degenerative alterations, different stages of degeneration will require different approaches. Putative biological therapies will need to be tailored for the treatment of a defined grade of degeneration. Therefore, criteria will need to be defined to decide if and with which technique a certain degenerated disc might be treated.
Taken together, these concerns might indicate that the clinical application of gene therapy/tissue engineering techniques to regenerate degenerated IVD is still far away. With our current technology, gene therapy of the degenerate disc might be compared to attempts to jumpstart a car that ran out of fuel. However, based on the recent insights into signal transduction mechanisms that might lead to the induction of pain by degenerated discs, it is conceivable that therapies targeting pro-inflammatory signaling pathways might be successful in the foreseeable future. Such therapies might not have the ability to reverse the progressing tissue destruction which occurs with ageing but may transform a symptomatic to an asymptomatic disc degeneration and thereby greatly improve life quality of the affected patients.
Footnotes
This study was supported by a grant from the AO Spine (SRN 02/103).
References
- 1.Adachi N, Sato K, Usas A, Fu FH, Ochi M, Han CW, Niyibizi C, Huard J. Muscle derived, cell based ex vivo gene therapy for treatment of full thickness articular cartilage defects. J Rheumatol. 2002;29:1920–1930. [PubMed] [Google Scholar]
- 2.Adams MA, Dolan P. Spine biomechanics. J Biomech. 2005;38:1972–1983. doi: 10.1016/j.jbiomech.2005.03.028. [DOI] [PubMed] [Google Scholar]
- 3.Adams MA, Freeman BJ, Morrison HP, Nelson IW, Dolan P. Mechanical initiation of intervertebral disc degeneration. Spine. 2000;25:1625–1636. doi: 10.1097/00007632-200007010-00005. [DOI] [PubMed] [Google Scholar]
- 4.Ahn SH, Cho YW, Ahn MW, Jang SH, Sohn YK, Kim HS. mRNA expression of cytokines and chemokines in herniated lumbar intervertebral discs. Spine. 2002;27:911–917. doi: 10.1097/00007632-200205010-00005. [DOI] [PubMed] [Google Scholar]
- 5.Akiyama H, Chaboissier MC, Martin JF, Schedl A, Crombrugghe B. The transcription factor Sox9 has essential roles in successive steps of the chondrocyte differentiation pathway and is required for expression of Sox5 and Sox6. Genes Dev. 2002;16:2813–2828. doi: 10.1101/gad.1017802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ala-Kokko L. Genetic risk factors for lumbar disc disease. Ann Med. 2002;34:42–47. doi: 10.1080/078538902317338634. [DOI] [PubMed] [Google Scholar]
- 7.Allan DB, Waddell G. An historical perspective on low back pain and disability. Acta Orthop Scand. 1989;234(Suppl):1–23. doi: 10.3109/17453678909153916. [DOI] [PubMed] [Google Scholar]
- 8.An HS, Takegami K, Kamada H, Nguyen CM, Thonar EJ, Singh K, Andersson GB, Masuda K (2005) Intradiscal administration of osteogenic protein-1 increases intervertebral disc height and proteoglycan content in the nucleus pulposus in normal adolescent rabbits. Spine 30:25–31; discussion 31–22 [DOI] [PubMed]
- 9.An HS, Thonar EJ, Masuda K. Biological repair of intervertebral disc. Spine. 2003;28:S86–92. doi: 10.1097/01.BRS.0000076904.99434.40. [DOI] [PubMed] [Google Scholar]
- 10.Anderson DG, Izzo MW, Hall DJ, Vaccaro AR, Hilibrand A, Arnold W, Tuan RS, Albert TJ. Comparative gene expression profiling of normal and degenerative discs: analysis of a rabbit annular laceration model. Spine. 2002;27:1291–1296. doi: 10.1097/00007632-200206150-00009. [DOI] [PubMed] [Google Scholar]
- 11.Antoniou J, Goudsouzian NM, Heathfield TF, Winterbottom N, Steffen T, Poole AR, Aebi M, Alini M. The human lumbar endplate. Evidence of changes in biosynthesis and denaturation of the extracellular matrix with growth, maturation, aging, and degeneration. Spine. 1996;21:1153–1161. doi: 10.1097/00007632-199605150-00006. [DOI] [PubMed] [Google Scholar]
- 12.Antoniou J, Steffen T, Nelson F, Winterbottom N, Hollander AP, Poole RA, Aebi M, Alini M. The human lumbar intervertebral disc: evidence for changes in the biosynthesis and denaturation of the extracellular matrix with growth, maturation, ageing, and degeneration. J Clin Invest. 1996;98:996–1003. doi: 10.1172/JCI118884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ariga K, Yonenobu K, Nakase T, Kaneko M, Okuda S, Uchiyama Y, Yoshikawa H. Localization of cathepsins D, K, and L in degenerated human intervertebral discs. Spine. 2001;26:2666–2672. doi: 10.1097/00007632-200112150-00007. [DOI] [PubMed] [Google Scholar]
- 14.Aydelotte MB, Kuettner KE. Differences between sub-populations of cultured bovine articular chondrocytes. I. Morphology and cartilage matrix production. Connect Tissue Res. 1988;18:205–222. doi: 10.3109/03008208809016808. [DOI] [PubMed] [Google Scholar]
- 15.Baffi MO, Slattery E, Sohn P, Moses HL, Chytil A, Serra R. Conditional deletion of the TGF-beta type II receptor in Col2a expressing cells results in defects in the axial skeleton without alterations in chondrocyte differentiation or embryonic development of long bones. Dev Biol. 2004;276:124–142. doi: 10.1016/j.ydbio.2004.08.027. [DOI] [PubMed] [Google Scholar]
- 16.Bartels EM, Fairbank JC, Winlove CP, Urban JP (1998) Oxygen and lactate concentrations measured in vivo in the intervertebral discs of patients with scoliosis and back pain. Spine 23:1–7; discussion 8 [DOI] [PubMed]
- 17.Battie MC, Videman T, Gibbons LE, Fisher LD, Manninen H, Gill K. 1995 Volvo Award in clinical sciences. Determinants of lumbar disc degeneration. A study relating lifetime exposures and magnetic resonance imaging findings in identical twins. Spine. 1995;20:2601–2612. doi: 10.1097/00007632-199512150-00001. [DOI] [PubMed] [Google Scholar]
- 18.Bertram H, Kroeber M, Wang H, Unglaub F, Guehring T, Carstens C, Richter W. Matrix-assisted cell transfer for intervertebral disc cell therapy. Biochem Biophys Res Commun. 2005;331:1185–1192. doi: 10.1016/j.bbrc.2005.04.034. [DOI] [PubMed] [Google Scholar]
- 19.Bianco P, Gehron Robey P. Marrow stromal stem cells. J Clin Invest. 2000;105:1663–1668. doi: 10.1172/JCI10413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bianco P, Riminucci M, Gronthos S, Robey PG. Bone marrow stromal stem cells: nature, biology, and potential applications. Stem Cells. 2001;19:180–192. doi: 10.1634/stemcells.19-3-180. [DOI] [PubMed] [Google Scholar]
- 21.Bibby SR, Urban JP. Effect of nutrient deprivation on the viability of intervertebral disc cells. Eur Spine J. 2004;13:695–701. doi: 10.1007/s00586-003-0616-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bijkerk C, Houwing-Duistermaat JJ, Valkenburg HA, Meulenbelt I, Hofman A, Breedveld FC, Pols HA, Duijn CM, Slagboom PE. Heritabilities of radiologic osteoarthritis in peripheral joints and of disc degeneration of the spine. Arthritis Rheum. 1999;42:1729–1735. doi: 10.1002/1529-0131(199908)42:8<1729::AID-ANR23>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 23.Boden SD, Davis DO, Dina TS, Patronas NJ, Wiesel SW. Abnormal magnetic-resonance scans of the lumbar spine in asymptomatic subjects. A prospective investigation. J Bone Joint Surg Am. 1990;72:403–408. [PubMed] [Google Scholar]
- 24.Nerlich AG, Schaaf R, Walchli B, Boos N (2006) Temporo-spatial distribution of blood vessels in human lumbar intervertebral discs. Eur Spine J (in press) [DOI] [PMC free article] [PubMed]
- 25.Boos N, Weissbach S, Rohrbach H, Weiler C, Spratt KF, Nerlich AG. Classification of age-related changes in lumbar intervertebral discs: 2002 Volvo Award in basic science. Spine. 2002;27:2631–2644. doi: 10.1097/00007632-200212010-00002. [DOI] [PubMed] [Google Scholar]
- 26.Buckwalter JA. Aging and degeneration of the human intervertebral disc. Spine. 1995;20:1307–1314. doi: 10.1097/00007632-199506000-00022. [DOI] [PubMed] [Google Scholar]
- 27.Burke JG, Watson RW, Conhyea D, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Human nucleus pulposis can respond to a pro-inflammatory stimulus. Spine. 2003;28:2685–2693. doi: 10.1097/01.BRS.0000103341.45133.F3. [DOI] [PubMed] [Google Scholar]
- 28.Burke JG, Watson RW, McCormack D, Dowling FE, Walsh MG, Fitzpatrick JM. Intervertebral discs which cause low back pain secrete high levels of proinflammatory mediators. J Bone Joint Surg Br. 2002;84:196–201. doi: 10.1302/0301-620x.84b2.12511. [DOI] [PubMed] [Google Scholar]
- 29.Carreon LY, Ito T, Yamada M, Uchiyama S, Takahashi HE (1997) Neovascularization induced by anulus and its inhibition by cartilage endplate. Its role in disc absorption. Spine 22:1429–1434; discussion 1446–1427 [DOI] [PubMed]
- 30.Chelberg MK, Banks GM, Geiger DF, Oegema TR., Jr Identification of heterogeneous cell populations in normal human intervertebral disc. J Anat. 1995;186(Pt 1):43–53. [PMC free article] [PubMed] [Google Scholar]
- 31.Ching CT, Chow DH, Yao FY, Holmes AD. The effect of cyclic compression on the mechanical properties of the inter-vertebral disc: an in vivo study in a rat tail model. Clin Biomech (Bristol Avon) 2003;18:182–189. doi: 10.1016/s0268-0033(02)00188-2. [DOI] [PubMed] [Google Scholar]
- 32.Chow DH, Luk KD, Evans JH, Leong JC. Effects of short anterior lumbar interbody fusion on biomechanics of neighboring unfused segments. Spine. 1996;21:549–555. doi: 10.1097/00007632-199603010-00004. [DOI] [PubMed] [Google Scholar]
- 33.Coventry MB, Ghormley RK, Kernohan JW. The intervertebral disc: Its microscopic anatomy and pathology. Part II. Changes in the intervertebral disc concomittant with age. J Bone Joint Surg Am. 1945;27A:233–247. [Google Scholar]
- 34.Crean JK, Roberts S, Jaffray DC, Eisenstein SM, Duance VC. Matrix metalloproteinases in the human intervertebral disc: role in disc degeneration and scoliosis. Spine. 1997;22:2877–2884. doi: 10.1097/00007632-199712150-00010. [DOI] [PubMed] [Google Scholar]
- 35.Crevensten G, Walsh AJ, Ananthakrishnan D, Page P, Wahba GM, Lotz JC, Berven S. Intervertebral disc cell therapy for regeneration: mesenchymal stem cell implantation in rat intervertebral discs. Ann Biomed Eng. 2004;32:430–434. doi: 10.1023/b:abme.0000017545.84833.7c. [DOI] [PubMed] [Google Scholar]
- 36.Crock HV, Goldwasser M, Yoshizawa H. Vascular anatomy related to the intervertebral disc. In: Gosh P, editor. The biology of the intervertebral disc. Boca Raton: CRC Press; 1988. pp. 109–133. [Google Scholar]
- 37.Crock HV, Yoshizawa H. The blood supply of the lumbar vertebral column. Clin Orthop. 1976;115:6–21. [PubMed] [Google Scholar]
- 38.Darling EM, Athanasiou KA. Biomechanical strategies for articular cartilage regeneration. Ann Biomed Eng. 2003;31:1114–1124. doi: 10.1114/1.1603752. [DOI] [PubMed] [Google Scholar]
- 39.Bari C, Dell’Accio F, Tylzanowski P, Luyten FP. Multipotent mesenchymal stem cells from adult human synovial membrane. Arthritis Rheum. 2001;44:1928–1942. doi: 10.1002/1529-0131(200108)44:8<1928::AID-ART331>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 40.Deiss LP, Galinka H, Berissi H, Cohen O, Kimchi A. Cathepsin D protease mediates programmed cell death induced by interferon-gamma, Fas/APO-1 and TNF-alpha. Embo J. 1996;15:3861–3870. [PMC free article] [PubMed] [Google Scholar]
- 41.Deyo RA, Bass JE. Lifestyle and low-back pain. The influence of smoking and obesity. Spine. 1989;14:501–506. doi: 10.1097/00007632-198905000-00005. [DOI] [PubMed] [Google Scholar]
- 42.Doege KJ, Coulter SN, Meek LM, Maslen K, Wood JG. A human-specific polymorphism in the coding region of the aggrecan gene. Variable number of tandem repeats produce a range of core protein sizes in the general population. J Biol Chem. 1997;272:13974–13979. doi: 10.1074/jbc.272.21.13974. [DOI] [PubMed] [Google Scholar]
- 43.Doita M, Kanatani T, Harada T, Mizuno K. Immunohistologic study of the ruptured intervertebral disc of the lumbar spine. Spine. 1996;21:235–241. doi: 10.1097/00007632-199601150-00015. [DOI] [PubMed] [Google Scholar]
- 44.Doita M, Kanatani T, Ozaki T, Matsui N, Kurosaka M, Yoshiya S. Influence of macrophage infiltration of herniated disc tissue on the production of matrix metalloproteinases leading to disc resorption. Spine. 2001;26:1522–1527. doi: 10.1097/00007632-200107150-00004. [DOI] [PubMed] [Google Scholar]
- 45.Ebisui C, Tsujinaka T, Morimoto T, Kan K, Iijima S, Yano M, Kominami E, Tanaka K, Monden M. Interleukin-6 induces proteolysis by activating intracellular proteases (cathepsins B and L, proteasome) in C2C12 myotubes. Clin Sci (Lond) 1995;89:431–439. doi: 10.1042/cs0890431. [DOI] [PubMed] [Google Scholar]
- 46.Elfering A, Semmer N, Birkhofer D, Zanetti M, Hodler J, Boos N. Risk factors for lumbar disc degeneration: a 5-year prospective MRI study in asymptomatic individuals. Spine. 2002;27:125–134. doi: 10.1097/00007632-200201150-00002. [DOI] [PubMed] [Google Scholar]
- 47.Elfering A, Semmer NK, Schade V, Grund S, Boos N. Supportive colleague, unsupportive supervisor: the role of provider-specific constellations of social support at work in the development of low back pain. J Occup Health Psychol. 2002;7:130–140. doi: 10.1037//1076-8998.7.2.130. [DOI] [PubMed] [Google Scholar]
- 48.Evans CH, Robbins PD, Ghivizzani SC, Herndon JH, Kang R, Bahnson AB, Barranger JA, Elders EM, Gay S, Tomaino MM, Wasko MC, Watkins SC, Whiteside TL, Glorioso JC, Lotze MT, Wright TM. Clinical trial to assess the safety, feasibility, and efficacy of transferring a potentially anti-arthritic cytokine gene to human joints with rheumatoid arthritis. Hum Gene Ther. 1996;7:1261–1280. doi: 10.1089/hum.1996.7.10-1261. [DOI] [PubMed] [Google Scholar]
- 49.Eyre DR, Benya PD, Buckwalter J, et al. Basic science perspectives. In: Frymoyer JW, Gordon SL, et al., editors. New perspectives on low back pain. Park Ridge, IL: American Academy Orthopaedic Surgeons; 1988. pp. 147–214. [Google Scholar]
- 50.Fischer L, Boland G, Tuan RS. Wnt-3A enhances bone morphogenetic protein-2-mediated chondrogenesis of murine C3H10T1/2 mesenchymal cells. J Biol Chem. 2002;277:30870–30878. doi: 10.1074/jbc.M109330200. [DOI] [PubMed] [Google Scholar]
- 51.Freemont AJ, Jeziorska M, Hoyland JA, Rooney P, Kumar S. Mast cells in the pathogenesis of chronic back pain: a hypothesis. J Pathol. 2002;197:281–285. doi: 10.1002/path.1107. [DOI] [PubMed] [Google Scholar]
- 52.Freemont AJ, Watkins A, Le Maitre C, Baird P, Jeziorska M, Knight MT, Ross ER, O’Brien JP, Hoyland JA. Nerve growth factor expression and innervation of the painful intervertebral disc. J Pathol. 2002;197:286–292. doi: 10.1002/path.1108. [DOI] [PubMed] [Google Scholar]
- 53.Gafni Y, Turgeman G, Liebergal M, Pelled G, Gazit Z, Gazit D. Stem cells as vehicles for orthopedic gene therapy. Gene Ther. 2004;11:417–426. doi: 10.1038/sj.gt.3302197. [DOI] [PubMed] [Google Scholar]
- 54.Ganey T, Libera J, Moos V, Alasevic O, Fritsch KG, Meisel HJ, Hutton WC. Disc chondrocyte transplantation in a canine model: a treatment for degenerated or damaged intervertebral disc. Spine. 2003;28:2609–2620. doi: 10.1097/01.BRS.0000097891.63063.78. [DOI] [PubMed] [Google Scholar]
- 55.Ganey TM, Meisel HJ. A potential role for cell-based therapeutics in the treatment of intervertebral disc herniation. Eur Spine J. 2002;11(Suppl 2):S206–S214. doi: 10.1007/s00586-002-0494-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Gardlik R, Palffy R, Hodosy J, Lukacs J, Turna J, Celec P. Vectors and delivery systems in gene therapy. Med Sci Monit. 2005;11:RA110–RA121. [PubMed] [Google Scholar]
- 57.Gerber A, Welte T, Ansorge S, Buhling F. Expression of cathepsins B and L in human lung epithelial cells is regulated by cytokines. Adv Exp Med Biol. 2000;477:287–292. doi: 10.1007/0-306-46826-3_31. [DOI] [PubMed] [Google Scholar]
- 58.Gerber A, Wille A, Welte T, Ansorge S, Buhling F. Interleukin-6 and transforming growth factor-beta 1 control expression of cathepsins B and L in human lung epithelial cells. J Interferon Cytokine Res. 2001;21:11–19. doi: 10.1089/107999001459114. [DOI] [PubMed] [Google Scholar]
- 59.Gigante A, Bevilacqua C, Pagnotta A, Manzotti S, Toesca A, Greco F. Expression of NGF, Trka and p75 in human cartilage. Eur J Histochem. 2003;47:339–344. [PubMed] [Google Scholar]
- 60.Goupille P, Jayson MI, Valat JP, Freemont AJ. Matrix metalloproteinases: the clue to intervertebral disc degeneration? Spine. 1998;23:1612–1626. doi: 10.1097/00007632-199807150-00021. [DOI] [PubMed] [Google Scholar]
- 61.Gruber HE, Fisher EC, Jr, Desai B, Stasky AA, Hoelscher G, Hanley EN., Jr Human intervertebral disc cells from the annulus: three-dimensional culture in agarose or alginate and responsiveness to TGF-beta1. Exp Cell Res. 1997;235:13–21. doi: 10.1006/excr.1997.3647. [DOI] [PubMed] [Google Scholar]
- 62.Gruber HE, Hanley EN., Jr Analysis of aging and degeneration of the human intervertebral disc. Comparison of surgical specimens with normal controls. Spine. 1998;23:751–757. doi: 10.1097/00007632-199804010-00001. [DOI] [PubMed] [Google Scholar]
- 63.Gruber HE, Hanley EN., Jr Recent advances in disc cell biology. Spine. 2003;28:186–193. doi: 10.1097/00007632-200301150-00017. [DOI] [PubMed] [Google Scholar]
- 64.Gruber HE, Johnson TL, Leslie K, Ingram JA, Martin D, Hoelscher G, Banks D, Phieffer L, Coldham G, Hanley EN., Jr Autologous intervertebral disc cell implantation: a model using Psammomys obesus, the sand rat. Spine. 2002;27:1626–1633. doi: 10.1097/00007632-200208010-00007. [DOI] [PubMed] [Google Scholar]
- 65.Gruber HE, Norton HJ, Hanley EN., Jr Anti-apoptotic effects of IGF-1 and PDGF on human intervertebral disc cells in vitro. Spine. 2000;25:2153–2157. doi: 10.1097/00007632-200009010-00002. [DOI] [PubMed] [Google Scholar]
- 66.Handa T, Ishihara H, Ohshima H, Osada R, Tsuji H, Obata K. Effects of hydrostatic pressure on matrix synthesis and matrix metalloproteinase production in the human lumbar intervertebral disc. Spine. 1997;22:1085–1091. doi: 10.1097/00007632-199705150-00006. [DOI] [PubMed] [Google Scholar]
- 67.Haro H, Kato T, Komori H, Osada M, Shinomiya K. Vascular endothelial growth factor (VEGF)-induced angiogenesis in herniated disc resorption. J Orthop Res. 2002;20:409–415. doi: 10.1016/S0736-0266(01)00150-4. [DOI] [PubMed] [Google Scholar]
- 68.Hastreiter D, Ozuna RM, Spector M. Regional variations in certain cellular characteristics in human lumbar intervertebral discs, including the presence of alpha-smooth muscle actin. J Orthop Res. 2001;19:597–604. doi: 10.1016/S0736-0266(00)00069-3. [DOI] [PubMed] [Google Scholar]
- 69.Heikkila JK, Koskenvuo M, Heliovaara M, Kurppa K, Riihimaki H, Heikkila K, Rita H, Videman T. Genetic and environmental factors in sciatica. Evidence from a nationwide panel of 9365 adult twin pairs. Ann Med. 1989;21:393–398. doi: 10.3109/07853898909149227. [DOI] [PubMed] [Google Scholar]
- 70.Heliovaara M. Risk factors for low back pain and sciatica. Ann Med. 1989;21:257–264. doi: 10.3109/07853898909149202. [DOI] [PubMed] [Google Scholar]
- 71.Hestbaek L, Iachine IA, Leboeuf-Yde C, Kyvik KO, Manniche C. Heredity of low back pain in a young population: a classical twin study. Twin Res. 2004;7:16–26. doi: 10.1375/13690520460741408. [DOI] [PubMed] [Google Scholar]
- 72.Hickey DG, Frenkel SR, Di Cesare PE. Clinical applications of growth factors for articular cartilage repair. Am J Orthop. 2003;32:70–76. [PubMed] [Google Scholar]
- 73.High K. AAV-mediated gene transfer for hemophilia. Genet Med. 2002;4:56S–61S. doi: 10.1097/00125817-200211001-00012. [DOI] [PubMed] [Google Scholar]
- 74.Hoffmann A, Czichos S, Kaps C, Bachner D, Mayer H, Kurkalli BG, Zilberman Y, Turgeman G, Pelled G, Gross G, Gazit D. The T-box transcription factor Brachyury mediates cartilage development in mesenchymal stem cell line C3H10T1/2. J Cell Sci. 2002;115:769–781. doi: 10.1242/jcs.115.4.769. [DOI] [PubMed] [Google Scholar]
- 75.Holm S, Maroudas A, Urban JP, Selstam G, Nachemson A. Nutrition of the intervertebral disc: solute transport and metabolism. Connect Tissue Res. 1981;8:101–119. doi: 10.3109/03008208109152130. [DOI] [PubMed] [Google Scholar]
- 76.Horner HA, Urban JP. 2001 Volvo Award Winner in basic science studies: Effect of nutrient supply on the viability of cells from the nucleus pulposus of the intervertebral disc. Spine. 2001;26:2543–2549. doi: 10.1097/00007632-200112010-00006. [DOI] [PubMed] [Google Scholar]
- 77.Horwitz EM, Gordon PL, Koo WK, Marx JC, Neel MD, McNall RY, Muul L, Hofmann T. Isolated allogeneic bone marrow-derived mesenchymal cells engraft and stimulate growth in children with osteogenesis imperfecta: Implications for cell therapy of bone. Proc Natl Acad Sci USA. 2002;99:8932–8937. doi: 10.1073/pnas.132252399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Horwitz EM, Prockop DJ, Fitzpatrick LA, Koo WW, Gordon PL, Neel M, Sussman M, Orchard P, Marx JC, Pyeritz RE, Brenner MK. Transplantability and therapeutic effects of bone marrow-derived mesenchymal cells in children with osteogenesis imperfecta. Nat Med. 1999;5:309–313. doi: 10.1038/6529. [DOI] [PubMed] [Google Scholar]
- 79.Hutton WC, Ganey TM, Elmer WA, Kozlowska E, Ugbo JL, Doh ES, Whitesides TE., Jr Does long-term compressive loading on the intervertebral disc cause degeneration? Spine. 2000;25:2993–3004. doi: 10.1097/00007632-200012010-00006. [DOI] [PubMed] [Google Scholar]
- 80.Iatridis JC, Mente PL, Stokes IA, Aronsson DD, Alini M. Compression-induced changes in intervertebral disc properties in a rat tail model. Spine. 1999;24:996–1002. doi: 10.1097/00007632-199905150-00013. [DOI] [PubMed] [Google Scholar]
- 81.Ikehara S. Pluripotent hemopoietic stem cells in mice and humans. Proc Soc Exp Biol Med. 2000;223:149–155. doi: 10.1046/j.1525-1373.2000.22320.x. [DOI] [PubMed] [Google Scholar]
- 82.Inoue H. Three-dimensional architecture of lumbar intervertebral discs. Spine. 1981;6:139–146. doi: 10.1097/00007632-198103000-00006. [DOI] [PubMed] [Google Scholar]
- 83.Jim JJ, Noponen-Hietala N, Cheung KM, Ott J, Karppinen J, Sahraravand A, Luk KD, Yip SP, Sham PC, Song YQ, Leong JC, Cheah KS, Ala-Kokko L, Chan D. The TRP2 allele of COL9A2 is an age-dependent risk factor for the development and severity of intervertebral disc degeneration. Spine. 2005;30:2735–2742. doi: 10.1097/01.brs.0000190828.85331.ef. [DOI] [PubMed] [Google Scholar]
- 84.Jimbo K, Park JS, Yokosuka K, Sato K, Nagata K. Positive feedback loop of interleukin-1beta upregulating production of inflammatory mediators in human intervertebral disc cells in vitro. J Neurosurg Spine. 2005;2:589–595. doi: 10.3171/spi.2005.2.5.0589. [DOI] [PubMed] [Google Scholar]
- 85.Johnson WE, Eisenstein SM, Roberts S. Cell cluster formation in degenerate lumbar intervertebral discs is associated with increased disc cell proliferation. Connect Tissue Res. 2001;42:197–207. doi: 10.3109/03008200109005650. [DOI] [PubMed] [Google Scholar]
- 86.Johnson WE, Evans H, Menage J, Eisenstein SM, El Haj A, Roberts S. Immunohistochemical detection of Schwann cells in innervated and vascularized human intervertebral discs. Spine. 2001;26:2550–2557. doi: 10.1097/00007632-200112010-00007. [DOI] [PubMed] [Google Scholar]
- 87.Jones G, White C, Sambrook P, Eisman J. Allelic variation in the vitamin D receptor, lifestyle factors and lumbar spinal degenerative disease. Ann Rheum Dis. 1998;57:94–99. doi: 10.1136/ard.57.2.94. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Jullig M, Zhang WV, Stott NS. Gene therapy in orthopaedic surgery: the current status. ANZ J Surg. 2004;74:46–54. doi: 10.1046/j.1445-1433.2003.documendoc.doc.x. [DOI] [PubMed] [Google Scholar]
- 89.Kalajzic I, Stover ML, Liu P, Kalajzic Z, Rowe DW, Lichtler AC. Use of VSV-G pseudotyped retroviral vectors to target murine osteoprogenitor cells. Virology. 2001;284:37–45. doi: 10.1006/viro.2001.0903. [DOI] [PubMed] [Google Scholar]
- 90.Kanemoto M, Hukuda S, Komiya Y, Katsuura A, Nishioka J. Immunohistochemical study of matrix metalloproteinase-3 and tissue inhibitor of metalloproteinase-1 human intervertebral discs. Spine. 1996;21:1–8. doi: 10.1097/00007632-199601010-00001. [DOI] [PubMed] [Google Scholar]
- 91.Kang JD, Georgescu HI, McIntyre-Larkin L, Stefanovic-Racic M, Donaldson WF, 3rd, Evans CH. Herniated lumbar intervertebral discs spontaneously produce matrix metalloproteinases, nitric oxide, interleukin-6, and prostaglandin E2. Spine. 1996;21:271–277. doi: 10.1097/00007632-199602010-00003. [DOI] [PubMed] [Google Scholar]
- 92.Kang JD, Stefanovic-Racic M, McIntyre LA, Georgescu HI, Evans CH. Toward a biochemical understanding of human intervertebral disc degeneration and herniation. Contributions of nitric oxide, interleukins, prostaglandin E2, and matrix metalloproteinases. Spine. 1997;22:1065–1073. doi: 10.1097/00007632-199705150-00003. [DOI] [PubMed] [Google Scholar]
- 93.Karppinen J, Paakko E, Paassilta P, Lohiniva J, Kurunlahti M, Tervonen O, Nieminen P, Goring HH, Malmivaara A, Vanharanta H, Ala-Kokko L. Radiologic phenotypes in lumbar MR imaging for a gene defect in the COL9A3 gene of type IX collagen. Radiology. 2003;227:143–148. doi: 10.1148/radiol.2271011821. [DOI] [PubMed] [Google Scholar]
- 94.Karppinen J, Paakko E, Raina S, Tervonen O, Kurunlahti M, Nieminen P, Ala-Kokko L, Malmivaara A, Vanharanta H. Magnetic resonance imaging findings in relation to the COL9A2 tryptophan allele among patients with sciatica. Spine. 2002;27:78–83. doi: 10.1097/00007632-200201010-00018. [DOI] [PubMed] [Google Scholar]
- 95.Kawaguchi Y, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T. The association of lumbar disc disease with vitamin-D receptor gene polymorphism. J Bone Joint Surg Am. 2002;84-A:2022–2028. doi: 10.2106/00004623-200211000-00018. [DOI] [PubMed] [Google Scholar]
- 96.Kawaguchi Y, Osada R, Kanamori M, Ishihara H, Ohmori K, Matsui H, Kimura T. Association between an aggrecan gene polymorphism and lumbar disc degeneration. Spine. 1999;24:2456–2460. doi: 10.1097/00007632-199912010-00006. [DOI] [PubMed] [Google Scholar]
- 97.Kawakami M, Matsumoto T, Hashizume H, Kuribayashi K, Chubinskaya S, Yoshida M. Osteogenic protein-1 (osteogenic protein-1/bone morphogenetic protein-7) inhibits degeneration and pain-related behavior induced by chronically compressed nucleus pulposus in the rat. Spine. 2005;30:1933–1939. doi: 10.1097/01.brs.0000176319.78887.64. [DOI] [PubMed] [Google Scholar]
- 98.Kay MA, Manno CS, Ragni MV, Larson PJ, Couto LB, McClelland A, Glader B, Chew AJ, Tai SJ, Herzog RW, Arruda V, Johnson F, Scallan C, Skarsgard E, Flake AW, High KA. Evidence for gene transfer and expression of factor IX in haemophilia B patients treated with an AAV vector. Nat Genet. 2000;24:257–261. doi: 10.1038/73464. [DOI] [PubMed] [Google Scholar]
- 99.Kim S, Le Visage C, Tateno K, Sieber A, Kostuik J, Leong K. Interaction of human mesenchymal stem cells with disc cells: changes in biosynthesis of extracellular matrix. Spine J. 2002;2:107S. doi: 10.1097/01.brs.0000231442.05245.87. [DOI] [PubMed] [Google Scholar]
- 100.Kim YE, Goel VK, Weinstein JN, Lim TH. Effect of disc degeneration at one level on the adjacent level in axial mode. Spine. 1991;16:331–335. doi: 10.1097/00007632-199103000-00013. [DOI] [PubMed] [Google Scholar]
- 101.Klein RG, Eek BC, O’Neill CW, Elin C, Mooney V, Derby RR. Biochemical injection treatment for discogenic low back pain: a pilot study. Spine J. 2003;3:220–226. doi: 10.1016/s1529-9430(02)00669-1. [DOI] [PubMed] [Google Scholar]
- 102.Koch H, Reinecke JA, Meijer H, Wehling P. Spontaneous secretion of interleukin 1 receptor antagonist (IL-1ra) by cells isolated from herniated lumbar discal tissue after discectomy. Cytokine. 1998;10:703–705. doi: 10.1006/cyto.1998.0353. [DOI] [PubMed] [Google Scholar]
- 103.Konttinen YT, Kaapa E, Hukkanen M, Gu XH, Takagi M, Santavirta S, Alaranta H, Li TF, Suda A. Cathepsin G in degenerating and healthy discal tissue. Clin Exp Rheumatol. 1999;17:197–204. [PubMed] [Google Scholar]
- 104.Konttinen YT, Kemppinen P, Li TF, Waris E, Pihlajamaki H, Sorsa T, Takagi M, Santavirta S, Schultz GS, Humphreys-Beher MG. Transforming and epidermal growth factors in degenerated intervertebral discs. J Bone Joint Surg Br. 1999;81:1058–1063. doi: 10.1302/0301-620x.81b6.9321. [DOI] [PubMed] [Google Scholar]
- 105.Kramer J, Hegert C, Guan K, Wobus AM, Muller PK, Rohwedel J. Embryonic stem cell-derived chondrogenic differentiation in vitro: activation by BMP-2 and BMP-4. Mech Dev. 2000;92:193–205. doi: 10.1016/s0925-4773(99)00339-1. [DOI] [PubMed] [Google Scholar]
- 106.Kroeber MW, Unglaub F, Wang H, Schmid C, Thomsen M, Nerlich A, Richter W. New in vivo animal model to create intervertebral disc degeneration and to investigate the effects of therapeutic strategies to stimulate disc regeneration. Spine. 2002;27:2684–2690. doi: 10.1097/00007632-200212010-00007. [DOI] [PubMed] [Google Scholar]
- 107.Kuettner KE, Pauli BU, Gall G, Memoli VA, Schenk RK. Synthesis of cartilage matrix by mammalian chondrocytes in vitro. I. Isolation, culture characteristics, and morphology. J Cell Biol. 1982;93:743–750. doi: 10.1083/jcb.93.3.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Kuhlcke K, Fehse B, Schilz A, Loges S, Lindemann C, Ayuk F, Lehmann F, Stute N, Fauser AA, Zander AR, Eckert HG. Highly efficient retroviral gene transfer based on centrifugation-mediated vector preloading of tissue culture vessels. Mol Ther. 2002;5:473–478. doi: 10.1006/mthe.2002.0566. [DOI] [PubMed] [Google Scholar]
- 109.Lattermann C, Oxner WM, Xiao X, Li J, Gilbertson LG, Robbins PD, Kang JD. The adeno associated viral vector as a strategy for intradiscal gene transfer in immune competent and pre-exposed rabbits. Spine. 2005;30:497–504. doi: 10.1097/01.brs.0000154764.62072.44. [DOI] [PubMed] [Google Scholar]
- 110.Le Blanc K, Tammik C, Rosendahl K, Zetterberg E, Ringden O. HLA expression and immunologic properties of differentiated and undifferentiated mesenchymal stem cells. Exp Hematol. 2003;31:890–896. doi: 10.1016/s0301-472x(03)00110-3. [DOI] [PubMed] [Google Scholar]
- 111.Le Maitre CL, Freemont AJ, Hoyland JA. Localization of degradative enzymes and their inhibitors in the degenerate human intervertebral disc. J Pathol. 2004;204:47–54. doi: 10.1002/path.1608. [DOI] [PubMed] [Google Scholar]
- 112.Le Maitre CL, Freemont AJ, Hoyland JA. The role of interleukin-1 in the pathogenesis of human intervertebral disc degeneration. Arthritis Res Ther. 2005;7:R732–745. doi: 10.1186/ar1732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Le Visage C, Tateno K, Sieber A, Kostuik J, Leong K (2003) Interaction of human mesenchymal stem cells with disc cells: changes in biosynthesis of extracellular matrix. In: 30th annual meeting. International Society for the Study of the Lumbar Spine [DOI] [PubMed]
- 114.Lee CK. Accelerated degeneration of the segment adjacent to a lumbar fusion. Spine. 1988;13:375–377. doi: 10.1097/00007632-198803000-00029. [DOI] [PubMed] [Google Scholar]
- 115.Legre J, Louis R, Serrano R, Debaene A. Anatomo-radiological considerations about lumbar discography. An experimental study. Neuroradiology. 1979;17:77–82. doi: 10.1007/BF00556022. [DOI] [PubMed] [Google Scholar]
- 116.Li H, Zou X, Baatrup A, Lind M, Bunger C. Cytokine profiles in conditioned media from cultured human intervertebral disc tissue. Implications of their effect on bone marrow stem cell metabolism. Acta Orthop. 2005;76:115–121. doi: 10.1080/00016470510030436. [DOI] [PubMed] [Google Scholar]
- 117.Li S, Huang L. Nonviral gene therapy: promises and challenges. Gene Ther. 2000;7:31–34. doi: 10.1038/sj.gt.3301110. [DOI] [PubMed] [Google Scholar]
- 118.Li S, Wu SP, Whitmore M, Loeffert EJ, Wang L, Watkins SC, Pitt BR, Huang L. Effect of immune response on gene transfer to the lung via systemic administration of cationic lipidic vectors. Am J Physiol. 1999;276:L796–804. doi: 10.1152/ajplung.1999.276.5.L796. [DOI] [PubMed] [Google Scholar]
- 119.Liu P, Kalajzic I, Stover ML, Rowe DW, Lichtler AC. Human bone marrow stromal cells are efficiently transduced by vesicular stomatitis virus-pseudotyped retrovectors without affecting subsequent osteoblastic differentiation. Bone. 2001;29:331–335. doi: 10.1016/s8756-3282(01)00590-7. [DOI] [PubMed] [Google Scholar]
- 120.Livshits G, Cohen Z, Higla O, Yakovenko K. Familial history, age and smoking are important risk factors for disc degeneration disease in Arabic pedigrees. Eur J Epidemiol. 2001;17:643–651. doi: 10.1023/a:1015503329989. [DOI] [PubMed] [Google Scholar]
- 121.Lotz JC, Chin JR. Intervertebral disc cell death is dependent on the magnitude and duration of spinal loading. Spine. 2000;25:1477–1483. doi: 10.1097/00007632-200006150-00005. [DOI] [PubMed] [Google Scholar]
- 122.Lotz JC, Colliou OK, Chin JR, Duncan NA, Liebenberg E. Compression-induced degeneration of the intervertebral disc: an in vivo mouse model and finite-element study. Spine. 1998;23:2493–2506. doi: 10.1097/00007632-199812010-00004. [DOI] [PubMed] [Google Scholar]
- 123.Luo J, Sun MH, Kang Q, Peng Y, Jiang W, Luu HH, Luo Q, Park JY, Li Y, Haydon RC, He TC. Gene therapy for bone regeneration. Curr Gene Ther. 2005;5:167–179. doi: 10.2174/1566523053544218. [DOI] [PubMed] [Google Scholar]
- 124.Lyons G, Eisenstein SM, Sweet MB. Biochemical changes in intervertebral disc degeneration. Biochim Biophys Acta. 1981;673:443–453. doi: 10.1016/0304-4165(81)90476-1. [DOI] [PubMed] [Google Scholar]
- 125.MacGregor AJ, Andrew T, Sambrook PN, Spector TD. Structural, psychological, and genetic influences on low back and neck pain: a study of adult female twins. Arthritis Rheum. 2004;51:160–167. doi: 10.1002/art.20236. [DOI] [PubMed] [Google Scholar]
- 126.Mahato RI, Kawabata K, Takakura Y, Hashida M. In vivo disposition characteristics of plasmid DNA complexed with cationic liposomes. J Drug Target. 1995;3:149–157. doi: 10.3109/10611869509059214. [DOI] [PubMed] [Google Scholar]
- 127.Maldonado BA, Oegema TR., Jr Initial characterization of the metabolism of intervertebral disc cells encapsulated in microspheres. J Orthop Res. 1992;10:677–690. doi: 10.1002/jor.1100100510. [DOI] [PubMed] [Google Scholar]
- 128.Manno CS, Chew AJ, Hutchison S, Larson PJ, Herzog RW, Arruda VR, Tai SJ, Ragni MV, Thompson A, Ozelo M, Couto LB, Leonard DG, Johnson FA, McClelland A, Scallan C, Skarsgard E, Flake AW, Kay MA, High KA, Glader B. AAV-mediated factor IX gene transfer to skeletal muscle in patients with severe hemophilia B. Blood. 2003;101:2963–2972. doi: 10.1182/blood-2002-10-3296. [DOI] [PubMed] [Google Scholar]
- 129.Marchand F, Ahmed AM. Investigation of the laminate structure of lumbar disc anulus fibrosus. Spine. 1990;15:402–410. doi: 10.1097/00007632-199005000-00011. [DOI] [PubMed] [Google Scholar]
- 130.Maroon JC. Current concepts in minimally invasive discectomy. Neurosurgery. 2002;51:137–145. [PubMed] [Google Scholar]
- 131.Maroudas A, Stockwell RA, Nachemson A, Urban J. Factors involved in the nutrition of the human lumbar intervertebral disc: cellularity and diffusion of glucose in vitro. J Anat. 1975;120:113–130. [PMC free article] [PubMed] [Google Scholar]
- 132.Mason JM, Breitbart AS, Barcia M, Porti D, Pergolizzi RG, Grande DA. Cartilage and bone regeneration using gene-enhanced tissue engineering. Clin Orthop Relat Res. 2000;379(Suppl):S171–S178. doi: 10.1097/00003086-200010001-00023. [DOI] [PubMed] [Google Scholar]
- 133.Masuda K, Oegema TR, Jr, An HS. Growth factors and treatment of intervertebral disc degeneration. Spine. 2004;29:2757–2769. doi: 10.1097/01.brs.0000146048.14946.af. [DOI] [PubMed] [Google Scholar]
- 134.Matsui H, Kanamori M, Ishihara H, Yudoh K, Naruse Y, Tsuji H. Familial predisposition for lumbar degenerative disc disease. A case-control study. Spine. 1998;23:1029–1034. doi: 10.1097/00007632-199805010-00013. [DOI] [PubMed] [Google Scholar]
- 135.Miyamoto H, Saura R, Harada T, Doita M, Mizuno K. The role of cyclooxygenase-2 and inflammatory cytokines in pain induction of herniated lumbar intervertebral disc. Kobe J Med Sci. 2000;46:13–28. [PubMed] [Google Scholar]
- 136.Moon SH, Gilbertson LG, Nishida K, Knaub M, Muzzonigro T, Robbins PD, Evans CH, Kang JD. Human intervertebral disc cells are genetically modifiable by adenovirus-mediated gene transfer: implications for the clinical management of intervertebral disc disorders. Spine. 2000;25:2573–2579. doi: 10.1097/00007632-200010150-00006. [DOI] [PubMed] [Google Scholar]
- 137.Moon SH, Nishida K, Gilbertson L, Hall A, Robbins P, Kang JD (2001) Biologic response of human intervertebral disc cell to gene therapy cocktail. In: International Society for the Study of the Lumbar Spine. San Francisco, CA [DOI] [PMC free article] [PubMed]
- 138.Nagase H, Okada Y. Proteinases and matrix degeneration. In: Kelley W, Harris E, Ruddy S, editors. Rheumatology. Philadelphia: WB Saunders; 1997. pp. 323–341. [Google Scholar]
- 139.Nemoto O, Yamagishi M, Yamada H, Kikuchi T, Takaishi H. Matrix metalloproteinase-3 production by human degenerated intervertebral disc. J Spinal Disord. 1997;10:493–498. [PubMed] [Google Scholar]
- 140.Nerlich AG, Bachmeier BE, Boos N. Expression of fibronectin and TGF-beta1 mRNA and protein suggest altered regulation of extracellular matrix in degenerated disc tissue. Eur Spine J. 2005;14:17–26. doi: 10.1007/s00586-004-0745-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Nishida K, Gilbertson LG, Robbins PD, Evans CH, Kang JD. Potential applications of gene therapy to the treatment of intervertebral disc disorders. Clin Orthop Relat Res. 2000;379(Suppl):S234–S241. doi: 10.1097/00003086-200010001-00031. [DOI] [PubMed] [Google Scholar]
- 142.Nishida K, Kang JD, Gilbertson LG, Moon SH, Suh JK, Vogt MT, Robbins PD, Evans CH. Modulation of the biologic activity of the rabbit intervertebral disc by gene therapy: an in vivo study of adenovirus-mediated transfer of the human transforming growth factor beta 1 encoding gene. Spine. 1999;24:2419–2425. doi: 10.1097/00007632-199912010-00002. [DOI] [PubMed] [Google Scholar]
- 143.Nishida T. Kinetics of tissue and serum matrix metalloproteinase-3 and tissue inhibitor of metalloproteinases-1 in intervertebral disc degeneration and disc herniation. Kurume Med J. 1999;46:39–50. doi: 10.2739/kurumemedj.46.39. [DOI] [PubMed] [Google Scholar]
- 144.Noponen-Hietala N, Kyllonen E, Mannikko M, Ilkko E, Karppinen J, Ott J, Ala-Kokko L. Sequence variations in the collagen IX and XI genes are associated with degenerative lumbar spinal stenosis. Ann Rheum Dis. 2003;62:1208–1214. doi: 10.1136/ard.2003.008334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Noponen-Hietala N, Virtanen I, Karttunen R, Schwenke S, Jakkula E, Li H, Merikivi R, Barral S, Ott J, Karppinen J, Ala-Kokko L. Genetic variations in IL6 associate with intervertebral disc disease characterized by sciatica. Pain. 2005;114:186–194. doi: 10.1016/j.pain.2004.12.015. [DOI] [PubMed] [Google Scholar]
- 146.Nygaard OP, Mellgren SI, Osterud B. The inflammatory properties of contained and noncontained lumbar disc herniation. Spine. 1997;22:2484–2488. doi: 10.1097/00007632-199711010-00004. [DOI] [PubMed] [Google Scholar]
- 147.Ohshima H, Urban JP. The effect of lactate and pH on proteoglycan and protein synthesis rates in the intervertebral disc. Spine. 1992;17:1079–1082. doi: 10.1097/00007632-199209000-00012. [DOI] [PubMed] [Google Scholar]
- 148.Okuda S, Myoui A, Ariga K, Nakase T, Yonenobu K, Yoshikawa H. Mechanisms of age-related decline in insulin-like growth factor-I dependent proteoglycan synthesis in rat intervertebral disc cells. Spine. 2001;26:2421–2426. doi: 10.1097/00007632-200111150-00005. [DOI] [PubMed] [Google Scholar]
- 149.Osada R, Ohshima H, Ishihara H, Yudoh K, Sakai K, Matsui H, Tsuji H. Autocrine/paracrine mechanism of insulin-like growth factor-1 secretion, and the effect of insulin-like growth factor-1 on proteoglycan synthesis in bovine intervertebral discs. J Orthop Res. 1996;14:690–699. doi: 10.1002/jor.1100140503. [DOI] [PubMed] [Google Scholar]
- 150.Ozaki S, Muro T, Ito S, Mizushima M. Neovascularization of the outermost area of herniated lumbar intervertebral discs. J Orthop Sci. 1999;4:286–292. doi: 10.1007/s007760050105. [DOI] [PubMed] [Google Scholar]
- 151.Pai RR, D’sa B, Raghuveer CV, Kamath A. Neovascularization of nucleus pulposus. A diagnostic feature of intervertebral disc prolapse. Spine. 1999;24:739–741. doi: 10.1097/00007632-199904150-00002. [DOI] [PubMed] [Google Scholar]
- 152.Pattison ST, Melrose J, Ghosh P, Taylor TK. Regulation of gelatinase-A (MMP-2) production by ovine intervertebral disc nucleus pulposus cells grown in alginate bead culture by Transforming Growth Factor-beta(1)and insulin like growth factor-I. Cell Biol Int. 2001;25:679–689. doi: 10.1006/cbir.2000.0718. [DOI] [PubMed] [Google Scholar]
- 153.Paul R, Haydon RC, Cheng H, Ishikawa A, Nenadovich N, Jiang W, Zhou L, Breyer B, Feng T, Gupta P, He TC, Phillips FM. Potential use of Sox9 gene therapy for intervertebral degenerative disc disease. Spine. 2003;28:755–763. [PMC free article] [PubMed] [Google Scholar]
- 154.Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, Moorman MA, Simonetti DW, Craig S, Marshak DR. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 155.Pluijm SM, Essen HW, Bravenboer N, Uitterlinden AG, Smit JH, Pols HA, Lips P. Collagen type I alpha1 Sp1 polymorphism, osteoporosis, and intervertebral disc degeneration in older men and women. Ann Rheum Dis. 2004;63:71–77. doi: 10.1136/ard.2002.002287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Powell MC, Wilson M, Szypryt P, Symonds EM, Worthington BS. Prevalence of lumbar disc degeneration observed by magnetic resonance in symptomless women. Lancet. 1986;2:1366–1367. doi: 10.1016/s0140-6736(86)92008-8. [DOI] [PubMed] [Google Scholar]
- 157.Puustjarvi K, Lammi M, Helminen H, Inkinen R, Tammi M. Proteoglycans in the intervertebral disc of young dogs following strenuous running exercise. Connect Tissue Res. 1994;30:225–240. doi: 10.3109/03008209409061974. [DOI] [PubMed] [Google Scholar]
- 158.Rainov NG. A phase III clinical evaluation of herpes simplex virus type 1 thymidine kinase and ganciclovir gene therapy as an adjuvant to surgical resection and radiation in adults with previously untreated glioblastoma multiforme. Hum Gene Ther. 2000;11:2389–2401. doi: 10.1089/104303400750038499. [DOI] [PubMed] [Google Scholar]
- 159.Rannou F, Lee TS, Zhou RH, Chin J, Lotz JC, Mayoux-Benhamou MA, Barbet JP, Chevrot A, Shyy JY. Intervertebral disc degeneration: the role of the mitochondrial pathway in annulus fibrosus cell apoptosis induced by overload. Am J Pathol. 2004;164:915–924. doi: 10.1016/S0002-9440(10)63179-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 160.Reinecke JA, Wehling P, Robbins P, Evans CH, Sager M, Schulze-Allen G, Koch H. [In vitro transfer of genes in spinal tissue] Z Orthop Ihre Grenzgeb. 1997;135:412–416. doi: 10.1055/s-2008-1039409. [DOI] [PubMed] [Google Scholar]
- 161.Repanti M, Korovessis PG, Stamatakis MV, Spastris P, Kosti P. Evolution of disc degeneration in lumbar spine: a comparative histological study between herniated and postmortem retrieved disc specimens. J Spinal Disord. 1998;11:41–45. [PubMed] [Google Scholar]
- 162.Richardson SM, Walker RV, Parker S, Rhodes NP, Hunt JA, Freemont AJ, Hoyland JA. Intervertebral Disc Cell Mediated Mesenchymal Stem Cell Differentiation. Stem Cells. 2005;24:707–716. doi: 10.1634/stemcells.2005-0205. [DOI] [PubMed] [Google Scholar]
- 163.Risbud MV, Izzo MW, Adams CS, al. e (2003) Mesenchymal stem cells respond to their microenvironment in vitro to assume nucleus pulposus-like phenotype. In: 30th annual meeting. International Society for the Study of the Lumbar Spine
- 164.Ritter T, Lehmann M, Volk HD. Improvements in gene therapy: averting the immune response to adenoviral vectors. Biodrugs. 2002;16:3–10. doi: 10.2165/00063030-200216010-00001. [DOI] [PubMed] [Google Scholar]
- 165.Roberts S, Caterson B, Menage J, Evans EH, Jaffray DC, Eisenstein SM. Matrix metalloproteinases and aggrecanase: their role in disorders of the human intervertebral disc. Spine. 2000;25:3005–3013. doi: 10.1097/00007632-200012010-00007. [DOI] [PubMed] [Google Scholar]
- 166.Roberts S, Menage J, Duance V, Wotton S, Ayad S. 1991 Volvo Award in basic sciences. Collagen types around the cells of the intervertebral disc and cartilage end plate: an immunolocalization study. Spine. 1991;16:1030–1038. [PubMed] [Google Scholar]
- 167.Roberts S, Menage J, Eisenstein SM. The cartilage end-plate and intervertebral disc in scoliosis: calcification and other sequelae. J Orthop Res. 1993;11:747–757. doi: 10.1002/jor.1100110517. [DOI] [PubMed] [Google Scholar]
- 168.Romero NB, Benveniste O, Payan C, Braun S, Squiban P, Herson S, Fardeau M. Current protocol of a research phase I clinical trial of full-length dystrophin plasmid DNA in Duchenne/Becker muscular dystrophies. Part II: clinical protocol. Neuromuscul Disord. 2002;12(Suppl 1):S45–48. doi: 10.1016/s0960-8966(02)00081-0. [DOI] [PubMed] [Google Scholar]
- 169.Saal JS, Franson RC, Dobrow R, Saal JA, White AH, Goldthwaite N. High levels of inflammatory phospholipase A2 activity in lumbar disc herniations. Spine. 1990;15:674–678. doi: 10.1097/00007632-199007000-00011. [DOI] [PubMed] [Google Scholar]
- 170.Sakai D, Mochida J, Iwashina T, Hiyama A, Omi H, Imai M, Nakai T, Ando K, Hotta T. Regenerative effects of transplanting mesenchymal stem cells embedded in atelocollagen to the degenerated intervertebral disc. Biomaterials. 2006;27:335–345. doi: 10.1016/j.biomaterials.2005.06.038. [DOI] [PubMed] [Google Scholar]
- 171.Sakai D, Mochida J, Iwashina T, Watanabe T, Nakai T, Ando K, Hotta T. Differentiation of mesenchymal stem cells transplanted to a rabbit degenerative disc model: potential and limitations for stem cell therapy in disc regeneration. Spine. 2005;30:2379–2387. doi: 10.1097/01.brs.0000184365.28481.e3. [DOI] [PubMed] [Google Scholar]
- 172.Sakai D, Mochida J, Yamamoto Y, Nomura T, Okuma M, Nishimura K, Nakai T, Ando K, Hotta T. Transplantation of mesenchymal stem cells embedded in Atelocollagen gel to the intervertebral disc: a potential therapeutic model for disc degeneration. Biomaterials. 2003;24:3531–3541. doi: 10.1016/s0142-9612(03)00222-9. [DOI] [PubMed] [Google Scholar]
- 173.Sambrook PN, MacGregor AJ, Spector TD. Genetic influences on cervical and lumbar disc degeneration: a magnetic resonance imaging study in twins. Arthritis Rheum. 1999;42:366–372. doi: 10.1002/1529-0131(199902)42:2<366::AID-ANR20>3.0.CO;2-6. [DOI] [PubMed] [Google Scholar]
- 174.Sandover J. Dynamic loading as a possible source of low-back disorders. Spine. 1983;8:652–658. doi: 10.1097/00007632-198309000-00011. [DOI] [PubMed] [Google Scholar]
- 175.Sato M, Asazuma T, Ishihara M, Kikuchi T, Kikuchi M, Fujikawa K. An experimental study of the regeneration of the intervertebral disc with an allograft of cultured annulus fibrosus cells using a tissue-engineering method. Spine. 2003;28:548–553. doi: 10.1097/01.BRS.0000049909.09102.60. [DOI] [PubMed] [Google Scholar]
- 176.Sato M, Asazuma T, Ishihara M, Kikuchi T, Masuoka K, Ichimura S, Kikuchi M, Kurita A, Fujikawa K. An atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS-scaffold) for the culture of annulus fibrosus cells from an intervertebral disc. J Biomed Mater Res. 2003;64A:248–256. doi: 10.1002/jbm.a.10287. [DOI] [PubMed] [Google Scholar]
- 177.Sato M, Kikuchi M, Ishihara M, Asazuma T, Kikuchi T, Masuoka K, Hattori H, Fujikawa K. Tissue engineering of the intervertebral disc with cultured annulus fibrosus cells using atelocollagen honeycomb-shaped scaffold with a membrane seal (ACHMS scaffold) Med Biol Eng Comput. 2003;41:365–371. doi: 10.1007/BF02348444. [DOI] [PubMed] [Google Scholar]
- 178.Scapinelli R. Lumbar disc herniation in eight siblings with a positive family history for disc disease. Acta Orthop Belg. 1993;59:371–376. [PubMed] [Google Scholar]
- 179.Seki S, Kawaguchi Y, Chiba K, Mikami Y, Kizawa H, Oya T, Mio F, Mori M, Miyamoto Y, Masuda I, Tsunoda T, Kamata M, Kubo T, Toyama Y, Kimura T, Nakamura Y, Ikegawa S. A functional SNP in CILP, encoding cartilage intermediate layer protein, is associated with susceptibility to lumbar disc disease. Nat Genet. 2005;37:607–612. doi: 10.1038/ng1557. [DOI] [PubMed] [Google Scholar]
- 180.Shen B, Melrose J, Ghosh P, Taylor F. Induction of matrix metalloproteinase-2 and -3 activity in ovine nucleus pulposus cells grown in three-dimensional agarose gel culture by interleukin-1beta: a potential pathway of disc degeneration. Eur Spine J. 2003;12:66–75. doi: 10.1007/s00586-002-0454-2. [DOI] [PubMed] [Google Scholar]
- 181.Shieh SJ, Vacanti JP. State-of-the-art tissue engineering: from tissue engineering to organ building. Surgery. 2005;137:1–7. doi: 10.1016/j.surg.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 182.Sobajima S, Vadala G, Niyibizi C, al. e (2003) Stem cell therapy for degenerative disc disease: An in-vitro feasibility study. In: 30th annual meeting. International Society for the Study of the Lumbar Spine
- 183.Solovieva S, Kouhia S, Leino-Arjas P, Ala-Kokko L, Luoma K, Raininko R, Saarela J, Riihimaki H. Interleukin 1 polymorphisms and intervertebral disc degeneration. Epidemiology. 2004;15:626–633. doi: 10.1097/01.ede.0000135179.04563.35. [DOI] [PubMed] [Google Scholar]
- 184.Solovieva S, Leino-Arjas P, Saarela J, Luoma K, Raininko R, Riihimaki H. Possible association of interleukin 1 gene locus polymorphisms with low back pain. Pain. 2004;109:8–19. doi: 10.1016/j.pain.2003.10.020. [DOI] [PubMed] [Google Scholar]
- 185.Solovieva S, Lohiniva J, Leino-Arjas P, Raininko R, Luoma K, Ala-Kokko L, Riihimaki H. COL9A3 gene polymorphism and obesity in intervertebral disc degeneration of the lumbar spine: evidence of gene-environment interaction. Spine. 2002;27:2691–2696. doi: 10.1097/00007632-200212010-00008. [DOI] [PubMed] [Google Scholar]
- 186.Somia N, Verma IM. Gene therapy: trials and tribulations. Nat Rev Genet. 2000;1:91–99. doi: 10.1038/35038533. [DOI] [PubMed] [Google Scholar]
- 187.Specchia N, Pagnotta A, Toesca A, Greco F. Cytokines and growth factors in the protruded intervertebral disc of the lumbar spine. Eur Spine J. 2002;11:145–151. doi: 10.1007/s00586-001-0361-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 188.Stabler A, Weiss M, Scheidler J, Krodel A, Seiderer M, Reiser M. Degenerative disk vascularization on MRI: correlation with clinical and histopathologic findings. Skelet Radiol. 1996;25:119–126. doi: 10.1007/s002560050047. [DOI] [PubMed] [Google Scholar]
- 189.Stamenkovic I. Matrix metalloproteinases in tumor invasion and metastasis. Semin Cancer Biol. 2000;10:415–433. doi: 10.1006/scbi.2000.0379. [DOI] [PubMed] [Google Scholar]
- 190.Steck E, Bertram H, Abel R, Chen B, Winter A, Richter W. Induction of intervertebral disc-like cells from adult mesenchymal stem cells. Stem Cells. 2005;23:403–411. doi: 10.1634/stemcells.2004-0107. [DOI] [PubMed] [Google Scholar]
- 191.Sztrolovics R, Alini M, Roughley PJ, Mort JS. Aggrecan degradation in human intervertebral disc and articular cartilage. Biochem J. 1997;326(Pt 1):235–241. doi: 10.1042/bj3260235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Takahashi H, Suguro T, Okazima Y, Motegi M, Okada Y, Kakiuchi T. Inflammatory cytokines in the herniated disc of the lumbar spine. Spine. 1996;21:218–224. doi: 10.1097/00007632-199601150-00011. [DOI] [PubMed] [Google Scholar]
- 193.Takahashi M, Haro H, Wakabayashi Y, Kawa-uchi T, Komori H, Shinomiya K. The association of degeneration of the intervertebral disc with 5a/6a polymorphism in the promoter of the human matrix metalloproteinase-3 gene. J Bone Joint Surg Br. 2001;83:491–495. doi: 10.1302/0301-620x.83b4.11617. [DOI] [PubMed] [Google Scholar]
- 194.Takegami K, Masuda K, An H. In vivo administration of osteogenic protein-1 increases proteoglycan content and disc height in rabbit intervertebral disc. Orthop Res Soc Trans. 2000;25:338. doi: 10.1097/01.brs.0000148002.68656.4d. [DOI] [PubMed] [Google Scholar]
- 195.Thompson JP, Oegema TR, Jr, Bradford DS. Stimulation of mature canine intervertebral disc by growth factors. Spine. 1991;16:253–260. doi: 10.1097/00007632-199103000-00001. [DOI] [PubMed] [Google Scholar]
- 196.Tolonen J, Gronblad M, Vanharanta H, Virri J, Guyer RD, Rytomaa T, Karaharju EO. Growth factor expression in degenerated intervertebral disc tissue An immunohistochemical analysis of transforming growth factor beta, fibroblast growth factor and platelet-derived growth factor. Eur Spine J. 2005;15:588–596. doi: 10.1007/s00586-005-0930-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 197.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju E. Basic fibroblast growth factor immunoreactivity in blood vessels and cells of disc herniations. Spine. 1995;20:271–276. doi: 10.1097/00007632-199502000-00003. [DOI] [PubMed] [Google Scholar]
- 198.Tolonen J, Gronblad M, Virri J, Seitsalo S, Rytomaa T, Karaharju E. Transforming growth factor beta receptor induction in herniated intervertebral disc tissue: an immunohistochemical study. Eur Spine J. 2001;10:172–176. doi: 10.1007/s005860000213. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.Trout JJ, Buckwalter JA, Moore KC. Ultrastructure of the human intervertebral disc: II. Cells of the nucleus pulposus. Anat Rec. 1982;204:307–314. doi: 10.1002/ar.1092040403. [DOI] [PubMed] [Google Scholar]
- 200.Tsujinaka T, Kishibuchi M, Yano M, Morimoto T, Ebisui C, Fujita J, Ogawa A, Shiozaki H, Kominami E, Monden M. Involvement of interleukin-6 in activation of lysosomal cathepsin and atrophy of muscle fibers induced by intramuscular injection of turpentine oil in mice. J Biochem (Tokyo) 1997;122:595–600. doi: 10.1093/oxfordjournals.jbchem.a021794. [DOI] [PubMed] [Google Scholar]
- 201.Tsuru M, Nagata K, Ueno T, Jimi A, Irie K, Yamada A, Nishida T, Sata M. Electron microscopic observation of established chondrocytes derived from human intervertebral disc hernia (KTN-1) and role of macrophages in spontaneous regression of degenerated tissues. Spine J. 2001;1:422–431. doi: 10.1016/s1529-9430(01)00055-9. [DOI] [PubMed] [Google Scholar]
- 202.Urban JP, Holm S, Maroudas A. Diffusion of small solutes into the intervertebral disc: as in vivo study. Biorheology. 1978;15:203–221. doi: 10.3233/bir-1978-153-409. [DOI] [PubMed] [Google Scholar]
- 203.Varlotta GP, Brown MD, Kelsey JL, Golden AL. Familial predisposition for herniation of a lumbar disc in patients who are less than twenty-one years old. J Bone Joint Surg Am. 1991;73:124–128. [PubMed] [Google Scholar]
- 204.Vernon-Roberts B. Age-related and degenerative pathology of intervertebral discs and apophyseal joints. In: Jayson MI, editor. The lumbar spine and back pain. Edinburh: Churchill Livingstone; 1992. pp. 17–41. [Google Scholar]
- 205.Vernon-Roberts B. Disc pathology and disease states. In: Gosh P, editor. The biology of the intervertebral disc. Boca Raton, Florida: CRC Press; 1988. pp. 73–119. [Google Scholar]
- 206.Videman T, Battie MC. The influence of occupation on lumbar degeneration. Spine. 1999;24:1164–1168. doi: 10.1097/00007632-199906010-00020. [DOI] [PubMed] [Google Scholar]
- 207.Videman T, Gibbons LE, Battie MC, Maravilla K, Vanninen E, Leppavuori J, Kaprio J, Peltonen L. The relative roles of intragenic polymorphisms of the vitamin d receptor gene in lumbar spine degeneration and bone density. Spine. 2001;26:E7–E12. doi: 10.1097/00007632-200102010-00003. [DOI] [PubMed] [Google Scholar]
- 208.Videman T, Leppavuori J, Kaprio J, Battie MC, Gibbons LE, Peltonen L, Koskenvuo M. Intragenic polymorphisms of the vitamin D receptor gene associated with intervertebral disc degeneration. Spine. 1998;23:2477–2485. doi: 10.1097/00007632-199812010-00002. [DOI] [PubMed] [Google Scholar]
- 209.Wallach CJ, Sobajima S, Watanabe Y, Kim JS, Georgescu HI, Robbins P, Gilbertson LG, Kang JD. Gene transfer of the catabolic inhibitor TIMP-1 increases measured proteoglycans in cells from degenerated human intervertebral discs. Spine. 2003;28:2331–2337. doi: 10.1097/01.BRS.0000085303.67942.94. [DOI] [PubMed] [Google Scholar]
- 210.Walmsley R. The development and growth of the intervertebral disc. Edinburgh Med J. 1953;60:341–364. [PMC free article] [PubMed] [Google Scholar]
- 211.Wang F, Duan R, Chirgwin J, Safe SH. Transcriptional activation of cathepsin D gene expression by growth factors. J Mol Endocrinol. 2000;24:193–202. doi: 10.1677/jme.0.0240193. [DOI] [PubMed] [Google Scholar]
- 212.Wehling P, Schulitz KP, Robbins PD, Evans CH, Reinecke JA. Transfer of genes to chondrocytic cells of the lumbar spine. Proposal for a treatment strategy of spinal disorders by local gene therapy. Spine. 1997;22:1092–1097. doi: 10.1097/00007632-199705150-00008. [DOI] [PubMed] [Google Scholar]
- 213.Weiler C, Nerlich AG, Bachmeier BE, Boos N (2005) Expression and distribution of tumor necrosis factor alpha in human lumbar intervertebral discs: a study in surgical specimen and autopsy controls. Spine 30:44–53; discussion 54 [DOI] [PubMed]
- 214.Weiler C, Nerlich AG, Zipperer J, Bachmeier BE, Boos N. 2002 SSE Award Competition in Basic Science: expression of major matrix metalloproteinases is associated with intervertebral disc degradation and resorption. Eur Spine J. 2002;11:308–320. doi: 10.1007/s00586-002-0472-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Weishaupt D, Zanetti M, Hodler J, Boos N. MR imaging of the lumbar spine: prevalence of intervertebral disk extrusion and sequestration, nerve root compression, end plate abnormalities, and osteoarthritis of the facet joints in asymptomatic volunteers. Radiology. 1998;209:661–666. doi: 10.1148/radiology.209.3.9844656. [DOI] [PubMed] [Google Scholar]
- 216.Wolff JA, Malone RW, Williams P, Chong W, Acsadi G, Jani A, Felgner PL. Direct gene transfer into mouse muscle in vivo. Science. 1990;247:1465–1468. doi: 10.1126/science.1690918. [DOI] [PubMed] [Google Scholar]
- 217.Yamazaki S, Banes AJ, Weinhold PS, Tsuzaki M, Kawakami M, Minchew JT. Vibratory loading decreases extracellular matrix and matrix metalloproteinase gene expression in rabbit annulus cells. Spine J. 2002;2:415–420. doi: 10.1016/s1529-9430(02)00427-8. [DOI] [PubMed] [Google Scholar]
- 218.Yang NS, Burkholder J, Roberts B, Martinell B, McCabe D. In vivo and in vitro gene transfer to mammalian somatic cells by particle bombardment. Proc Natl Acad Sci USA. 1990;87:9568–9572. doi: 10.1073/pnas.87.24.9568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Yoon ST, Park JS, Kim KS, Li J, Attallah-Wasif ES, Hutton WC, Boden SD. ISSLS prize winner: LMP-1 upregulates intervertebral disc cell production of proteoglycans and BMPs in vitro and in vivo. Spine. 2004;29:2603–2611. doi: 10.1097/01.brs.0000146103.94600.85. [DOI] [PubMed] [Google Scholar]
- 220.Young HE, Steele TA, Bray RA, Hudson J, Floyd JA, Hawkins K, Thomas K, Austin T, Edwards C, Cuzzourt J, Duenzl M, Lucas PA, Black AC., Jr Human reserve pluripotent mesenchymal stem cells are present in the connective tissues of skeletal muscle and dermis derived from fetal, adult, and geriatric donors. Anat Rec. 2001;264:51–62. doi: 10.1002/ar.1128. [DOI] [PubMed] [Google Scholar]
- 221.Yu J. Elastic tissues of the intervertebral disc. Biochem Soc Trans. 2002;30:848–852. doi: 10.1042/bst0300848. [DOI] [PubMed] [Google Scholar]
- 222.Yu J, Winlove PC, Roberts S, Urban JP. Elastic fibre organization in the intervertebral discs of the bovine tail. J Anat. 2002;201:465–475. doi: 10.1046/j.1469-7580.2002.00111.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Zhang YG, Guo X, Xu P, Kang LL, Li J. Bone mesenchymal stem cells transplanted into rabbit intervertebral discs can increase proteoglycans. Clin Orthop Relat Res. 2005;430:219–226. doi: 10.1097/01.blo.0000146534.31120.cf. [DOI] [PubMed] [Google Scholar]