Abstract
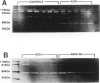
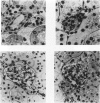
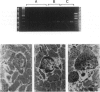
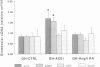
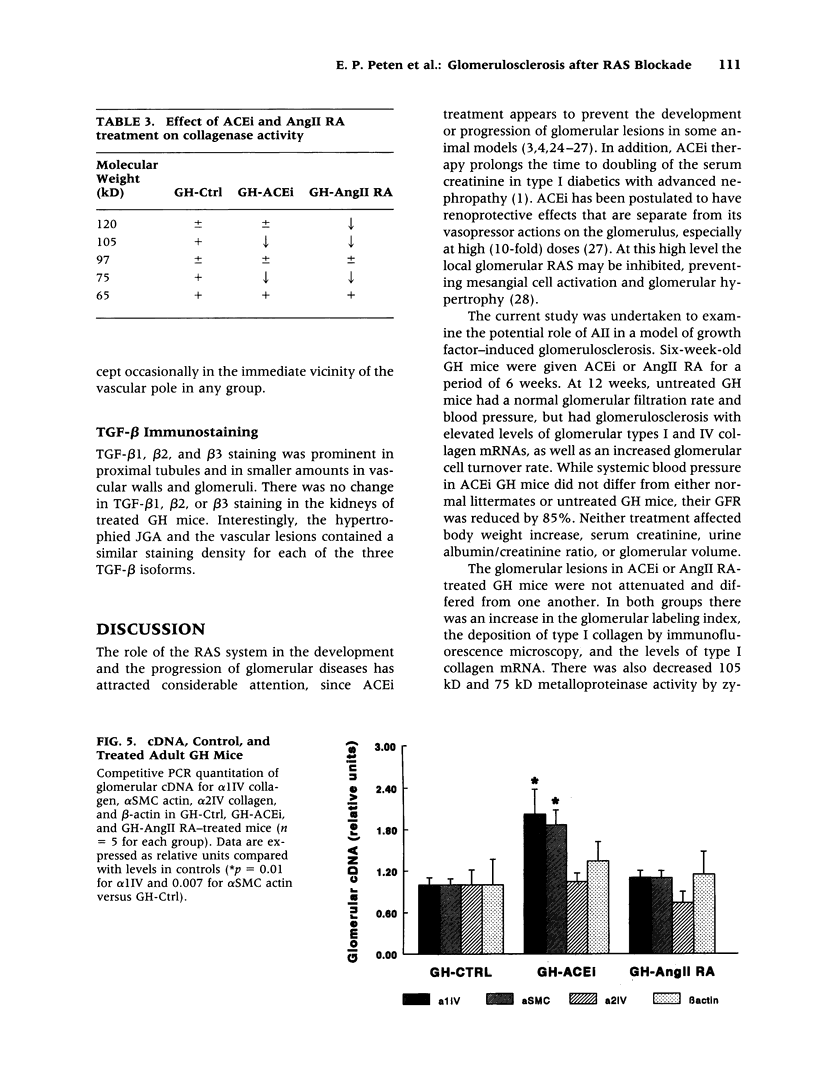
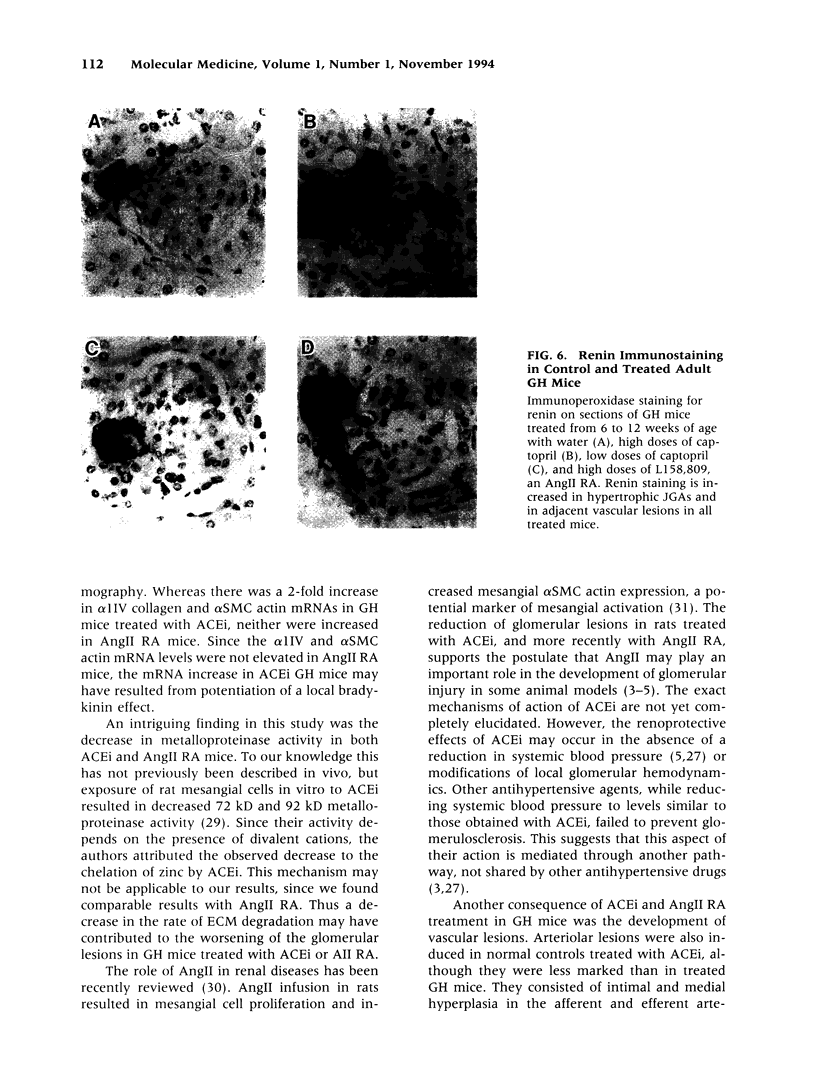
BACKGROUND: Angiotensin converting enzyme inhibitor (ACEi) therapy delays the onset of renal failure in diabetic nephropathy and inhibits or delays the onset of proteinuria in several animal models. MATERIALS AND METHODS: We examined this question using a transgenic model of chronic glomerulosclerosis caused by an excess production of growth hormone (GH) in which there is progressive glomerular scarring leading to uremia. In addition, since GH mice do not have systemic hypertension or an elevated glomerular filtration rate, we could address the question of whether ACEi or angiotensin II receptor antagonists (AII RA) had an effect on the development of glomerulosclerosis under these conditions. Since excess matrix accumulates in glomerulosclerosis because of alterations in the balance between its synthesis and degradation, we examined the effect of ACEi and AII RA on these parameters. RESULTS: Systemic blood pressure was unaffected by ACEi treatment, but the glomerular filtration rate decreased 85%. ACEi-treated mice had increased mesangial deposition of type I collagen and decreased 105 kD complex collagenase activity. In addition, ACEi-treated GH mice had increased glomerular alpha 1 type I collagen, alpha 1 type IV collagen, and alpha-smooth muscle cell actin mRNAs. No changes were noted in beta actin, or 72 kD metalloproteinase mRNAs. The result of these changes was a net increase in sclerosis. Surprisingly, GH mice treated with ACEi or AngII RA developed marked renal arteriolar lesions. CONCLUSIONS: In some forms of glomerulosclerosis, the lesions develop independently of angiotensin II. Pharmacological inhibition of angiotensin II, in this circumstance, may aggravate the lesions through disregulation of the levels and the balance between glomerular matrix synthesis and degradation.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anderson S., Diamond J. R., Karnovsky M. J., Brenner B. M. Mechanisms underlying transition from acute glomerular injury to late glomerular sclerosis in a rat model of nephrotic syndrome. J Clin Invest. 1988 Nov;82(5):1757–1768. doi: 10.1172/JCI113789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anderson S., Rennke H. G., Brenner B. M. Therapeutic advantage of converting enzyme inhibitors in arresting progressive renal disease associated with systemic hypertension in the rat. J Clin Invest. 1986 Jun;77(6):1993–2000. doi: 10.1172/JCI112528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T., Striker L. J., Kimata K., Peten E. P., Yamada Y., Striker G. E. Glomerulosclerosis in mice transgenic for growth hormone. Increased mesangial extracellular matrix is correlated with kidney mRNA levels. J Exp Med. 1991 May 1;173(5):1287–1290. doi: 10.1084/jem.173.5.1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T., Striker L. J., Quaife C., Conti F. G., Palmiter R., Behringer R., Brinster R., Striker G. E. Progressive glomerulosclerosis develops in transgenic mice chronically expressing growth hormone and growth hormone releasing factor but not in those expressing insulinlike growth factor-1. Am J Pathol. 1988 Jun;131(3):398–403. [PMC free article] [PubMed] [Google Scholar]
- FUHR J., KACZMARCZYK J., KRUTTGEN C. D. Eine einfache colorimetrische Methode zur Inulinbestimmung für Nieren-Clearance-Untersuchungen bei Stoffwechselgesunden und Diabetikern. Klin Wochenschr. 1955 Aug 1;33(29-30):729–730. doi: 10.1007/BF01473295. [DOI] [PubMed] [Google Scholar]
- Flanders K. C., Roberts A. B., Ling N., Fleurdelys B. E., Sporn M. B. Antibodies to peptide determinants in transforming growth factor beta and their applications. Biochemistry. 1988 Jan 26;27(2):739–746. doi: 10.1021/bi00402a037. [DOI] [PubMed] [Google Scholar]
- Hall R. L., Wilke W. L., Fettman M. J. The progression of adriamycin-induced nephrotic syndrome in rats and the effect of captopril. Toxicol Appl Pharmacol. 1986 Jan;82(1):164–174. doi: 10.1016/0041-008x(86)90448-5. [DOI] [PubMed] [Google Scholar]
- Horikoshi S., McCune B. K., Ray P. E., Kopp J. B., Sporn M. B., Klotman P. E. Water deprivation stimulates transforming growth factor-beta 2 accumulation in the juxtaglomerular apparatus of mouse kidney. J Clin Invest. 1991 Dec;88(6):2117–2122. doi: 10.1172/JCI115541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt M. K., Ramos S. P., Geary K. M., Norling L. L., Peach M. J., Gomez R. A., Carey R. M. Colocalization and release of angiotensin and renin in renal cortical cells. Am J Physiol. 1992 Sep;263(3 Pt 2):F363–F373. doi: 10.1152/ajprenal.1992.263.3.F363. [DOI] [PubMed] [Google Scholar]
- Ichikawi I., Harris R. C. Angiotensin actions in the kidney: renewed insight into the old hormone. Kidney Int. 1991 Oct;40(4):583–596. doi: 10.1038/ki.1991.249. [DOI] [PubMed] [Google Scholar]
- Ikoma M., Kawamura T., Kakinuma Y., Fogo A., Ichikawa I. Cause of variable therapeutic efficiency of angiotensin converting enzyme inhibitor on glomerular lesions. Kidney Int. 1991 Aug;40(2):195–202. doi: 10.1038/ki.1991.200. [DOI] [PubMed] [Google Scholar]
- Kakinuma Y., Kawamura T., Bills T., Yoshioka T., Ichikawa I., Fogo A. Blood pressure-independent effect of angiotensin inhibition on vascular lesions of chronic renal failure. Kidney Int. 1992 Jul;42(1):46–55. doi: 10.1038/ki.1992.259. [DOI] [PubMed] [Google Scholar]
- Kato H., Suzuki H., Tajima S., Ogata Y., Tominaga T., Sato A., Saruta T. Angiotensin II stimulates collagen synthesis in cultured vascular smooth muscle cells. J Hypertens. 1991 Jan;9(1):17–22. [PubMed] [Google Scholar]
- Lafayette R. A., Mayer G., Park S. K., Meyer T. W. Angiotensin II receptor blockade limits glomerular injury in rats with reduced renal mass. J Clin Invest. 1992 Sep;90(3):766–771. doi: 10.1172/JCI115949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majesky M. W., Lindner V., Twardzik D. R., Schwartz S. M., Reidy M. A. Production of transforming growth factor beta 1 during repair of arterial injury. J Clin Invest. 1991 Sep;88(3):904–910. doi: 10.1172/JCI115393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marinides G. N., Groggel G. C., Cohen A. H., Border W. A. Enalapril and low protein reverse chronic puromycin aminonucleoside nephropathy. Kidney Int. 1990 Feb;37(2):749–757. doi: 10.1038/ki.1990.42. [DOI] [PubMed] [Google Scholar]
- Marinides G. N., Groggel G. C., Cohen A. H., Cook T., Baranowski R. L., Westenfelder C., Border W. A. Failure of angiotensin converting enzyme inhibition to affect the course of chronic puromycin aminonucleoside nephropathy. Am J Pathol. 1987 Nov;129(2):394–401. [PMC free article] [PubMed] [Google Scholar]
- Pesce C. M., Striker L. J., Peten E., Elliot S. J., Striker G. E. Glomerulosclerosis at both early and late stages is associated with increased cell turnover in mice transgenic for growth hormone. Lab Invest. 1991 Nov;65(5):601–605. [PubMed] [Google Scholar]
- Peten E. P., Garcia-Perez A., Terada Y., Woodrow D., Martin B. M., Striker G. E., Striker L. J. Age-related changes in alpha 1- and alpha 2-chain type IV collagen mRNAs in adult mouse glomeruli: competitive PCR. Am J Physiol. 1992 Nov;263(5 Pt 2):F951–F957. doi: 10.1152/ajprenal.1992.263.5.F951. [DOI] [PubMed] [Google Scholar]
- Ray P. E., McCune B. K., Gomez R. A., Horikoshi S., Kopp J. B., Klotman P. E. Renal vascular induction of TGF-beta 2 and renin by potassium depletion. Kidney Int. 1993 Nov;44(5):1006–1013. doi: 10.1038/ki.1993.342. [DOI] [PubMed] [Google Scholar]
- Rosenberg M. E., Smith L. J., Correa-Rotter R., Hostetter T. H. The paradox of the renin-angiotensin system in chronic renal disease. Kidney Int. 1994 Feb;45(2):403–410. doi: 10.1038/ki.1994.52. [DOI] [PubMed] [Google Scholar]
- Smith H. W., Finkelstein N., Aliminosa L., Crawford B., Graber M. THE RENAL CLEARANCES OF SUBSTITUTED HIPPURIC ACID DERIVATIVES AND OTHER AROMATIC ACIDS IN DOG AND MAN. J Clin Invest. 1945 May;24(3):388–404. doi: 10.1172/JCI101618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sorbi D., Fadly M., Hicks R., Alexander S., Arbeit L. Captopril inhibits the 72 kDa and 92 kDa matrix metalloproteinases. Kidney Int. 1993 Dec;44(6):1266–1272. doi: 10.1038/ki.1993.378. [DOI] [PubMed] [Google Scholar]
- Wolf G., Haberstroh U., Neilson E. G. Angiotensin II stimulates the proliferation and biosynthesis of type I collagen in cultured murine mesangial cells. Am J Pathol. 1992 Jan;140(1):95–107. [PMC free article] [PubMed] [Google Scholar]
- Yang C. W., Striker L. J., Pesce C., Chen W. Y., Peten E. P., Elliot S., Doi T., Kopchick J. J., Striker G. E. Glomerulosclerosis and body growth are mediated by different portions of bovine growth hormone. Studies in transgenic mice. Lab Invest. 1993 Jan;68(1):62–70. [PubMed] [Google Scholar]
- Yoshida Y., Fogo A., Shiraga H., Glick A. D., Ichikawa I. Serial micropuncture analysis of single nephron function in subtotal renal ablation. Kidney Int. 1988 Apr;33(4):855–867. doi: 10.1038/ki.1988.77. [DOI] [PubMed] [Google Scholar]
- Yoshida Y., Kawamura T., Ikoma M., Fogo A., Ichikawa I. Effects of antihypertensive drugs on glomerular morphology. Kidney Int. 1989 Oct;36(4):626–635. doi: 10.1038/ki.1989.239. [DOI] [PubMed] [Google Scholar]
- Zatz R., Dunn B. R., Meyer T. W., Anderson S., Rennke H. G., Brenner B. M. Prevention of diabetic glomerulopathy by pharmacological amelioration of glomerular capillary hypertension. J Clin Invest. 1986 Jun;77(6):1925–1930. doi: 10.1172/JCI112521. [DOI] [PMC free article] [PubMed] [Google Scholar]