Abstract
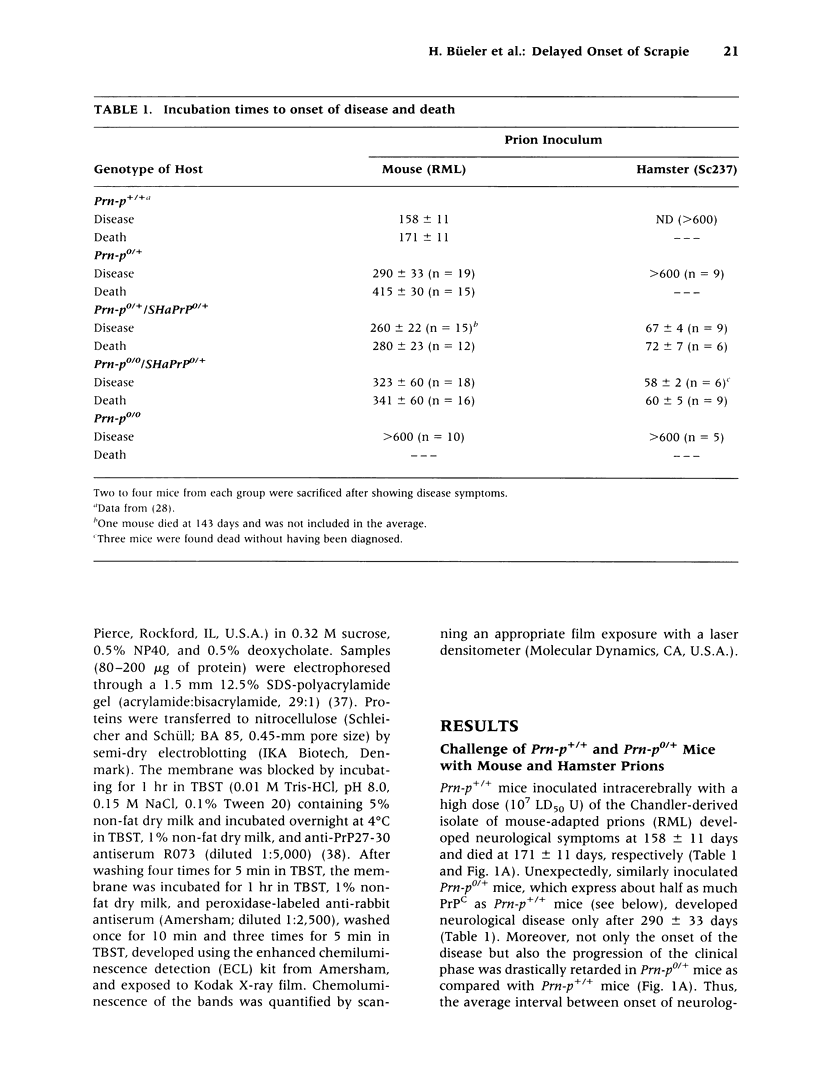
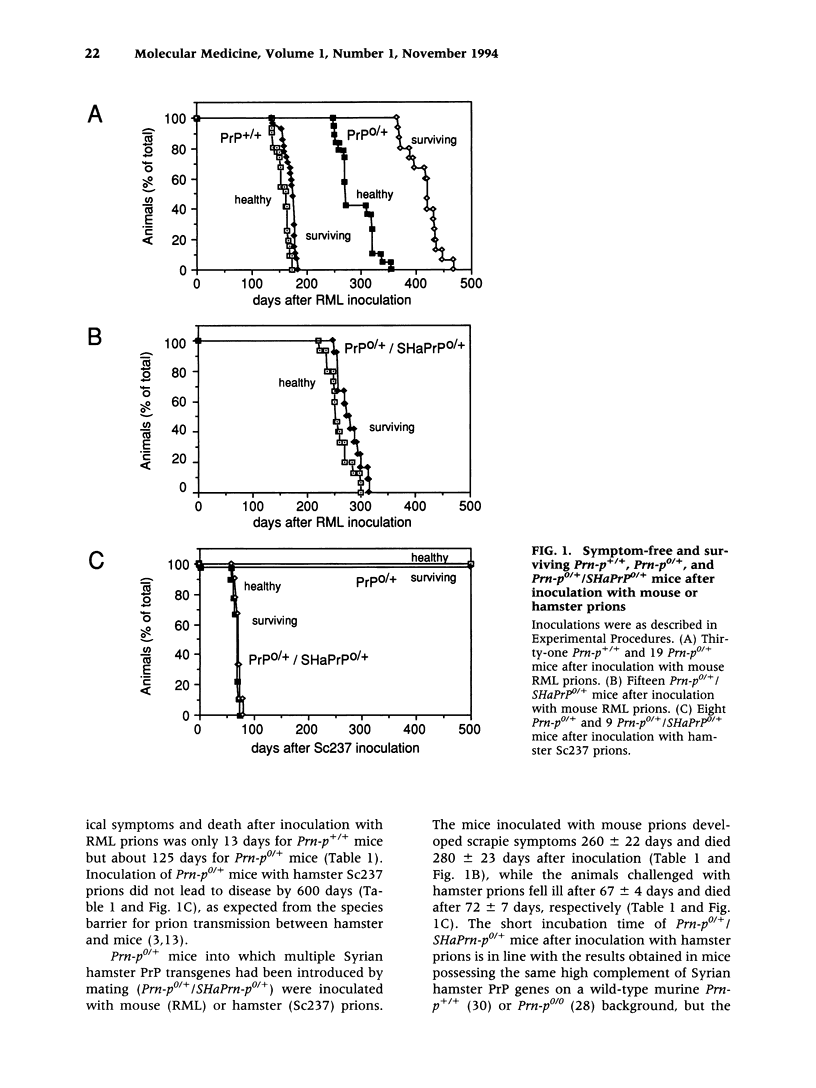
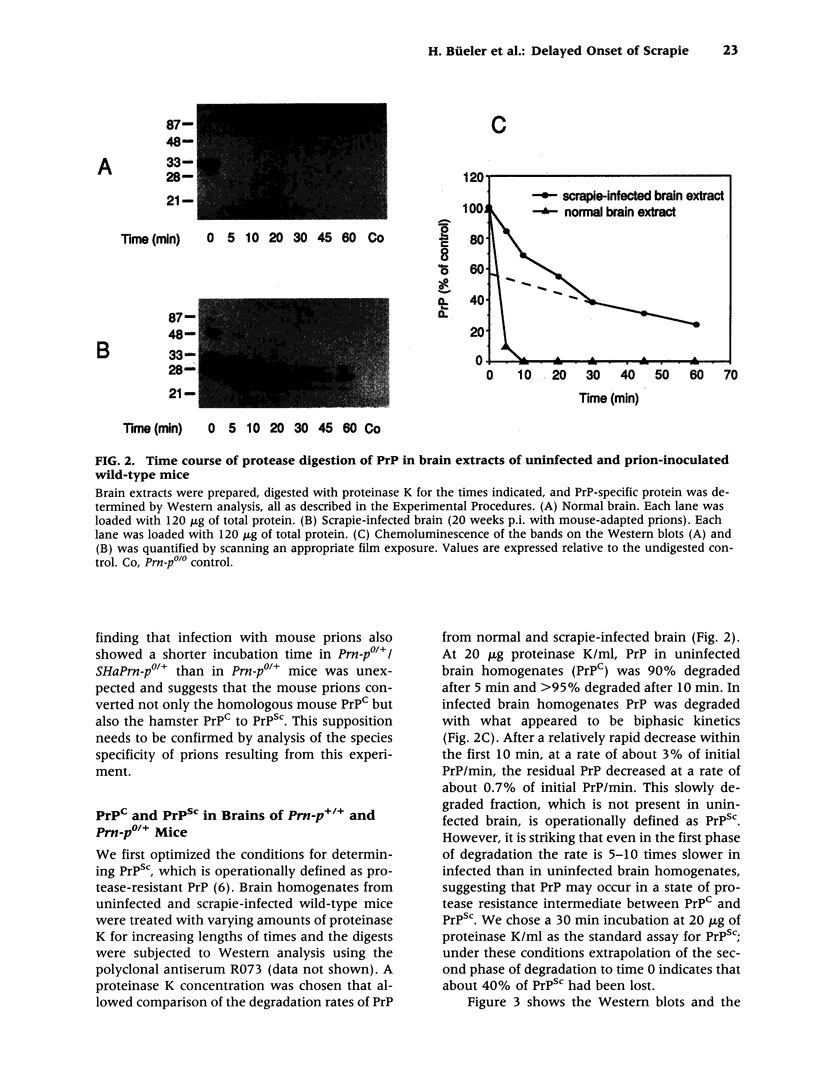
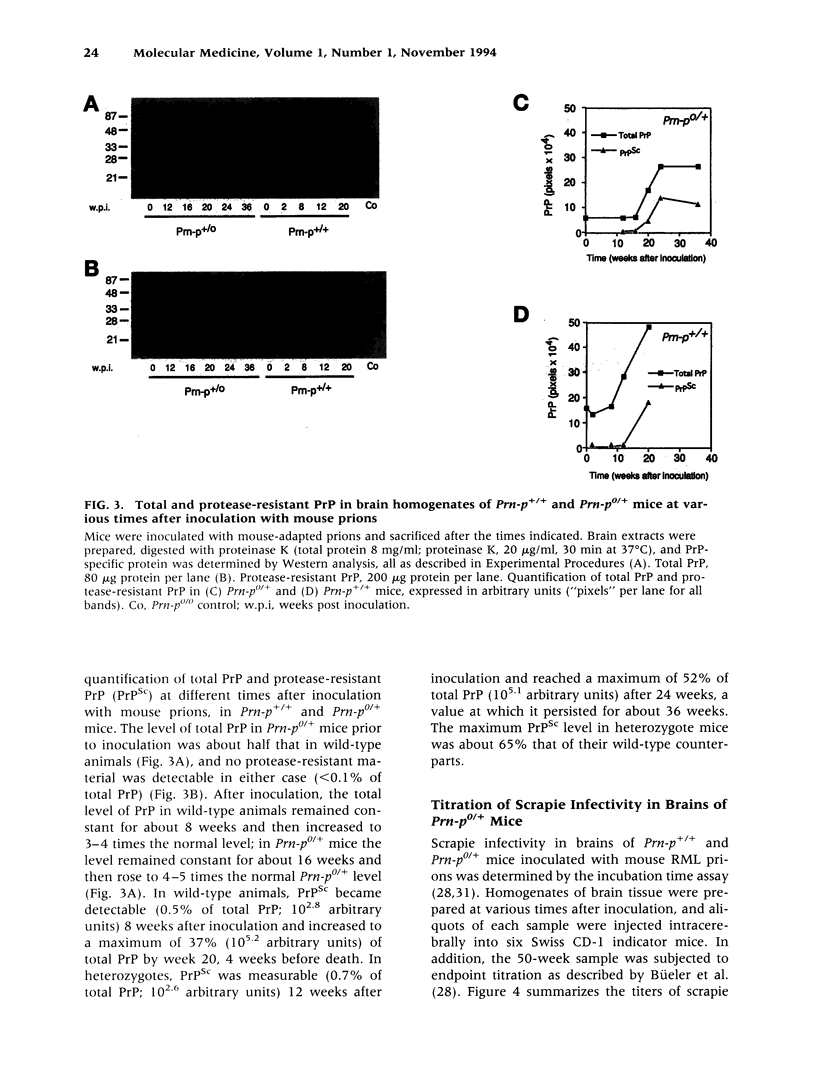
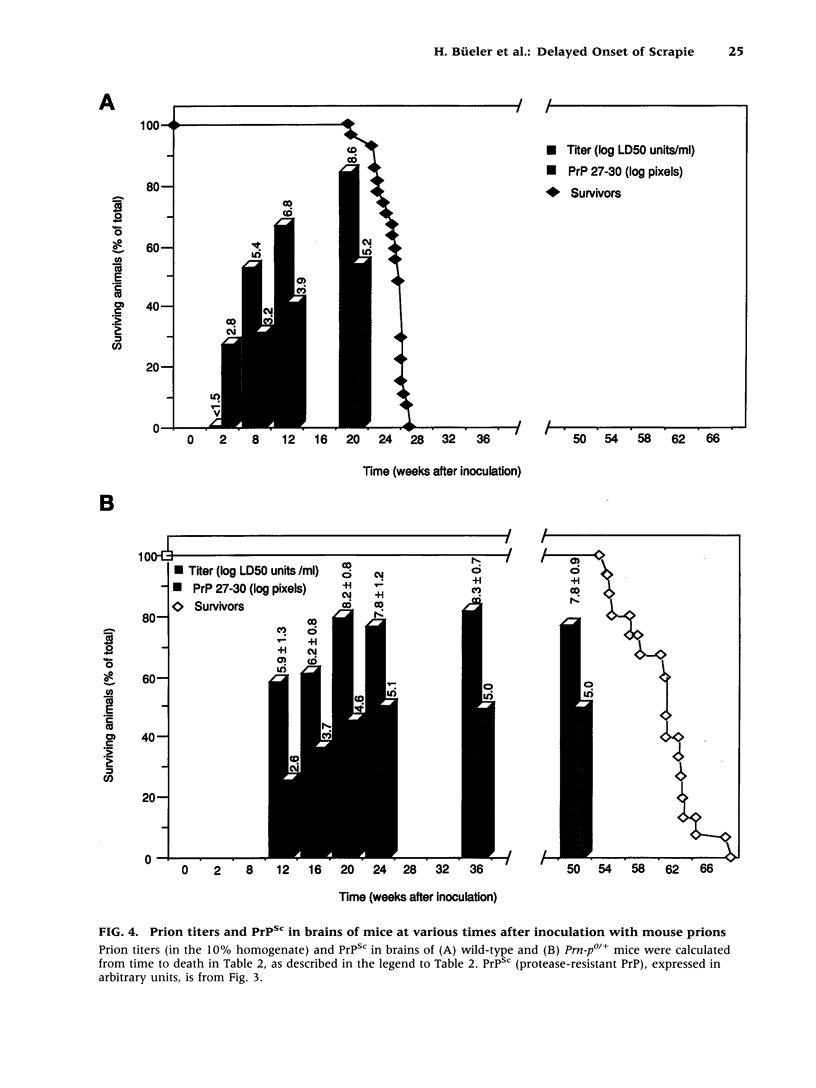
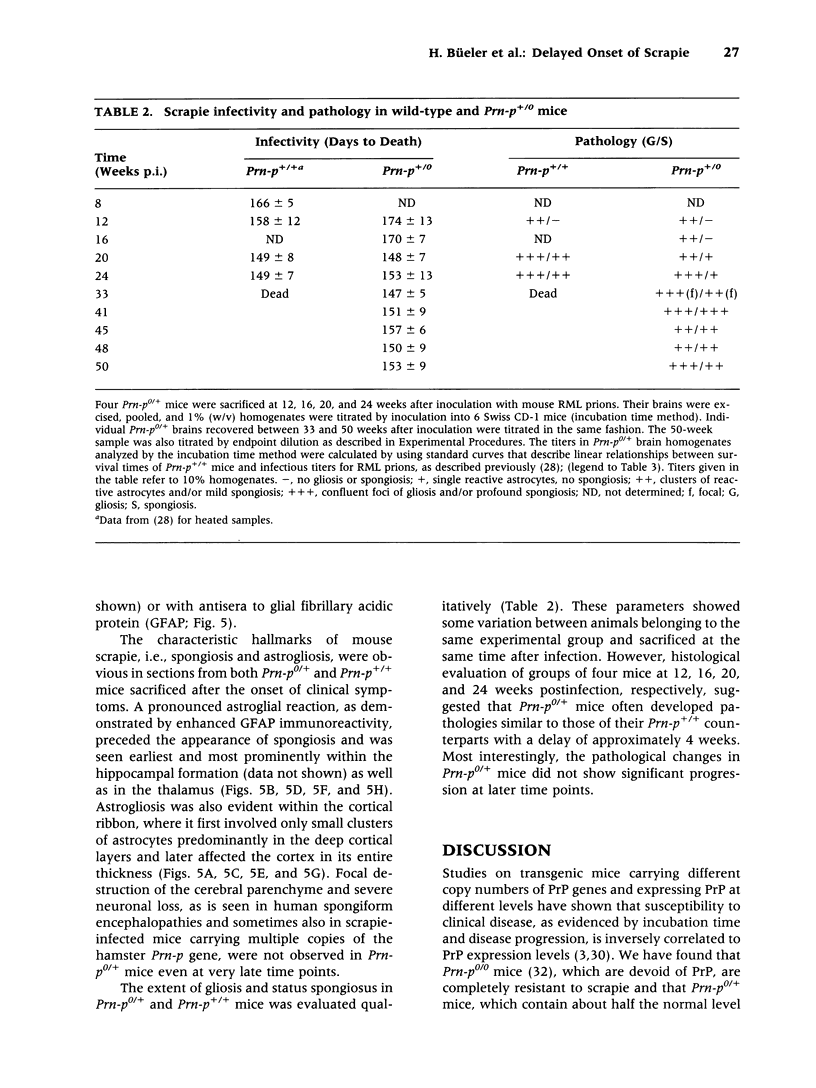
BACKGROUND: It has been proposed that the prion, the infectious agent of transmissible spongiform encephalopathies, is PrPSc, a post-translationally modified form of the normal host protein PrPC. We showed previously that mice devoid of PrPC (Prn-p0/0) are completely resistant to scrapie. We now report on the unexpected response of heterozygous (Prn-p0/+) mice to scrapie infection. MATERIALS AND METHODS: Prn-p0/+, Prn-p0/0 and Prn-p+/+ mice were obtained from crosses of Prn-p0/+ mice. Mice were inoculated intracerebrally with mouse-adapted scrapie agent and the clinical progression of the disease recorded. Mice were sacrificed at intervals, PrPSc was determined as protease-resistant PrP and the prion titer by the incubation time assay. RESULTS: Prn-p0/+ mice, which have about half the normal level of PrPC in their brains, show enhanced resistance to scrapie, as manifested by a significant delay in onset and progression of clinical disease. However, while in wild type animals an increase in prion titer and PrPSc levels is followed within weeks by scrapie symptoms and death, heterozygous Prn-p0/+ mice remain free of symptoms for many months despite similar levels of scrapie infectivity and PrPSc. CONCLUSIONS: Our findings extend previous reports showing an inverse relationship between PrP expression level and incubation time for scrapie. However, contrary to expectation, overall accumulation of PrPSc and prions to a high level do not necessarily lead to clinical disease. These findings raise the question whether high titers of prion infectivity could also persist for long periods under natural circumstances in the absence of clinical symptoms.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Basler K., Oesch B., Scott M., Westaway D., Wälchli M., Groth D. F., McKinley M. P., Prusiner S. B., Weissmann C. Scrapie and cellular PrP isoforms are encoded by the same chromosomal gene. Cell. 1986 Aug 1;46(3):417–428. doi: 10.1016/0092-8674(86)90662-8. [DOI] [PubMed] [Google Scholar]
- Bendheim P. E., Brown H. R., Rudelli R. D., Scala L. J., Goller N. L., Wen G. Y., Kascsak R. J., Cashman N. R., Bolton D. C. Nearly ubiquitous tissue distribution of the scrapie agent precursor protein. Neurology. 1992 Jan;42(1):149–156. doi: 10.1212/wnl.42.1.149. [DOI] [PubMed] [Google Scholar]
- Brown P., Kaur P., Sulima M. P., Goldfarb L. G., Gibbs C. J., Jr, Gajdusek D. C. Real and imagined clinicopathological limits of "prion dementia". Lancet. 1993 Jan 16;341(8838):127–129. doi: 10.1016/0140-6736(93)90001-w. [DOI] [PubMed] [Google Scholar]
- Brown P., Wolff A., Gajdusek D. C. A simple and effective method for inactivating virus infectivity in formalin-fixed tissue samples from patients with Creutzfeldt-Jakob disease. Neurology. 1990 Jun;40(6):887–890. doi: 10.1212/wnl.40.6.887. [DOI] [PubMed] [Google Scholar]
- Bruce M. E., Dickinson A. G. Biological evidence that scrapie agent has an independent genome. J Gen Virol. 1987 Jan;68(Pt 1):79–89. doi: 10.1099/0022-1317-68-1-79. [DOI] [PubMed] [Google Scholar]
- Büeler H., Aguzzi A., Sailer A., Greiner R. A., Autenried P., Aguet M., Weissmann C. Mice devoid of PrP are resistant to scrapie. Cell. 1993 Jul 2;73(7):1339–1347. doi: 10.1016/0092-8674(93)90360-3. [DOI] [PubMed] [Google Scholar]
- Büeler H., Fischer M., Lang Y., Bluethmann H., Lipp H. P., DeArmond S. J., Prusiner S. B., Aguet M., Weissmann C. Normal development and behaviour of mice lacking the neuronal cell-surface PrP protein. Nature. 1992 Apr 16;356(6370):577–582. doi: 10.1038/356577a0. [DOI] [PubMed] [Google Scholar]
- CHANDLER R. L. Encephalopathy in mice produced by inoculation with scrapie brain material. Lancet. 1961 Jun 24;1(7191):1378–1379. doi: 10.1016/s0140-6736(61)92008-6. [DOI] [PubMed] [Google Scholar]
- Carlson G. A., Ebeling C., Yang S. L., Telling G., Torchia M., Groth D., Westaway D., DeArmond S. J., Prusiner S. B. Prion isolate specified allotypic interactions between the cellular and scrapie prion proteins in congenic and transgenic mice. Proc Natl Acad Sci U S A. 1994 Jun 7;91(12):5690–5694. doi: 10.1073/pnas.91.12.5690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chesebro B., Race R., Wehrly K., Nishio J., Bloom M., Lechner D., Bergstrom S., Robbins K., Mayer L., Keith J. M. Identification of scrapie prion protein-specific mRNA in scrapie-infected and uninfected brain. Nature. 1985 May 23;315(6017):331–333. doi: 10.1038/315331a0. [DOI] [PubMed] [Google Scholar]
- Collinge J., Whittington M. A., Sidle K. C., Smith C. J., Palmer M. S., Clarke A. R., Jefferys J. G. Prion protein is necessary for normal synaptic function. Nature. 1994 Jul 28;370(6487):295–297. doi: 10.1038/370295a0. [DOI] [PubMed] [Google Scholar]
- Dickinson A. G., Outram G. W. Genetic aspects of unconventional virus infections: the basis of the virino hypothesis. Ciba Found Symp. 1988;135:63–83. doi: 10.1002/9780470513613.ch5. [DOI] [PubMed] [Google Scholar]
- Manson J., West J. D., Thomson V., McBride P., Kaufman M. H., Hope J. The prion protein gene: a role in mouse embryogenesis? Development. 1992 May;115(1):117–122. doi: 10.1242/dev.115.1.117. [DOI] [PubMed] [Google Scholar]
- McKinley M. P., Taraboulos A., Kenaga L., Serban D., Stieber A., DeArmond S. J., Prusiner S. B., Gonatas N. Ultrastructural localization of scrapie prion proteins in cytoplasmic vesicles of infected cultured cells. Lab Invest. 1991 Dec;65(6):622–630. [PubMed] [Google Scholar]
- Oesch B., Westaway D., Wälchli M., McKinley M. P., Kent S. B., Aebersold R., Barry R. A., Tempst P., Teplow D. B., Hood L. E. A cellular gene encodes scrapie PrP 27-30 protein. Cell. 1985 Apr;40(4):735–746. doi: 10.1016/0092-8674(85)90333-2. [DOI] [PubMed] [Google Scholar]
- Pan K. M., Baldwin M., Nguyen J., Gasset M., Serban A., Groth D., Mehlhorn I., Huang Z., Fletterick R. J., Cohen F. E. Conversion of alpha-helices into beta-sheets features in the formation of the scrapie prion proteins. Proc Natl Acad Sci U S A. 1993 Dec 1;90(23):10962–10966. doi: 10.1073/pnas.90.23.10962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B., Cochran S. P., Groth D. F., Downey D. E., Bowman K. A., Martinez H. M. Measurement of the scrapie agent using an incubation time interval assay. Ann Neurol. 1982 Apr;11(4):353–358. doi: 10.1002/ana.410110406. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Groth D., Serban A., Koehler R., Foster D., Torchia M., Burton D., Yang S. L., DeArmond S. J. Ablation of the prion protein (PrP) gene in mice prevents scrapie and facilitates production of anti-PrP antibodies. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10608–10612. doi: 10.1073/pnas.90.22.10608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology and genetics of neurodegenerative diseases caused by prions. Adv Virus Res. 1992;41:241–280. doi: 10.1016/s0065-3527(08)60038-x. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Molecular biology of prion diseases. Science. 1991 Jun 14;252(5012):1515–1522. doi: 10.1126/science.1675487. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Novel proteinaceous infectious particles cause scrapie. Science. 1982 Apr 9;216(4542):136–144. doi: 10.1126/science.6801762. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B., Scott M., Foster D., Pan K. M., Groth D., Mirenda C., Torchia M., Yang S. L., Serban D., Carlson G. A. Transgenetic studies implicate interactions between homologous PrP isoforms in scrapie prion replication. Cell. 1990 Nov 16;63(4):673–686. doi: 10.1016/0092-8674(90)90134-z. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Scrapie prions. Annu Rev Microbiol. 1989;43:345–374. doi: 10.1146/annurev.mi.43.100189.002021. [DOI] [PubMed] [Google Scholar]
- Prusiner S. B. Transgenetic investigations of prion diseases of humans and animals. Philos Trans R Soc Lond B Biol Sci. 1993 Feb 27;339(1288):239–254. doi: 10.1098/rstb.1993.0022. [DOI] [PubMed] [Google Scholar]
- Sailer A., Büeler H., Fischer M., Aguzzi A., Weissmann C. No propagation of prions in mice devoid of PrP. Cell. 1994 Jul 1;77(7):967–968. doi: 10.1016/0092-8674(94)90436-7. [DOI] [PubMed] [Google Scholar]
- Scott M., Foster D., Mirenda C., Serban D., Coufal F., Wälchli M., Torchia M., Groth D., Carlson G., DeArmond S. J. Transgenic mice expressing hamster prion protein produce species-specific scrapie infectivity and amyloid plaques. Cell. 1989 Dec 1;59(5):847–857. doi: 10.1016/0092-8674(89)90608-9. [DOI] [PubMed] [Google Scholar]
- Serban D., Taraboulos A., DeArmond S. J., Prusiner S. B. Rapid detection of Creutzfeldt-Jakob disease and scrapie prion proteins. Neurology. 1990 Jan;40(1):110–117. doi: 10.1212/wnl.40.1.110. [DOI] [PubMed] [Google Scholar]
- Stahl N., Baldwin M. A., Hecker R., Pan K. M., Burlingame A. L., Prusiner S. B. Glycosylinositol phospholipid anchors of the scrapie and cellular prion proteins contain sialic acid. Biochemistry. 1992 Jun 2;31(21):5043–5053. doi: 10.1021/bi00136a600. [DOI] [PubMed] [Google Scholar]
- Stahl N., Baldwin M. A., Teplow D. B., Hood L., Gibson B. W., Burlingame A. L., Prusiner S. B. Structural studies of the scrapie prion protein using mass spectrometry and amino acid sequencing. Biochemistry. 1993 Mar 2;32(8):1991–2002. doi: 10.1021/bi00059a016. [DOI] [PubMed] [Google Scholar]
- Taraboulos A., Rogers M., Borchelt D. R., McKinley M. P., Scott M., Serban D., Prusiner S. B. Acquisition of protease resistance by prion proteins in scrapie-infected cells does not require asparagine-linked glycosylation. Proc Natl Acad Sci U S A. 1990 Nov;87(21):8262–8266. doi: 10.1073/pnas.87.21.8262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboulos A., Serban D., Prusiner S. B. Scrapie prion proteins accumulate in the cytoplasm of persistently infected cultured cells. J Cell Biol. 1990 Jun;110(6):2117–2132. doi: 10.1083/jcb.110.6.2117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turk E., Teplow D. B., Hood L. E., Prusiner S. B. Purification and properties of the cellular and scrapie hamster prion proteins. Eur J Biochem. 1988 Sep 1;176(1):21–30. doi: 10.1111/j.1432-1033.1988.tb14246.x. [DOI] [PubMed] [Google Scholar]
- Weissmann C., Büeler H., Sailer A., Fischer M., Aguet M., Aguzzi A. Role of PrP in prion diseases. Br Med Bull. 1993 Oct;49(4):995–1011. doi: 10.1093/oxfordjournals.bmb.a072658. [DOI] [PubMed] [Google Scholar]