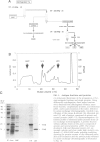
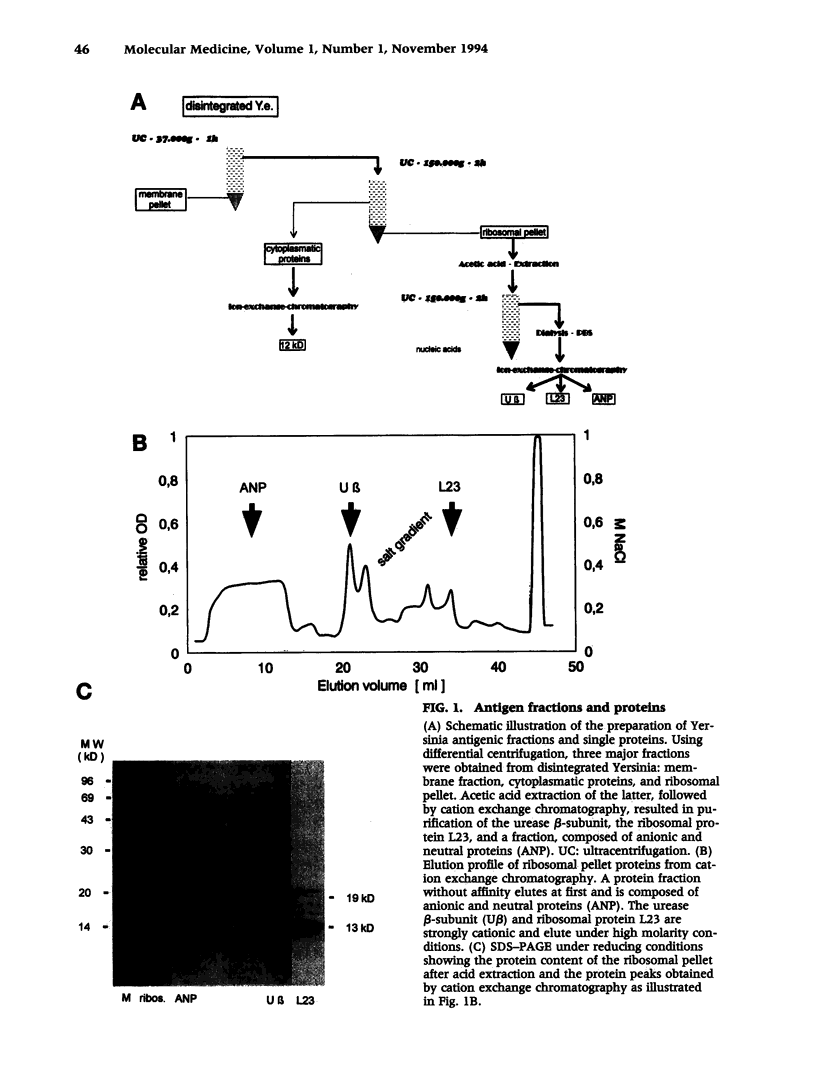
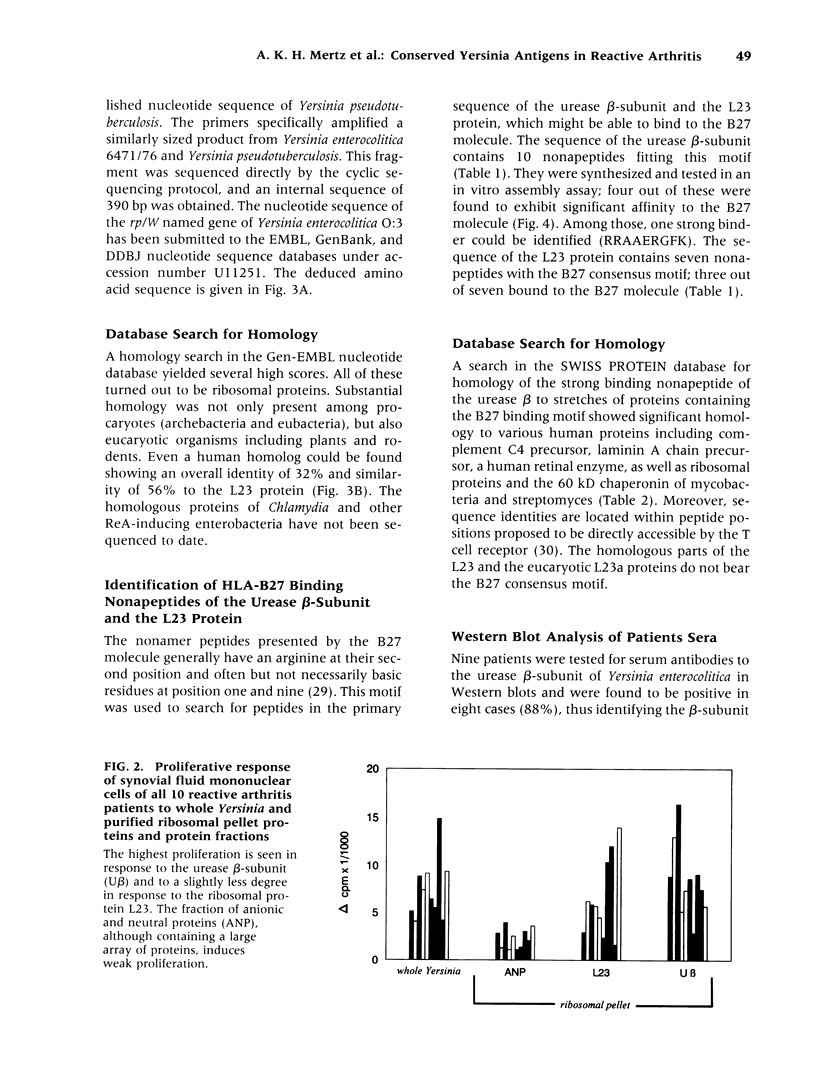
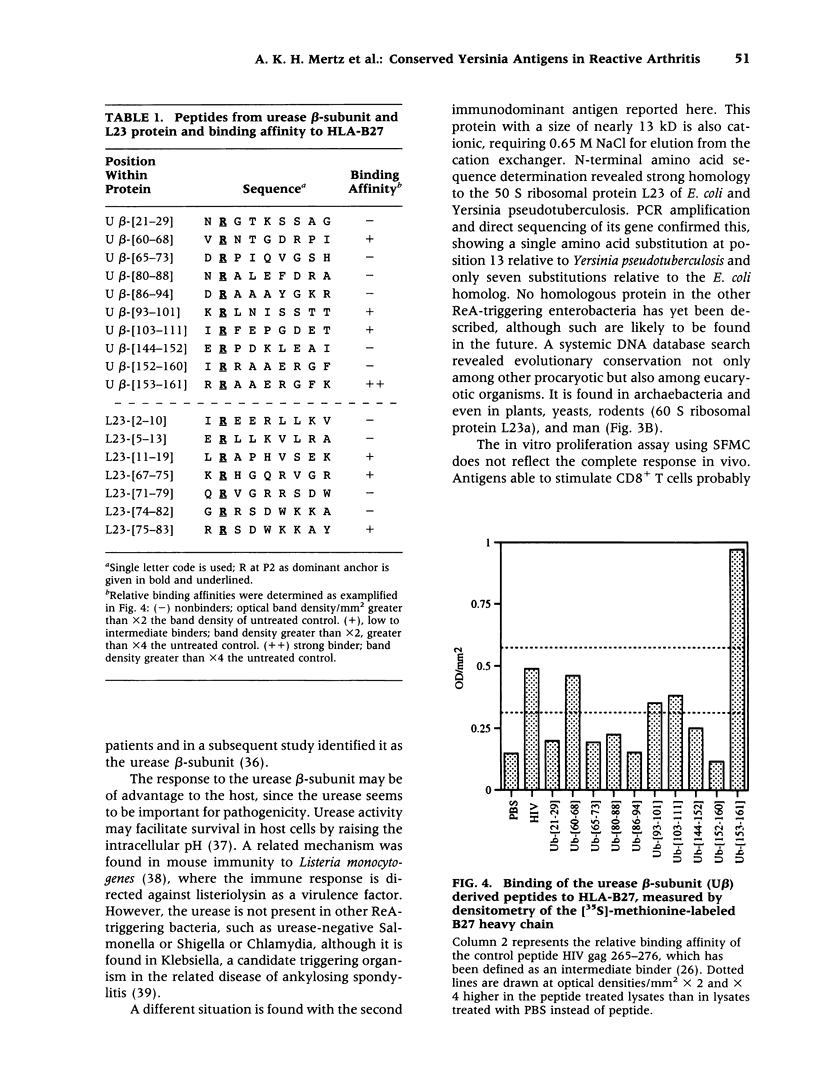
Abstract
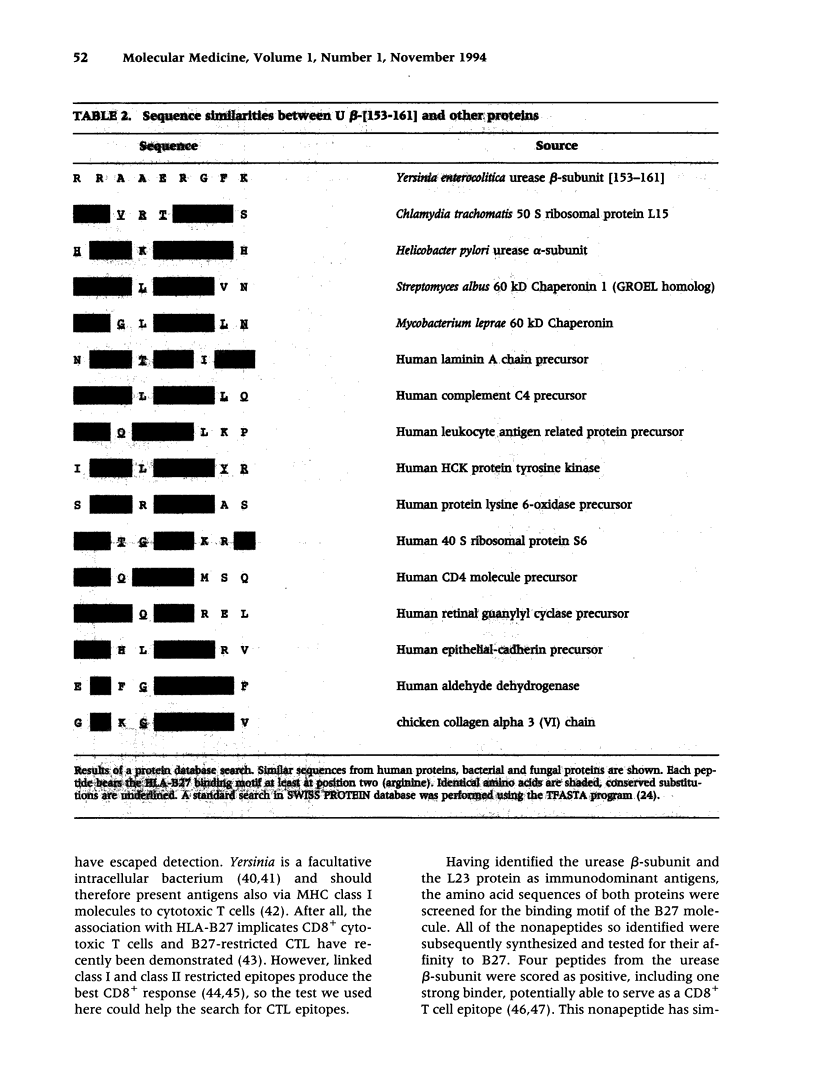
BACKGROUND: Reactive arthritis (ReA) is a T cell mediated inflammatory process. The immune response is primarily directed against a triggering organism, although autoimmunity has been invoked in long-lasting, antibiotic-resistant disease. Although a variety of different species are known to trigger Reactive arthritis, the clinical manifestations are strikingly similar as well as closely associated to the HLA-B27 (70%). MATERIALS AND METHODS: Various antigenic fractions and single antigens of Yersinia enterocolitica were prepared, and their immunological activity was assessed by proliferation of synovial fluid mononuclear cells from 10 Reactive arthritis patients. The gene encoding one hitherto unknown antigen has been sequenced. Nonapeptides deduced from sequences of the target antigens were tested in an assembly assay. RESULTS: Two immunodominant proteins of Yersinia enterocolitica were found, one being the urease beta-subunit and the other the 50 S ribosomal protein L23. The latter has been sequenced and belongs to the evolutionarily conserved ribosomal proteins with homology to procaryotes and eucaryotes. One nonapeptide derived from the urease beta-subunit was identified as a possible epitope for HLA-B27-restricted cytotoxic T cells by its high affinity. This epitope is also highly conserved. CONCLUSION: Sharing of conserved immunodominant proteins between different disease triggering microorganisms could provide an explanation of the shared clinical picture in Reactive arthritis. Moreover, autoimmunity in Reactive arthritis might be mediated by antigen mimicry between evolutionarily conserved epitopes of ribosomal proteins and their host analogs.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bölin I., Norlander L., Wolf-Watz H. Temperature-inducible outer membrane protein of Yersinia pseudotuberculosis and Yersinia enterocolitica is associated with the virulence plasmid. Infect Immun. 1982 Aug;37(2):506–512. doi: 10.1128/iai.37.2.506-512.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen I. R., Young D. B. Autoimmunity, microbial immunity and the immunological homunculus. Immunol Today. 1991 Apr;12(4):105–110. doi: 10.1016/0167-5699(91)90093-9. [DOI] [PubMed] [Google Scholar]
- Daser A., Urlaub H., Henklein P. HLA-B27 binding peptides derived from the 57 kD heat shock protein of Chlamydia trachomatis: novel insights into the peptide binding rules. Mol Immunol. 1994 Apr;31(5):331–336. doi: 10.1016/0161-5890(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Devereux J., Haeberli P., Smithies O. A comprehensive set of sequence analysis programs for the VAX. Nucleic Acids Res. 1984 Jan 11;12(1 Pt 1):387–395. doi: 10.1093/nar/12.1part1.387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiBrino M., Parker K. C., Shiloach J., Turner R. V., Tsuchida T., Garfield M., Biddison W. E., Coligan J. E. Endogenous peptides with distinct amino acid anchor residue motifs bind to HLA-A1 and HLA-B8. J Immunol. 1994 Jan 15;152(2):620–631. [PubMed] [Google Scholar]
- DiBrino M., Tsuchida T., Turner R. V., Parker K. C., Coligan J. E., Biddison W. E. HLA-A1 and HLA-A3 T cell epitopes derived from influenza virus proteins predicted from peptide binding motifs. J Immunol. 1993 Dec 1;151(11):5930–5935. [PubMed] [Google Scholar]
- Ellis S. A., Taylor C., McMichael A. Recognition of HLA-B27 and related antigen by a monoclonal antibody. Hum Immunol. 1982 Aug;5(1):49–59. doi: 10.1016/0198-8859(82)90030-1. [DOI] [PubMed] [Google Scholar]
- Evavold B. D., Sloan-Lancaster J., Allen P. M. Tickling the TCR: selective T-cell functions stimulated by altered peptide ligands. Immunol Today. 1993 Dec;14(12):602–609. doi: 10.1016/0167-5699(93)90200-5. [DOI] [PubMed] [Google Scholar]
- Ford D. K. Lymphocytes from the site of disease in reactive arthritis indicate antigen-specific immunopathology. J Infect Dis. 1991 Nov;164(5):1032–1033. doi: 10.1093/infdis/164.5.1032. [DOI] [PubMed] [Google Scholar]
- Gaston J. S., Life P. F., Granfors K., Merilahti-Palo R., Bailey L., Consalvey S., Toivanen A., Bacon P. A. Synovial T lymphocyte recognition of organisms that trigger reactive arthritis. Clin Exp Immunol. 1989 Jun;76(3):348–353. [PMC free article] [PubMed] [Google Scholar]
- Granfors K., Jalkanen S., von Essen R., Lahesmaa-Rantala R., Isomäki O., Pekkola-Heino K., Merilahti-Palo R., Saario R., Isomäki H., Toivanen A. Yersinia antigens in synovial-fluid cells from patients with reactive arthritis. N Engl J Med. 1989 Jan 26;320(4):216–221. doi: 10.1056/NEJM198901263200404. [DOI] [PubMed] [Google Scholar]
- Hammer M., Zeidler H., Klimsa S., Heesemann J. Yersinia enterocolitica in the synovial membrane of patients with Yersinia-induced arthritis. Arthritis Rheum. 1990 Dec;33(12):1795–1800. doi: 10.1002/art.1780331206. [DOI] [PubMed] [Google Scholar]
- Hassell A. B., Reynolds D. J., Deacon M., Gaston J. S., Pearce J. H. Identification of T-cell stimulatory antigens of Chlamydia trachomatis using synovial fluid-derived T-cell clones. Immunology. 1993 Aug;79(4):513–519. [PMC free article] [PubMed] [Google Scholar]
- Hermann E., Fleischer B., Mayet W. J., Poralla T., Meyer zum Büschenfelde K. H. Response of synovial fluid T cell clones to Yersinia enterocolitica antigens in patients with reactive Yersinia arthritis. Clin Exp Immunol. 1989 Mar;75(3):365–370. [PMC free article] [PubMed] [Google Scholar]
- Hermann E., Yu D. T., Meyer zum Büschenfelde K. H., Fleischer B. HLA-B27-restricted CD8 T cells derived from synovial fluids of patients with reactive arthritis and ankylosing spondylitis. Lancet. 1993 Sep 11;342(8872):646–650. doi: 10.1016/0140-6736(93)91760-j. [DOI] [PubMed] [Google Scholar]
- Jardetzky T. S., Lane W. S., Robinson R. A., Madden D. R., Wiley D. C. Identification of self peptides bound to purified HLA-B27. Nature. 1991 Sep 26;353(6342):326–329. doi: 10.1038/353326a0. [DOI] [PubMed] [Google Scholar]
- Kaufmann S. H. Immunity to intracellular bacteria. Annu Rev Immunol. 1993;11:129–163. doi: 10.1146/annurev.iy.11.040193.001021. [DOI] [PubMed] [Google Scholar]
- Keat A. Reiter's syndrome and reactive arthritis in perspective. N Engl J Med. 1983 Dec 29;309(26):1606–1615. doi: 10.1056/NEJM198312293092604. [DOI] [PubMed] [Google Scholar]
- Klempner M. S., Styrt B. Alkalinizing the intralysosomal pH inhibits degranulation of human neutrophils. J Clin Invest. 1983 Nov;72(5):1793–1800. doi: 10.1172/JCI111139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Lahesmaa R., Yssel H., Batsford S., Luukkainen R., Möttönen T., Steinman L., Peltz G. Yersinia enterocolitica activates a T helper type 1-like T cell subset in reactive arthritis. J Immunol. 1992 May 15;148(10):3079–3085. [PubMed] [Google Scholar]
- Lipman D. J., Pearson W. R. Rapid and sensitive protein similarity searches. Science. 1985 Mar 22;227(4693):1435–1441. doi: 10.1126/science.2983426. [DOI] [PubMed] [Google Scholar]
- Luo A. M., Garza K. M., Hunt D., Tung K. S. Antigen mimicry in autoimmune disease sharing of amino acid residues critical for pathogenic T cell activation. J Clin Invest. 1993 Nov;92(5):2117–2123. doi: 10.1172/JCI116812. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madden D. R., Gorga J. C., Strominger J. L., Wiley D. C. The three-dimensional structure of HLA-B27 at 2.1 A resolution suggests a general mechanism for tight peptide binding to MHC. Cell. 1992 Sep 18;70(6):1035–1048. doi: 10.1016/0092-8674(92)90252-8. [DOI] [PubMed] [Google Scholar]
- Mertz A. K., Batsford S. R., Curschellas E., Kist M. J., Gondolf K. B. Cationic Yersinia antigen-induced chronic allergic arthritis in rats. A model for reactive arthritis in humans. J Clin Invest. 1991 Aug;88(2):632–642. doi: 10.1172/JCI115348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijman H. W., Houbiers J. G., van der Burg S. H., Vierboom M. P., Kenemans P., Kast W. M., Melief C. J. Characterization of cytotoxic T lymphocyte epitopes of a self-protein, p53, and a non-self-protein, influenza matrix: relationship between major histocompatibility complex peptide binding affinity and immune responsiveness to peptides. J Immunother Emphasis Tumor Immunol. 1993 Aug;14(2):121–126. [PubMed] [Google Scholar]
- Pamer E. G., Harty J. T., Bevan M. J. Precise prediction of a dominant class I MHC-restricted epitope of Listeria monocytogenes. Nature. 1991 Oct 31;353(6347):852–855. doi: 10.1038/353852a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardoll D. M. Tumour antigens. A new look for the 1990s. Nature. 1994 Jun 2;369(6479):357–357. doi: 10.1038/369357a0. [DOI] [PubMed] [Google Scholar]
- Probst P., Hermann E., Meyer zum Büschenfelde K. H., Fleischer B. Identification of the Yersinia enterocolitica urease beta subunit as a target antigen for human synovial T lymphocytes in reactive arthritis. Infect Immun. 1993 Oct;61(10):4507–4509. doi: 10.1128/iai.61.10.4507-4509.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Probst P., Hermann E., Meyer zum Büschenfelde K. H., Fleischer B. Multiclonal synovial T cell response to Yersinia enterocolitica in reactive arthritis: the Yersinia 61-kDa heat-shock protein is not the major target antigen. J Infect Dis. 1993 Feb;167(2):385–391. doi: 10.1093/infdis/167.2.385. [DOI] [PubMed] [Google Scholar]
- Salari S. H., Ward M. E. Polypeptide composition of Chlamydia trachomatis. J Gen Microbiol. 1981 Apr;123(2):197–207. doi: 10.1099/00221287-123-2-197. [DOI] [PubMed] [Google Scholar]
- Schumacher H. R., Jr, Magge S., Cherian P. V., Sleckman J., Rothfuss S., Clayburne G., Sieck M. Light and electron microscopic studies on the synovial membrane in Reiter's syndrome. Immunocytochemical identification of chlamydial antigen in patients with early disease. Arthritis Rheum. 1988 Aug;31(8):937–946. doi: 10.1002/art.1780310801. [DOI] [PubMed] [Google Scholar]
- Scofield R. H., Warren W. L., Koelsch G., Harley J. B. A hypothesis for the HLA-B27 immune dysregulation in spondyloarthropathy: contributions from enteric organisms, B27 structure, peptides bound by B27, and convergent evolution. Proc Natl Acad Sci U S A. 1993 Oct 15;90(20):9330–9334. doi: 10.1073/pnas.90.20.9330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieper J., Braun J., Wu P., Kingsley G. T cells are responsible for the enhanced synovial cellular immune response to triggering antigen in reactive arthritis. Clin Exp Immunol. 1993 Jan;91(1):96–102. doi: 10.1111/j.1365-2249.1993.tb03361.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sieper J., Kingsley G., Palacios-Boix A., Pitzalis C., Treharne J., Hughes R., Keat A., Panayi G. S. Synovial T lymphocyte-specific immune response to Chlamydia trachomatis in Reiter's disease. Arthritis Rheum. 1991 May;34(5):588–598. doi: 10.1002/art.1780340511. [DOI] [PubMed] [Google Scholar]
- Simon A. K., Seipelt E., Sieper J. Divergent T-cell cytokine patterns in inflammatory arthritis. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8562–8566. doi: 10.1073/pnas.91.18.8562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skeiky Y. A., Benson D. R., Guderian J. A., Sleath P. R., Parsons M., Reed S. G. Trypanosoma cruzi acidic ribosomal P protein gene family. Novel P proteins encoding unusual cross-reactive epitopes. J Immunol. 1993 Nov 15;151(10):5504–5515. [PubMed] [Google Scholar]
- Skurnik M., Batsford S., Mertz A., Schiltz E., Toivanen P. The putative arthritogenic cationic 19-kilodalton antigen of Yersinia enterocolitica is a urease beta-subunit. Infect Immun. 1993 Jun;61(6):2498–2504. doi: 10.1128/iai.61.6.2498-2504.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skurnik M. Lack of correlation between the presence of plasmids and fimbriae in Yersinia enterocolitica and Yersinia pseudotuberculosis. J Appl Bacteriol. 1984 Jun;56(3):355–363. doi: 10.1111/j.1365-2672.1984.tb01362.x. [DOI] [PubMed] [Google Scholar]
- Small P. L., Ramakrishnan L., Falkow S. Remodeling schemes of intracellular pathogens. Science. 1994 Feb 4;263(5147):637–639. doi: 10.1126/science.8303269. [DOI] [PubMed] [Google Scholar]
- Takahashi H., Germain R. N., Moss B., Berzofsky J. A. An immunodominant class I-restricted cytotoxic T lymphocyte determinant of human immunodeficiency virus type 1 induces CD4 class II-restricted help for itself. J Exp Med. 1990 Feb 1;171(2):571–576. doi: 10.1084/jem.171.2.571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend A., Elliott T., Cerundolo V., Foster L., Barber B., Tse A. Assembly of MHC class I molecules analyzed in vitro. Cell. 1990 Jul 27;62(2):285–295. doi: 10.1016/0092-8674(90)90366-m. [DOI] [PubMed] [Google Scholar]
- Viner N. J., Bailey L. C., Life P. F., Bacon P. A., Gaston J. S. Isolation of Yersinia-specific T cell clones from the synovial membrane and synovial fluid of a patient with reactive arthritis. Arthritis Rheum. 1991 Sep;34(9):1151–1157. doi: 10.1002/art.1780340911. [DOI] [PubMed] [Google Scholar]
- Wuorela M., Jalkanen S., Toivanen P., Granfors K. Intracellular pathogens and professional phagocytes in reactive arthritis. Pathobiology. 1991;59(3):162–165. doi: 10.1159/000163636. [DOI] [PubMed] [Google Scholar]