Abstract
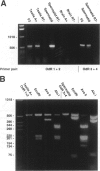
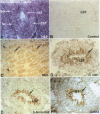
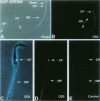
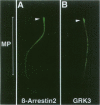
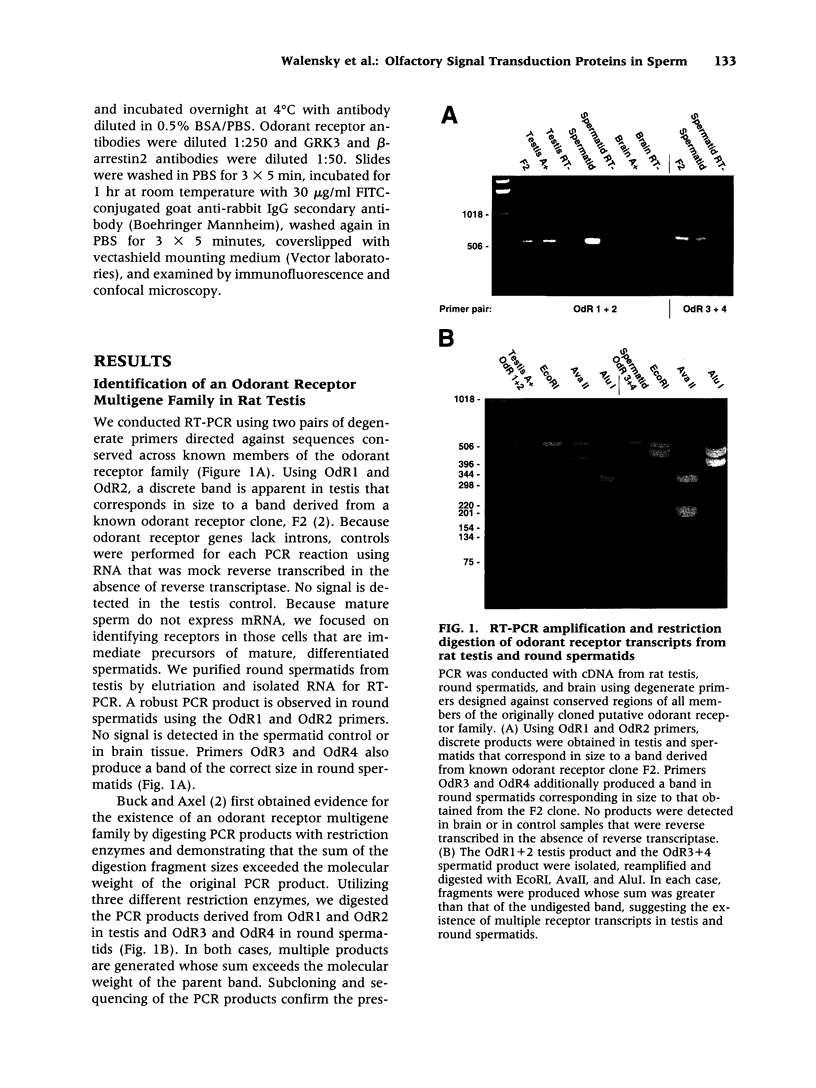
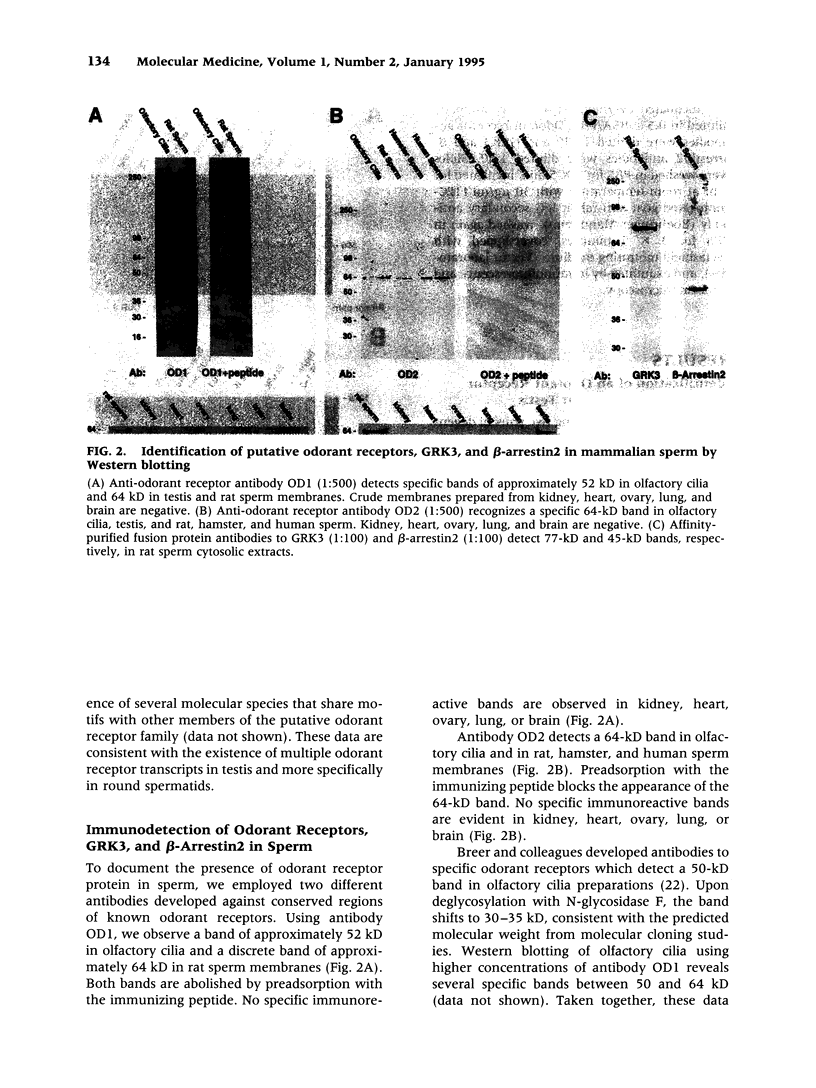
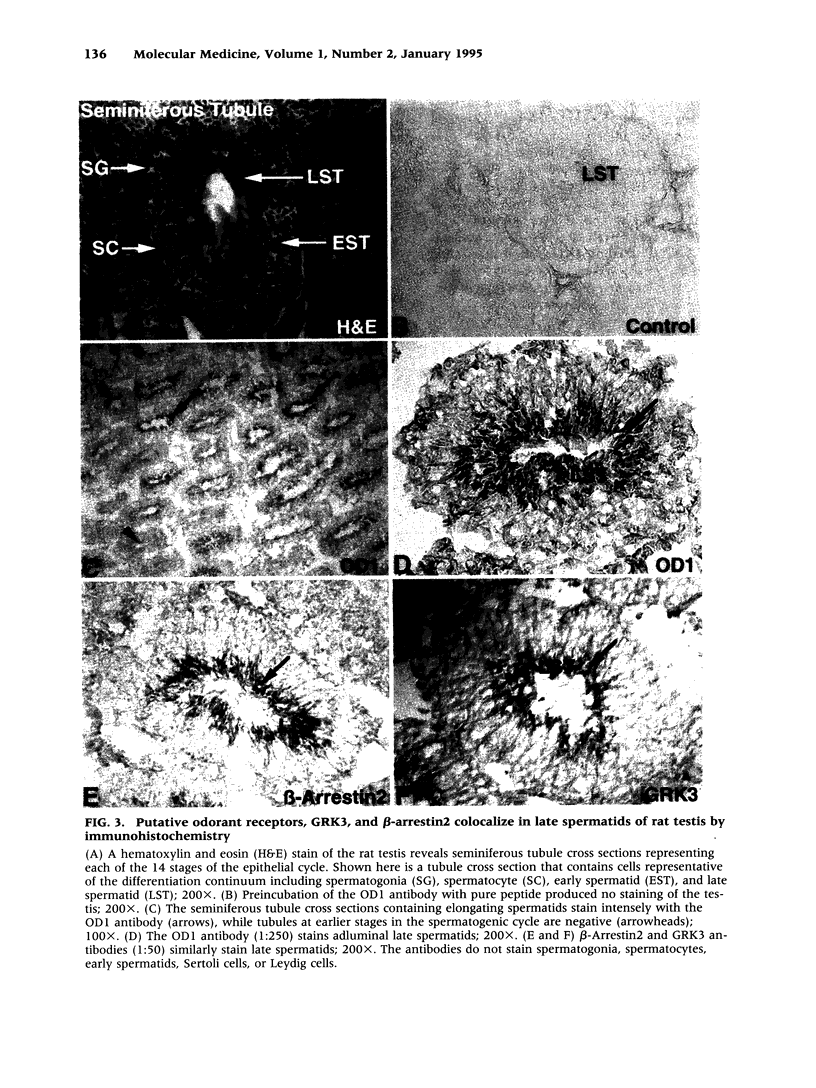
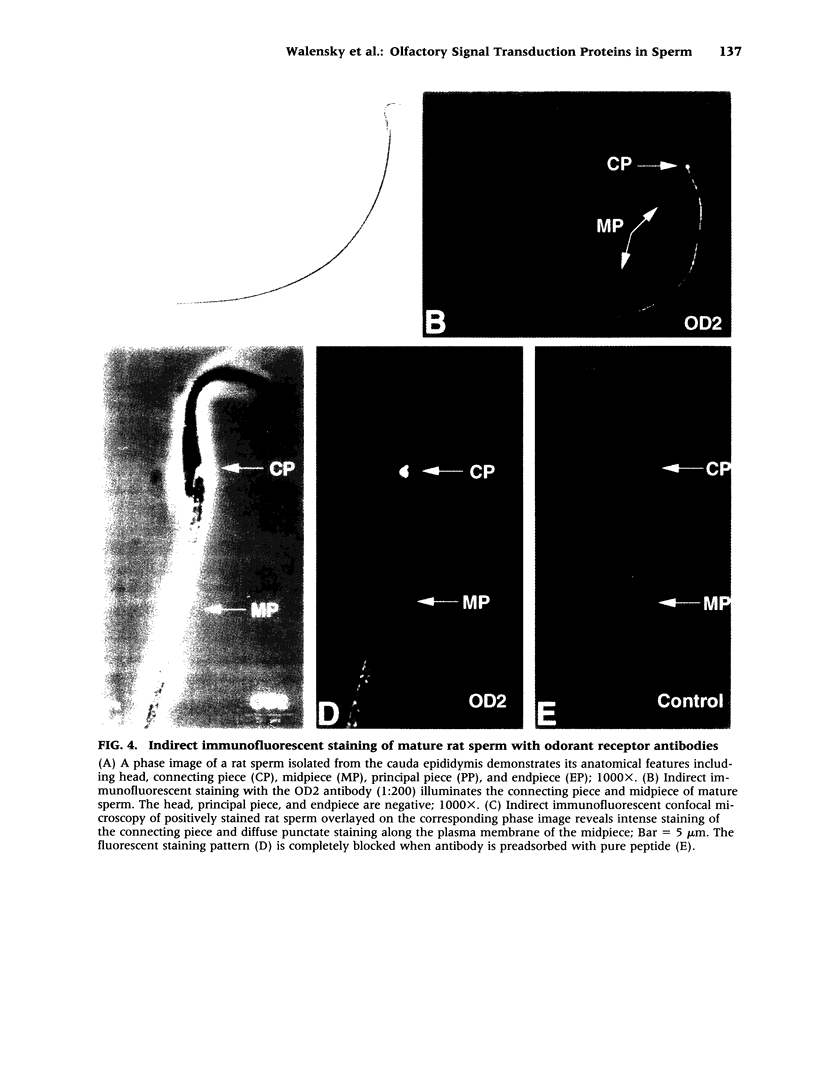
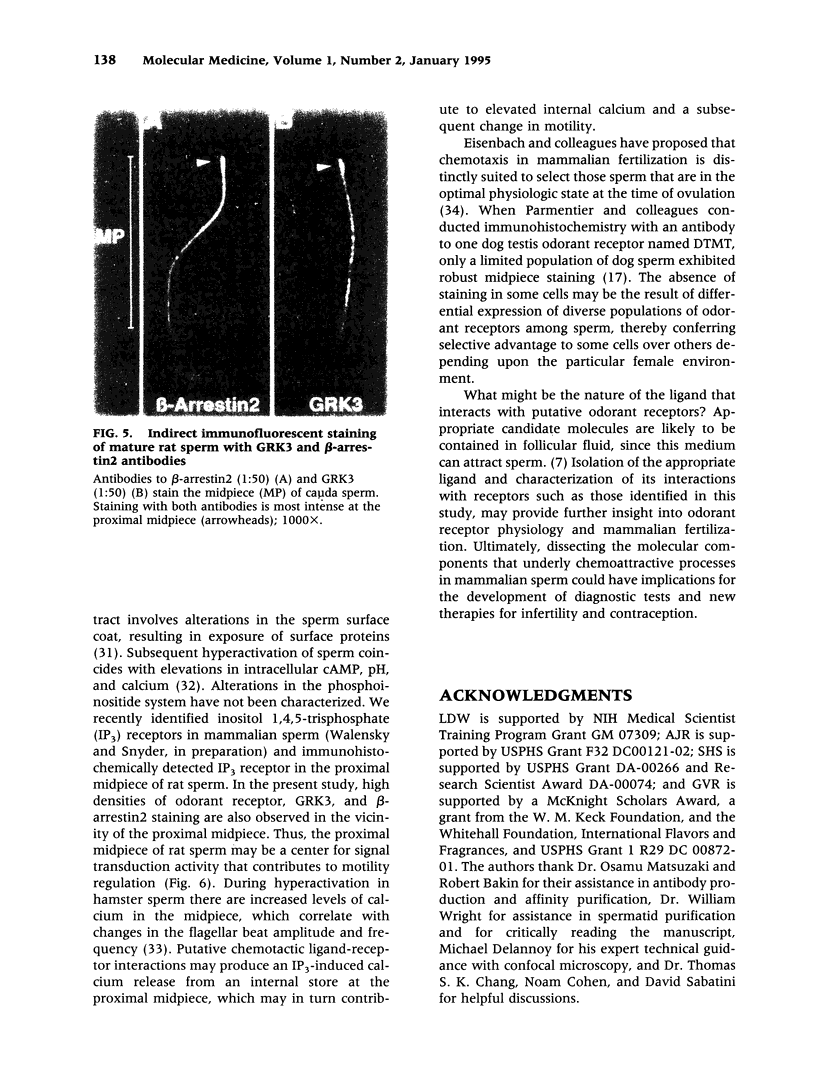
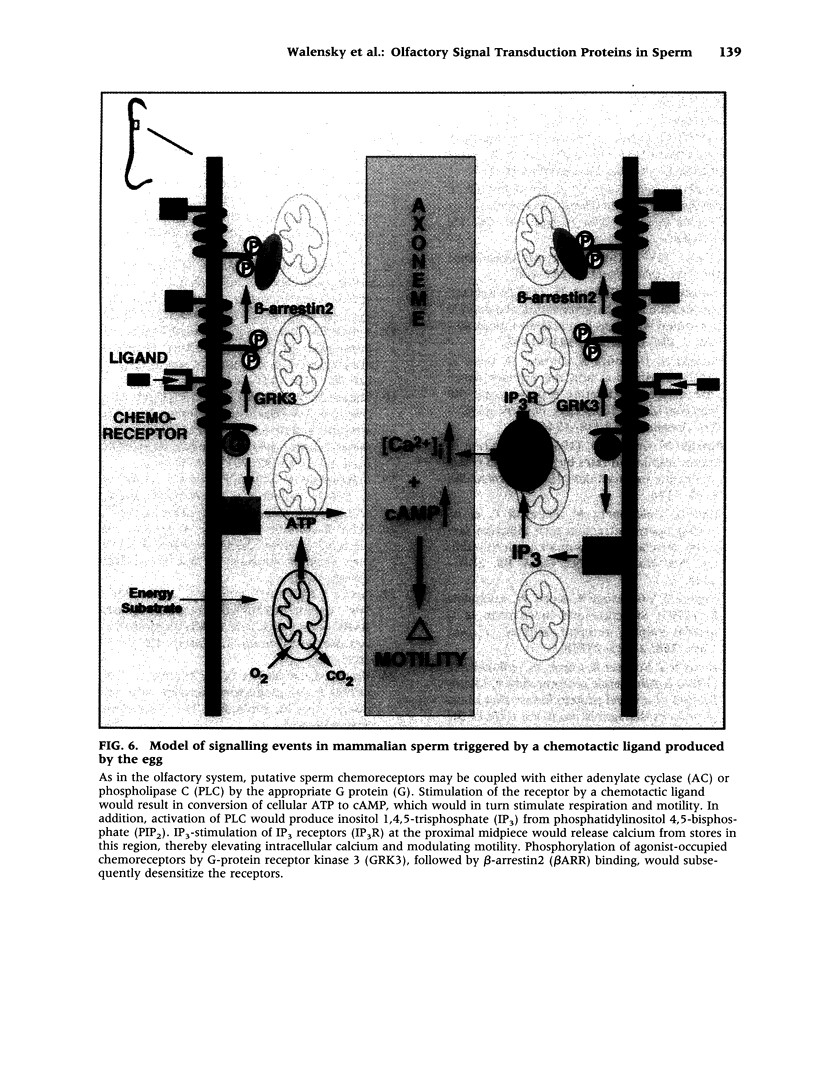
BACKGROUND: The identification of transcripts encoding putative olfactory receptors in mammalian germ cells (1) has generated the hypothesis that olfactory receptors may serve a chemosensory role in sperm chemotaxis during fertilization. We have sought to identify and localize these receptors and their regulatory machinery in rat sperm in order to gain further insight into mammalian sperm chemotaxis and odorant receptor physiology. MATERIALS AND METHODS: We conducted reverse transcription-polymerase chain reaction (RT-PCR) using degenerate primers directed against sequences conserved across members of the known odorant receptor family to identify transcripts from testis and round spermatids. Western analysis and immunohistochemistry were performed using antibodies raised against two peptide sequences conserved among odorant receptors and using fusion protein antibodies to G-protein receptor kinase 3 (GRK3/beta ARK2) and beta-arrestin2. RESULTS: We detected transcripts encoding putative odorant receptors in both testis and round spermatids of the adult rat. Restriction digests of the PCR products demonstrated the existence of multiple gene products. Two anti-odorant receptor antibodies specifically recognized a 64 kD band in rat sperm preparations by Western blot. The proteins GRK3 and beta-arrestin2, implicated in olfactory desensitization, were detected in sperm cytosolic extracts using Western analysis. Immunohistochemistry colocalized putative odorant receptors, GRK3 and beta-arrestin2 to elongating spermatids in the testis and to the midpiece of mature sperm. CONCLUSIONS: The specific localization of odorant receptors to the respiratory center of mature sperm is consistent with a role for these proteins in transducing chemotactic signals. Based on the colocalization, it is plausible that GRK3 and beta-arrestin2 function in sperm to regulate putative chemoreceptor responses.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arriza J. L., Dawson T. M., Simerly R. B., Martin L. J., Caron M. G., Snyder S. H., Lefkowitz R. J. The G-protein-coupled receptor kinases beta ARK1 and beta ARK2 are widely distributed at synapses in rat brain. J Neurosci. 1992 Oct;12(10):4045–4055. doi: 10.1523/JNEUROSCI.12-10-04045.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Attramadal H., Arriza J. L., Aoki C., Dawson T. M., Codina J., Kwatra M. M., Snyder S. H., Caron M. G., Lefkowitz R. J. Beta-arrestin2, a novel member of the arrestin/beta-arrestin gene family. J Biol Chem. 1992 Sep 5;267(25):17882–17890. [PubMed] [Google Scholar]
- Benovic J. L., Mayor F., Jr, Staniszewski C., Lefkowitz R. J., Caron M. G. Purification and characterization of the beta-adrenergic receptor kinase. J Biol Chem. 1987 Jul 5;262(19):9026–9032. [PubMed] [Google Scholar]
- Benovic J. L., Strasser R. H., Caron M. G., Lefkowitz R. J. Beta-adrenergic receptor kinase: identification of a novel protein kinase that phosphorylates the agonist-occupied form of the receptor. Proc Natl Acad Sci U S A. 1986 May;83(9):2797–2801. doi: 10.1073/pnas.83.9.2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bentley J. K., Shimomura H., Garbers D. L. Retention of a functional resact receptor in isolated sperm plasma membranes. Cell. 1986 Apr 25;45(2):281–288. doi: 10.1016/0092-8674(86)90392-2. [DOI] [PubMed] [Google Scholar]
- Bentley J. K., Tubb D. J., Garbers D. L. Receptor-mediated activation of spermatozoan guanylate cyclase. J Biol Chem. 1986 Nov 15;261(32):14859–14862. [PubMed] [Google Scholar]
- Breer H., Boekhoff I., Tareilus E. Rapid kinetics of second messenger formation in olfactory transduction. Nature. 1990 May 3;345(6270):65–68. doi: 10.1038/345065a0. [DOI] [PubMed] [Google Scholar]
- Buck L. B. The olfactory multigene family. Curr Opin Neurobiol. 1992 Jun;2(3):282–288. doi: 10.1016/0959-4388(92)90116-3. [DOI] [PubMed] [Google Scholar]
- Buck L., Axel R. A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell. 1991 Apr 5;65(1):175–187. doi: 10.1016/0092-8674(91)90418-x. [DOI] [PubMed] [Google Scholar]
- Dawson T. M., Arriza J. L., Jaworsky D. E., Borisy F. F., Attramadal H., Lefkowitz R. J., Ronnett G. V. Beta-adrenergic receptor kinase-2 and beta-arrestin-2 as mediators of odorant-induced desensitization. Science. 1993 Feb 5;259(5096):825–829. doi: 10.1126/science.8381559. [DOI] [PubMed] [Google Scholar]
- Dym M., Clermont Y. Role of spermatogonia in the repair of the seminiferous epithelium following x-irradiation of the rat testis. Am J Anat. 1970 Jul;128(3):265–282. doi: 10.1002/aja.1001280302. [DOI] [PubMed] [Google Scholar]
- Eisenbach M., Ralt D. Precontact mammalian sperm-egg communication and role in fertilization. Am J Physiol. 1992 May;262(5 Pt 1):C1095–C1101. doi: 10.1152/ajpcell.1992.262.5.C1095. [DOI] [PubMed] [Google Scholar]
- Hansbrough J. R., Garbers D. L. Speract. Purification and characterization of a peptide associated with eggs that activates spermatozoa. J Biol Chem. 1981 Feb 10;256(3):1447–1452. [PubMed] [Google Scholar]
- Hunter R. H. Human fertilization in vivo, with special reference to progression, storage and release of competent spermatozoa. Hum Reprod. 1987 May;2(4):329–332. doi: 10.1093/oxfordjournals.humrep.a136544. [DOI] [PubMed] [Google Scholar]
- Inglese J., Freedman N. J., Koch W. J., Lefkowitz R. J. Structure and mechanism of the G protein-coupled receptor kinases. J Biol Chem. 1993 Nov 15;268(32):23735–23738. [PubMed] [Google Scholar]
- Krieger J., Schleicher S., Strotmann J., Wanner I., Boekhoff I., Raming K., De Geus P., Breer H. Probing olfactory receptors with sequence-specific antibodies. Eur J Biochem. 1994 Feb 1;219(3):829–835. doi: 10.1111/j.1432-1033.1994.tb18564.x. [DOI] [PubMed] [Google Scholar]
- Lohse M. J., Benovic J. L., Codina J., Caron M. G., Lefkowitz R. J. beta-Arrestin: a protein that regulates beta-adrenergic receptor function. Science. 1990 Jun 22;248(4962):1547–1550. doi: 10.1126/science.2163110. [DOI] [PubMed] [Google Scholar]
- MacDonald R. J., Swift G. H., Przybyla A. E., Chirgwin J. M. Isolation of RNA using guanidinium salts. Methods Enzymol. 1987;152:219–227. doi: 10.1016/0076-6879(87)52023-7. [DOI] [PubMed] [Google Scholar]
- Pace U., Hanski E., Salomon Y., Lancet D. Odorant-sensitive adenylate cyclase may mediate olfactory reception. Nature. 1985 Jul 18;316(6025):255–258. doi: 10.1038/316255a0. [DOI] [PubMed] [Google Scholar]
- Parmentier M., Libert F., Schurmans S., Schiffmann S., Lefort A., Eggerickx D., Ledent C., Mollereau C., Gérard C., Perret J. Expression of members of the putative olfactory receptor gene family in mammalian germ cells. Nature. 1992 Jan 30;355(6359):453–455. doi: 10.1038/355453a0. [DOI] [PubMed] [Google Scholar]
- Ralt D., Goldenberg M., Fetterolf P., Thompson D., Dor J., Mashiach S., Garbers D. L., Eisenbach M. Sperm attraction to a follicular factor(s) correlates with human egg fertilizability. Proc Natl Acad Sci U S A. 1991 Apr 1;88(7):2840–2844. doi: 10.1073/pnas.88.7.2840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ronnett G. V., Parfitt D. J., Hester L. D., Snyder S. H. Odorant-sensitive adenylate cyclase: rapid, potent activation and desensitization in primary olfactory neuronal cultures. Proc Natl Acad Sci U S A. 1991 Mar 15;88(6):2366–2369. doi: 10.1073/pnas.88.6.2366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaper N. L., Wright W. W., Shaper J. H. Murine beta 1,4-galactosyltransferase: both the amounts and structure of the mRNA are regulated during spermatogenesis. Proc Natl Acad Sci U S A. 1990 Jan;87(2):791–795. doi: 10.1073/pnas.87.2.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shinohara T., Dietzschold B., Craft C. M., Wistow G., Early J. J., Donoso L. A., Horwitz J., Tao R. Primary and secondary structure of bovine retinal S antigen (48-kDa protein). Proc Natl Acad Sci U S A. 1987 Oct;84(20):6975–6979. doi: 10.1073/pnas.84.20.6975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sklar P. B., Anholt R. R., Snyder S. H. The odorant-sensitive adenylate cyclase of olfactory receptor cells. Differential stimulation by distinct classes of odorants. J Biol Chem. 1986 Nov 25;261(33):15538–15543. [PubMed] [Google Scholar]
- Suarez S. S., Varosi S. M., Dai X. Intracellular calcium increases with hyperactivation in intact, moving hamster sperm and oscillates with the flagellar beat cycle. Proc Natl Acad Sci U S A. 1993 May 15;90(10):4660–4664. doi: 10.1073/pnas.90.10.4660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Garbers D. L. Stimulation of sperm respiration rates by speract and resact at alkaline extracellular pH. Biol Reprod. 1984 Jun;30(5):1167–1174. doi: 10.1095/biolreprod30.5.1167. [DOI] [PubMed] [Google Scholar]
- Suzuki N., Nomura K., Ohtake H., Isaka S. Purification and the primary structure of sperm-activity peptides from the jelly coat of sea urchin eggs. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1238–1244. doi: 10.1016/0006-291x(81)90752-x. [DOI] [PubMed] [Google Scholar]
- Vanderhaeghen P., Schurmans S., Vassart G., Parmentier M. Olfactory receptors are displayed on dog mature sperm cells. J Cell Biol. 1993 Dec;123(6 Pt 1):1441–1452. doi: 10.1083/jcb.123.6.1441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward G. E., Brokaw C. J., Garbers D. L., Vacquier V. D. Chemotaxis of Arbacia punctulata spermatozoa to resact, a peptide from the egg jelly layer. J Cell Biol. 1985 Dec;101(6):2324–2329. doi: 10.1083/jcb.101.6.2324. [DOI] [PMC free article] [PubMed] [Google Scholar]