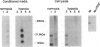Abstract
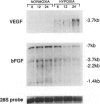
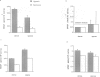
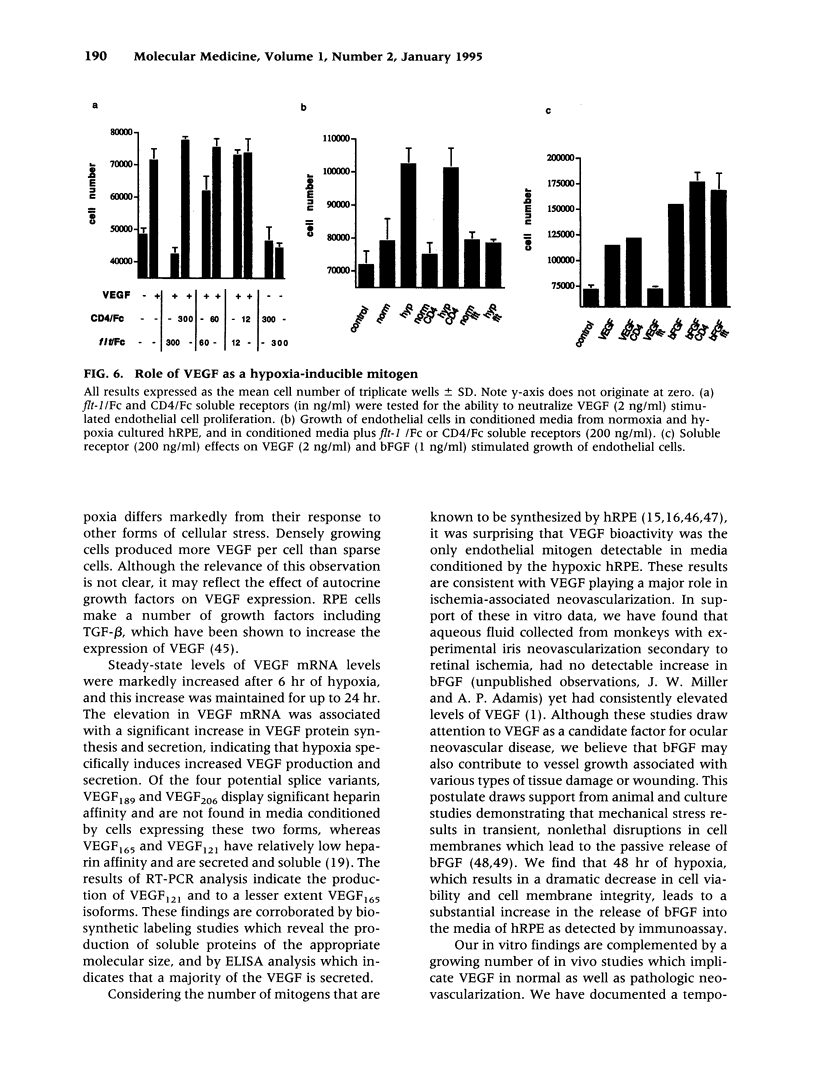
BACKGROUND: New vessel growth is often associated with ischemia, and hypoxic tissue has been identified as a potential source of angiogenic factors. In particular, ischemia is associated with the development of neovascularization in a number of ocular pathologies. For this reason, we have studied the induction of endothelial cell mitogens by hypoxia in retinal cells. MATERIALS AND METHODS: Human retinal pigment epithelium (hRPE) were grown under normoxic and hypoxic conditions and examined for the production of endothelial mitogens. Northern analysis, biosynthetic labeling and immunoprecipitation, and ELISA were used to assess the levels of vascular endothelial growth factor/vascular permeability factor (VEGF) and basic fibroblast growth factor (bFGF), two endothelial cell mitogens and potent angiogenic factors. Soluble receptors for VEGF were employed as competitive inhibitors to determine the contribution of the growth factor to the hypoxia-stimulated mitogen production. RESULTS: Following 6-24 hr of hypoxia, confluent and growing cultures of hRPE increase their levels of VEGF mRNA and protein synthesis. Biosynthetic labeling studies and RT-PCR analysis indicate that the cells secrete VEGF121 and VEGF165, the soluble forms of the angiogenic factor. In contrast, hRPE cultured under hypoxic conditions show reduced steady-state levels of basic fibroblast growth factor (bFGF) mRNA and decreased bFGF protein synthesis. Unlike VEGF, bFGF is not found in conditioned media of hRPE following 24 hr of hypoxia. Using a soluble high-affinity VEGF receptor as a competitive inhibitor of VEGF, we demonstrate that a VEGF-like activity is the sole hypoxia-inducible endothelial mitogen produced by cultured hRPE. CONCLUSIONS: From this comparison we conclude that hRPE do not respond to hypoxia with a general, nonspecific increase in the overall levels of growth factors, as is seen during cell wounding responses or serum stimulation. The physiological relevance of data from this in vitro model are affirmed by separate studies in an animal model of retinal ischemia-induced ocular neovascularization (1) in which retina-derived VEGF levels have been shown to correlate spatio-temporally with the onset of angiogenesis. Taken together, these data support the hypothesis that the induction of VEGF by hypoxia mediates the rapid, initial angiogenic response to retinal ischemia.
Full text
PDF







Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ASHTON N., WARD B., SERPELL G. Effect of oxygen on developing retinal vessels with particular reference to the problem of retrolental fibroplasia. Br J Ophthalmol. 1954 Jul;38(7):397–432. doi: 10.1136/bjo.38.7.397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adamis A. P., Miller J. W., Bernal M. T., D'Amico D. J., Folkman J., Yeo T. K., Yeo K. T. Increased vascular endothelial growth factor levels in the vitreous of eyes with proliferative diabetic retinopathy. Am J Ophthalmol. 1994 Oct 15;118(4):445–450. doi: 10.1016/s0002-9394(14)75794-0. [DOI] [PubMed] [Google Scholar]
- Adamis A. P., Shima D. T., Yeo K. T., Yeo T. K., Brown L. F., Berse B., D'Amore P. A., Folkman J. Synthesis and secretion of vascular permeability factor/vascular endothelial growth factor by human retinal pigment epithelial cells. Biochem Biophys Res Commun. 1993 Jun 15;193(2):631–638. doi: 10.1006/bbrc.1993.1671. [DOI] [PubMed] [Google Scholar]
- Bost L. M., Aotaki-Keen A. E., Hjelmeland L. M. Coexpression of FGF-5 and bFGF by the retinal pigment epithelium in vitro. Exp Eye Res. 1992 Nov;55(5):727–734. doi: 10.1016/0014-4835(92)90177-t. [DOI] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Dvorak H. F., Senger D. R. Increased expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in kidney and bladder carcinomas. Am J Pathol. 1993 Nov;143(5):1255–1262. [PMC free article] [PubMed] [Google Scholar]
- Brown L. F., Berse B., Jackman R. W., Tognazzi K., Manseau E. J., Senger D. R., Dvorak H. F. Expression of vascular permeability factor (vascular endothelial growth factor) and its receptors in adenocarcinomas of the gastrointestinal tract. Cancer Res. 1993 Oct 1;53(19):4727–4735. [PubMed] [Google Scholar]
- Campochiaro P. A. Cytokine production by retinal pigmented epithelial cells. Int Rev Cytol. 1993;146:75–82. doi: 10.1016/s0074-7696(08)60380-0. [DOI] [PubMed] [Google Scholar]
- Clauss M., Gerlach M., Gerlach H., Brett J., Wang F., Familletti P. C., Pan Y. C., Olander J. V., Connolly D. T., Stern D. Vascular permeability factor: a tumor-derived polypeptide that induces endothelial cell and monocyte procoagulant activity, and promotes monocyte migration. J Exp Med. 1990 Dec 1;172(6):1535–1545. doi: 10.1084/jem.172.6.1535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- D'Amore P. A. Modes of FGF release in vivo and in vitro. Cancer Metastasis Rev. 1990 Nov;9(3):227–238. doi: 10.1007/BF00046362. [DOI] [PubMed] [Google Scholar]
- Dvorak H. F., Sioussat T. M., Brown L. F., Berse B., Nagy J. A., Sotrel A., Manseau E. J., Van de Water L., Senger D. R. Distribution of vascular permeability factor (vascular endothelial growth factor) in tumors: concentration in tumor blood vessels. J Exp Med. 1991 Nov 1;174(5):1275–1278. doi: 10.1084/jem.174.5.1275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elner V. M., Strieter R. M., Elner S. G., Baggiolini M., Lindley I., Kunkel S. L. Neutrophil chemotactic factor (IL-8) gene expression by cytokine-treated retinal pigment epithelial cells. Am J Pathol. 1990 Apr;136(4):745–750. [PMC free article] [PubMed] [Google Scholar]
- Ferrara N., Houck K. A., Jakeman L. B., Winer J., Leung D. W. The vascular endothelial growth factor family of polypeptides. J Cell Biochem. 1991 Nov;47(3):211–218. doi: 10.1002/jcb.240470305. [DOI] [PubMed] [Google Scholar]
- Ferrara N., Leung D. W., Cachianes G., Winer J., Henzel W. J. Purification and cloning of vascular endothelial growth factor secreted by pituitary folliculostellate cells. Methods Enzymol. 1991;198:391–405. doi: 10.1016/0076-6879(91)98040-d. [DOI] [PubMed] [Google Scholar]
- Finklestein S. P., Apostolides P. J., Caday C. G., Prosser J., Philips M. F., Klagsbrun M. Increased basic fibroblast growth factor (bFGF) immunoreactivity at the site of focal brain wounds. Brain Res. 1988 Sep 20;460(2):253–259. doi: 10.1016/0006-8993(88)90370-8. [DOI] [PubMed] [Google Scholar]
- Goldberg M. A., Dunning S. P., Bunn H. F. Regulation of the erythropoietin gene: evidence that the oxygen sensor is a heme protein. Science. 1988 Dec 9;242(4884):1412–1415. doi: 10.1126/science.2849206. [DOI] [PubMed] [Google Scholar]
- Heacock C. S., Sutherland R. M. Enhanced synthesis of stress proteins caused by hypoxia and relation to altered cell growth and metabolism. Br J Cancer. 1990 Aug;62(2):217–225. doi: 10.1038/bjc.1990.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houck K. A., Ferrara N., Winer J., Cachianes G., Li B., Leung D. W. The vascular endothelial growth factor family: identification of a fourth molecular species and characterization of alternative splicing of RNA. Mol Endocrinol. 1991 Dec;5(12):1806–1814. doi: 10.1210/mend-5-12-1806. [DOI] [PubMed] [Google Scholar]
- Kandel J., Bossy-Wetzel E., Radvanyi F., Klagsbrun M., Folkman J., Hanahan D. Neovascularization is associated with a switch to the export of bFGF in the multistep development of fibrosarcoma. Cell. 1991 Sep 20;66(6):1095–1104. doi: 10.1016/0092-8674(91)90033-u. [DOI] [PubMed] [Google Scholar]
- Keck P. J., Hauser S. D., Krivi G., Sanzo K., Warren T., Feder J., Connolly D. T. Vascular permeability factor, an endothelial cell mitogen related to PDGF. Science. 1989 Dec 8;246(4935):1309–1312. doi: 10.1126/science.2479987. [DOI] [PubMed] [Google Scholar]
- Kendall R. L., Thomas K. A. Inhibition of vascular endothelial cell growth factor activity by an endogenously encoded soluble receptor. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10705–10709. doi: 10.1073/pnas.90.22.10705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim K. J., Li B., Winer J., Armanini M., Gillett N., Phillips H. S., Ferrara N. Inhibition of vascular endothelial growth factor-induced angiogenesis suppresses tumour growth in vivo. Nature. 1993 Apr 29;362(6423):841–844. doi: 10.1038/362841a0. [DOI] [PubMed] [Google Scholar]
- Kitaoka T., Bost L. M., Ishigooka H., Aotaki-Keen A. E., Hjelmeland L. M. Increasing cell density down-regulates the expression of acidic FGF by human RPE cells in vitro. Curr Eye Res. 1993 Nov;12(11):993–999. doi: 10.3109/02713689309029225. [DOI] [PubMed] [Google Scholar]
- Klagsbrun M., Soker S. VEGF/VPF: the angiogenesis factor found? Curr Biol. 1993 Oct 1;3(10):699–702. doi: 10.1016/0960-9822(93)90073-w. [DOI] [PubMed] [Google Scholar]
- Knighton D. R., Hunt T. K., Scheuenstuhl H., Halliday B. J., Werb Z., Banda M. J. Oxygen tension regulates the expression of angiogenesis factor by macrophages. Science. 1983 Sep 23;221(4617):1283–1285. doi: 10.1126/science.6612342. [DOI] [PubMed] [Google Scholar]
- Korte G. E., Reppucci V., Henkind P. RPE destruction causes choriocapillary atrophy. Invest Ophthalmol Vis Sci. 1984 Oct;25(10):1135–1145. [PubMed] [Google Scholar]
- Kostyk S. K., D'Amore P. A., Herman I. M., Wagner J. A. Optic nerve injury alters basic fibroblast growth factor localization in the retina and optic tract. J Neurosci. 1994 Mar;14(3 Pt 2):1441–1449. doi: 10.1523/JNEUROSCI.14-03-01441.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., Hannan R. L., Faller D. V. Oxygen tension regulates the expression of the platelet-derived growth factor-B chain gene in human endothelial cells. J Clin Invest. 1990 Aug;86(2):670–674. doi: 10.1172/JCI114759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kourembanas S., Marsden P. A., McQuillan L. P., Faller D. V. Hypoxia induces endothelin gene expression and secretion in cultured human endothelium. J Clin Invest. 1991 Sep;88(3):1054–1057. doi: 10.1172/JCI115367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung D. W., Cachianes G., Kuang W. J., Goeddel D. V., Ferrara N. Vascular endothelial growth factor is a secreted angiogenic mitogen. Science. 1989 Dec 8;246(4935):1306–1309. doi: 10.1126/science.2479986. [DOI] [PubMed] [Google Scholar]
- Li L. X., Turner J. E. Inherited retinal dystrophy in the RCS rat: prevention of photoreceptor degeneration by pigment epithelial cell transplantation. Exp Eye Res. 1988 Dec;47(6):911–917. doi: 10.1016/0014-4835(88)90073-5. [DOI] [PubMed] [Google Scholar]
- McAvoy J. W., Chamberlain C. G. Growth factors in the eye. Prog Growth Factor Res. 1990;2(1):29–43. doi: 10.1016/0955-2235(90)90008-8. [DOI] [PubMed] [Google Scholar]
- McNeil P. L. Cellular and molecular adaptations to injurious mechanical stress. Trends Cell Biol. 1993 Sep;3(9):302–307. doi: 10.1016/0962-8924(93)90012-p. [DOI] [PubMed] [Google Scholar]
- Miller J. W., Adamis A. P., Shima D. T., D'Amore P. A., Moulton R. S., O'Reilly M. S., Folkman J., Dvorak H. F., Brown L. F., Berse B. Vascular endothelial growth factor/vascular permeability factor is temporally and spatially correlated with ocular angiogenesis in a primate model. Am J Pathol. 1994 Sep;145(3):574–584. [PMC free article] [PubMed] [Google Scholar]
- Nguyen M., Watanabe H., Budson A. E., Richie J. P., Folkman J. Elevated levels of the angiogenic peptide basic fibroblast growth factor in urine of bladder cancer patients. J Natl Cancer Inst. 1993 Feb 3;85(3):241–242. doi: 10.1093/jnci/85.3.241. [DOI] [PubMed] [Google Scholar]
- Padua R. R., Kardami E. Increased basic fibroblast growth factor (bFGF) accumulation and distinct patterns of localization in isoproterenol-induced cardiomyocyte injury. Growth Factors. 1993;8(4):291–306. doi: 10.3109/08977199308991574. [DOI] [PubMed] [Google Scholar]
- Pepper M. S., Ferrara N., Orci L., Montesano R. Potent synergism between vascular endothelial growth factor and basic fibroblast growth factor in the induction of angiogenesis in vitro. Biochem Biophys Res Commun. 1992 Dec 15;189(2):824–831. doi: 10.1016/0006-291x(92)92277-5. [DOI] [PubMed] [Google Scholar]
- Pertovaara L., Kaipainen A., Mustonen T., Orpana A., Ferrara N., Saksela O., Alitalo K. Vascular endothelial growth factor is induced in response to transforming growth factor-beta in fibroblastic and epithelial cells. J Biol Chem. 1994 Mar 4;269(9):6271–6274. [PubMed] [Google Scholar]
- Plate K. H., Breier G., Weich H. A., Risau W. Vascular endothelial growth factor is a potential tumour angiogenesis factor in human gliomas in vivo. Nature. 1992 Oct 29;359(6398):845–848. doi: 10.1038/359845a0. [DOI] [PubMed] [Google Scholar]
- Sarks S. H. Council Lecture. Drusen and their relationship to senile macular degeneration. Aust J Ophthalmol. 1980 May;8(2):117–130. doi: 10.1111/j.1442-9071.1980.tb01670.x. [DOI] [PubMed] [Google Scholar]
- Seaton A. D., Turner J. E. RPE transplants stabilize retinal vasculature and prevent neovascularization in the RCS rat. Invest Ophthalmol Vis Sci. 1992 Jan;33(1):83–91. [PubMed] [Google Scholar]
- Senger D. R., Galli S. J., Dvorak A. M., Perruzzi C. A., Harvey V. S., Dvorak H. F. Tumor cells secrete a vascular permeability factor that promotes accumulation of ascites fluid. Science. 1983 Feb 25;219(4587):983–985. doi: 10.1126/science.6823562. [DOI] [PubMed] [Google Scholar]
- Shing Y., Folkman J., Sullivan R., Butterfield C., Murray J., Klagsbrun M. Heparin affinity: purification of a tumor-derived capillary endothelial cell growth factor. Science. 1984 Mar 23;223(4642):1296–1299. doi: 10.1126/science.6199844. [DOI] [PubMed] [Google Scholar]
- Shreeniwas R., Ogawa S., Cozzolino F., Torcia G., Braunstein N., Butura C., Brett J., Lieberman H. B., Furie M. B., Joseph-Silverstein J. Macrovascular and microvascular endothelium during long-term hypoxia: alterations in cell growth, monolayer permeability, and cell surface coagulant properties. J Cell Physiol. 1991 Jan;146(1):8–17. doi: 10.1002/jcp.1041460103. [DOI] [PubMed] [Google Scholar]
- Shweiki D., Itin A., Soffer D., Keshet E. Vascular endothelial growth factor induced by hypoxia may mediate hypoxia-initiated angiogenesis. Nature. 1992 Oct 29;359(6398):843–845. doi: 10.1038/359843a0. [DOI] [PubMed] [Google Scholar]
- Sivalingam A., Kenney J., Brown G. C., Benson W. E., Donoso L. Basic fibroblast growth factor levels in the vitreous of patients with proliferative diabetic retinopathy. Arch Ophthalmol. 1990 Jun;108(6):869–872. doi: 10.1001/archopht.1990.01070080113046. [DOI] [PubMed] [Google Scholar]
- Sternfeld M. D., Robertson J. E., Shipley G. D., Tsai J., Rosenbaum J. T. Cultured human retinal pigment epithelial cells express basic fibroblast growth factor and its receptor. Curr Eye Res. 1989 Oct;8(10):1029–1037. doi: 10.3109/02713688908997395. [DOI] [PubMed] [Google Scholar]
- Watanabe H., Hori A., Seno M., Kozai Y., Igarashi K., Ichimori Y., Kondo K. A sensitive enzyme immunoassay for human basic fibroblast growth factor. Biochem Biophys Res Commun. 1991 Feb 28;175(1):229–235. doi: 10.1016/s0006-291x(05)81224-0. [DOI] [PubMed] [Google Scholar]
- Yeo K. T., Sioussat T. M., Faix J. D., Senger D. R., Yeo T. K. Development of time-resolved immunofluorometric assay of vascular permeability factor. Clin Chem. 1992 Jan;38(1):71–75. [PubMed] [Google Scholar]
- Yeo K. T., Wang H. H., Nagy J. A., Sioussat T. M., Ledbetter S. R., Hoogewerf A. J., Zhou Y., Masse E. M., Senger D. R., Dvorak H. F. Vascular permeability factor (vascular endothelial growth factor) in guinea pig and human tumor and inflammatory effusions. Cancer Res. 1993 Jun 15;53(12):2912–2918. [PubMed] [Google Scholar]
- Yeo T. K., Senger D. R., Dvorak H. F., Freter L., Yeo K. T. Glycosylation is essential for efficient secretion but not for permeability-enhancing activity of vascular permeability factor (vascular endothelial growth factor). Biochem Biophys Res Commun. 1991 Sep 30;179(3):1568–1575. doi: 10.1016/0006-291x(91)91752-x. [DOI] [PubMed] [Google Scholar]