Abstract
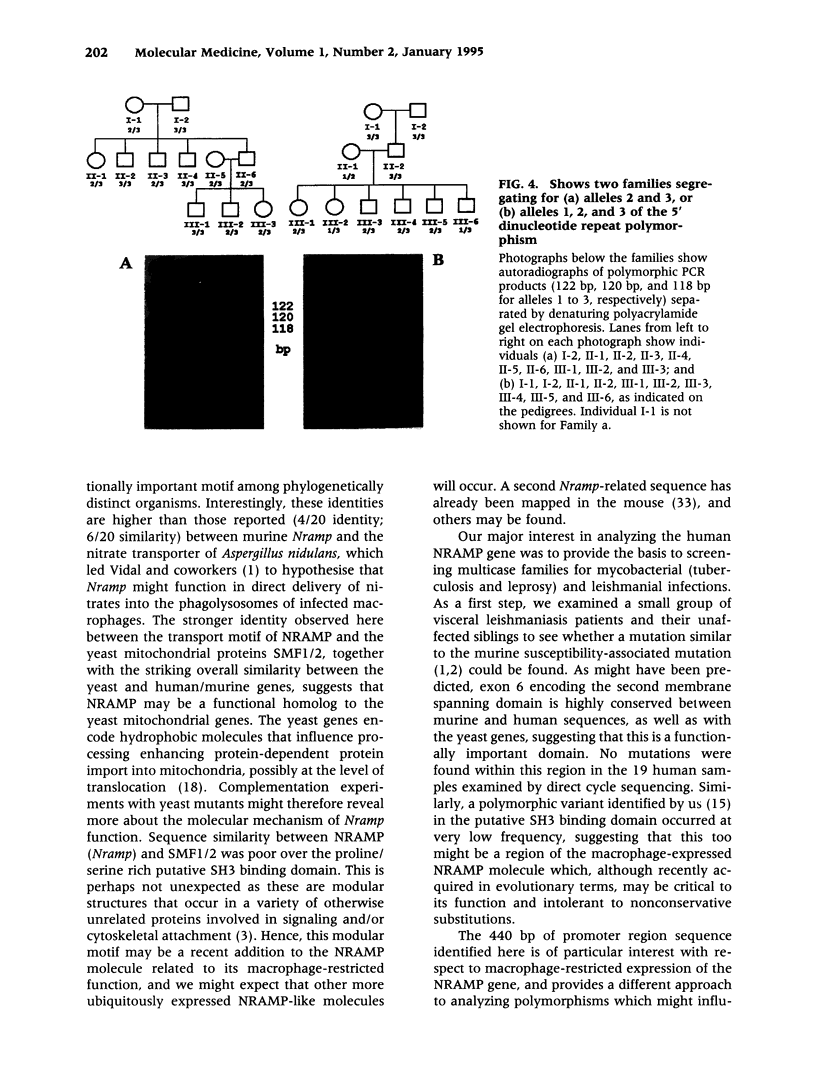
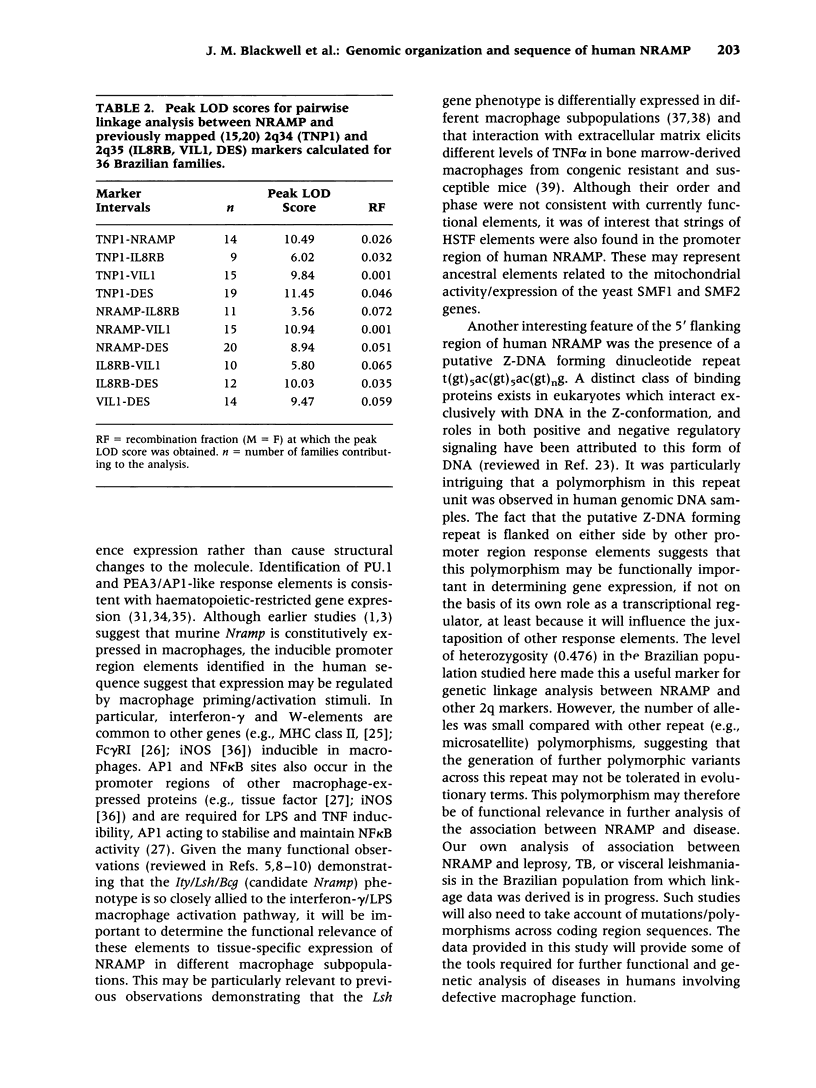
BACKGROUND: Murine Nramp is a candidate for the macrophage resistance gene Ity/Lsh/Bcg. Sequence analysis of human NRAMP was undertaken to determine its role in man. MATERIALS AND METHODS: A yeast artificial chromosome carrying NRAMP was subcloned and positive clones sequenced. The transcriptional start site was mapped using 5' RACE PCR. Polymorphic variants were amplified by PCR. Linkage analysis was used to map NRAMP. RESULTS: NRAMP spans 12kb and has 15 exons encoding a 550 amino acid protein showing 85% identity (92% similarity) with Nramp. Two conserved PKC sites occur in exon 2 encoding the Pro/Ser rich SH3 binding domain, and in exon 3. Striking sequence similarities (57 and 53%) were observed with yeast mitochondrial proteins, SMF1 and SMF2, especially within putative functional domains: exon 6 encoding the second transmembrane spanning domain, site of the murine susceptibility mutation; and exon 11 encoding a conserved transport motif. No mutations comparable to the murine susceptibility mutation were found. The transcriptional initiation site mapped 148 bp 5' of the translational initiation codon. 440bp of 5' flanking sequence contained putative promoter region elements: 6 interferon-gamma response elements, 3 W-elements, 3 NF kappa B binding sites and 1 AP-1 site. Nine purine-rich GGAA core motifs for the myeloid-specific PU.1 transcription factor were identified, two combining with imperfect AP1-like sites to create PEA3 motifs. TATA, GC and CCAAT boxes were absent. A possible enhancer element containing the Z-DNA forming dinucleotide repeat t(gt),ac(gt),ac(gt),g was polymorphic (4 alleles; n = 4,9,10,11), and was used to map NRAMP to 2q35. CONCLUSIONS: This analysis provides important resources to study the role of NRAMP in human disease.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barton C. H., White J. K., Roach T. I., Blackwell J. M. NH2-terminal sequence of macrophage-expressed natural resistance-associated macrophage protein (Nramp) encodes a proline/serine-rich putative Src homology 3-binding domain. J Exp Med. 1994 May 1;179(5):1683–1687. doi: 10.1084/jem.179.5.1683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blackwell J. M., Barton C. H., White J. K., Roach T. I., Shaw M. A., Whitehead S. H., Mock B. A., Searle S., Williams H., Baker A. M. Genetic regulation of leishmanial and mycobacterial infections: the Lsh/Ity/Bcg gene story continues. Immunol Lett. 1994 Dec;43(1-2):99–107. doi: 10.1016/0165-2478(94)00161-8. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M., Roach T. I., Atkinson S. E., Ajioka J. W., Barton C. H., Shaw M. A. Genetic regulation of macrophage priming/activation: the Lsh gene story. Immunol Lett. 1991 Oct;30(2):241–248. doi: 10.1016/0165-2478(91)90032-6. [DOI] [PubMed] [Google Scholar]
- Blackwell J. M. The macrophage resistance gene Lsh/Ity/Bcg. Res Immunol. 1989 Oct;140(8):767–769. [PubMed] [Google Scholar]
- Blackwell J. M., Toole S., King M., Dawda P., Roach T. I., Cooper A. Analysis of Lsh gene expression in congenic B10.L-Lshr mice. Curr Top Microbiol Immunol. 1988;137:301–309. doi: 10.1007/978-3-642-50059-6_45. [DOI] [PubMed] [Google Scholar]
- Boguski M. S., Lowe T. M., Tolstoshev C. M. dbEST--database for "expressed sequence tags". Nat Genet. 1993 Aug;4(4):332–333. doi: 10.1038/ng0893-332. [DOI] [PubMed] [Google Scholar]
- Cassady A. I., Stacey K. J., Nimmo K. A., Murphy K. M., von der Ahe D., Pearson D., Botteri F. M., Nagamine Y., Hume D. A. Constitutive expression of the urokinase plasminogen activator gene in murine RAW264 macrophages involves distal and 5' non-coding sequences that are conserved between mouse and pig. Nucleic Acids Res. 1991 Dec 25;19(24):6839–6847. doi: 10.1093/nar/19.24.6839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crocker P. R., Davies E. V., Blackwell J. M. Variable expression of the murine natural resistance gene Lsh in different macrophage populations infected in vitro with Leishmania donovani. Parasite Immunol. 1987 Nov;9(6):705–719. doi: 10.1111/j.1365-3024.1987.tb00540.x. [DOI] [PubMed] [Google Scholar]
- Davies E. V., Singleton A. M., Blackwell J. M. Differences in Lsh gene control over systemic Leishmania major and Leishmania donovani or Leishmania mexicana mexicana infections are caused by differential targeting to infiltrating and resident liver macrophage populations. Infect Immun. 1988 May;56(5):1128–1134. doi: 10.1128/iai.56.5.1128-1134.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dosik J. K., Barton C. H., Holiday D. L., Krall M. M., Blackwell J. M., Mock B. A. An Nramp-related sequence maps to mouse chromosome 17. Mamm Genome. 1994 Jul;5(7):458–460. doi: 10.1007/BF00357010. [DOI] [PubMed] [Google Scholar]
- Edwards J. B., Delort J., Mallet J. Oligodeoxyribonucleotide ligation to single-stranded cDNAs: a new tool for cloning 5' ends of mRNAs and for constructing cDNA libraries by in vitro amplification. Nucleic Acids Res. 1991 Oct 11;19(19):5227–5232. doi: 10.1093/nar/19.19.5227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feinman R., Qiu W. Q., Pearse R. N., Nikolajczyk B. S., Sen R., Sheffery M., Ravetch J. V. PU.1 and an HLH family member contribute to the myeloid-specific transcription of the Fc gamma RIIIA promoter. EMBO J. 1994 Aug 15;13(16):3852–3860. doi: 10.1002/j.1460-2075.1994.tb06696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Finan P., Shimizu Y., Gout I., Hsuan J., Truong O., Butcher C., Bennett P., Waterfield M. D., Kellie S. An SH3 domain and proline-rich sequence mediate an interaction between two components of the phagocyte NADPH oxidase complex. J Biol Chem. 1994 May 13;269(19):13752–13755. [PubMed] [Google Scholar]
- Formica S., Roach T. I., Blackwell J. M. Interaction with extracellular matrix proteins influences Lsh/Ity/Bcg (candidate Nramp) gene regulation of macrophage priming/activation for tumour necrosis factor-alpha and nitrite release. Immunology. 1994 May;82(1):42–50. [PMC free article] [PubMed] [Google Scholar]
- Hamada H., Kakunaga T. Potential Z-DNA forming sequences are highly dispersed in the human genome. Nature. 1982 Jul 22;298(5872):396–398. doi: 10.1038/298396a0. [DOI] [PubMed] [Google Scholar]
- Higgins D. G., Sharp P. M. CLUSTAL: a package for performing multiple sequence alignment on a microcomputer. Gene. 1988 Dec 15;73(1):237–244. doi: 10.1016/0378-1119(88)90330-7. [DOI] [PubMed] [Google Scholar]
- Karim F. D., Urness L. D., Thummel C. S., Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A., Gunther C. V., Nye J. A. The ETS-domain: a new DNA-binding motif that recognizes a purine-rich core DNA sequence. Genes Dev. 1990 Sep;4(9):1451–1453. doi: 10.1101/gad.4.9.1451. [DOI] [PubMed] [Google Scholar]
- Kaye P. M., Blackwell J. M. Lsh, antigen presentation and the development of CMI. Res Immunol. 1989 Oct;140(8):810–822. doi: 10.1016/0923-2494(89)90038-2. [DOI] [PubMed] [Google Scholar]
- Klemsz M. J., McKercher S. R., Celada A., Van Beveren C., Maki R. A. The macrophage and B cell-specific transcription factor PU.1 is related to the ets oncogene. Cell. 1990 Apr 6;61(1):113–124. doi: 10.1016/0092-8674(90)90219-5. [DOI] [PubMed] [Google Scholar]
- Kozak M. Point mutations define a sequence flanking the AUG initiator codon that modulates translation by eukaryotic ribosomes. Cell. 1986 Jan 31;44(2):283–292. doi: 10.1016/0092-8674(86)90762-2. [DOI] [PubMed] [Google Scholar]
- Larin Z., Monaco A. P., Lehrach H. Yeast artificial chromosome libraries containing large inserts from mouse and human DNA. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4123–4127. doi: 10.1073/pnas.88.10.4123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenardo M. J., Baltimore D. NF-kappa B: a pleiotropic mediator of inducible and tissue-specific gene control. Cell. 1989 Jul 28;58(2):227–229. doi: 10.1016/0092-8674(89)90833-7. [DOI] [PubMed] [Google Scholar]
- Mackman N., Brand K., Edgington T. S. Lipopolysaccharide-mediated transcriptional activation of the human tissue factor gene in THP-1 monocytic cells requires both activator protein 1 and nuclear factor kappa B binding sites. J Exp Med. 1991 Dec 1;174(6):1517–1526. doi: 10.1084/jem.174.6.1517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malo D., Vogan K., Vidal S., Hu J., Cellier M., Schurr E., Fuks A., Bumstead N., Morgan K., Gros P. Haplotype mapping and sequence analysis of the mouse Nramp gene predict susceptibility to infection with intracellular parasites. Genomics. 1994 Sep 1;23(1):51–61. doi: 10.1006/geno.1994.1458. [DOI] [PubMed] [Google Scholar]
- Pearse R. N., Feinman R., Ravetch J. V. Characterization of the promoter of the human gene encoding the high-affinity IgG receptor: transcriptional induction by gamma-interferon is mediated through common DNA response elements. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11305–11309. doi: 10.1073/pnas.88.24.11305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pringault E., Arpin M., Garcia A., Finidori J., Louvard D. A human villin cDNA clone to investigate the differentiation of intestinal and kidney cells in vivo and in culture. EMBO J. 1986 Dec 1;5(12):3119–3124. doi: 10.1002/j.1460-2075.1986.tb04618.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rich A., Nordheim A., Wang A. H. The chemistry and biology of left-handed Z-DNA. Annu Rev Biochem. 1984;53:791–846. doi: 10.1146/annurev.bi.53.070184.004043. [DOI] [PubMed] [Google Scholar]
- Schneemann M., Schoedon G., Hofer S., Blau N., Guerrero L., Schaffner A. Nitric oxide synthase is not a constituent of the antimicrobial armature of human mononuclear phagocytes. J Infect Dis. 1993 Jun;167(6):1358–1363. doi: 10.1093/infdis/167.6.1358. [DOI] [PubMed] [Google Scholar]
- Schurr E., Malo D., Radzioch D., Buschman E., Morgan K., Gros P., Skamene E. Genetic control of innate resistance to mycobacterial infections. Immunol Today. 1991 Mar;12(3):A42–A45. doi: 10.1016/S0167-5699(05)80012-X. [DOI] [PubMed] [Google Scholar]
- Schurr E., Radzioch D., Malo D., Gros P., Skamene E. Molecular genetics of inherited susceptibility to intracellular parasites. Behring Inst Mitt. 1991 Feb;(88):1–12. [PubMed] [Google Scholar]
- Shaw M. A., Atkinson S., Dockrell H., Hussain R., Lins-Lainson Z., Shaw J., Ramos F., Silveira F., Mehdi S. Q., Kaukab F. An RFLP map for 2q33-q37 from multicase mycobacterial and leishmanial disease families: no evidence for an Lsh/Ity/Bcg gene homologue influencing susceptibility to leprosy. Ann Hum Genet. 1993 Oct;57(Pt 4):251–271. doi: 10.1111/j.1469-1809.1993.tb00899.x. [DOI] [PubMed] [Google Scholar]
- Tsuchiya S., Yamabe M., Yamaguchi Y., Kobayashi Y., Konno T., Tada K. Establishment and characterization of a human acute monocytic leukemia cell line (THP-1). Int J Cancer. 1980 Aug;26(2):171–176. doi: 10.1002/ijc.2910260208. [DOI] [PubMed] [Google Scholar]
- Vidal S. M., Malo D., Vogan K., Skamene E., Gros P. Natural resistance to infection with intracellular parasites: isolation of a candidate for Bcg. Cell. 1993 May 7;73(3):469–485. doi: 10.1016/0092-8674(93)90135-d. [DOI] [PubMed] [Google Scholar]
- West A. H., Clark D. J., Martin J., Neupert W., Hartl F. U., Horwich A. L. Two related genes encoding extremely hydrophobic proteins suppress a lethal mutation in the yeast mitochondrial processing enhancing protein. J Biol Chem. 1992 Dec 5;267(34):24625–24633. [PubMed] [Google Scholar]
- White J. K., Shaw M. A., Barton C. H., Cerretti D. P., Williams H., Mock B. A., Carter N. P., Peacock C. S., Blackwell J. M. Genetic and physical mapping of 2q35 in the region of the NRAMP and IL8R genes: identification of a polymorphic repeat in exon 2 of NRAMP. Genomics. 1994 Nov 15;24(2):295–302. doi: 10.1006/geno.1994.1619. [DOI] [PubMed] [Google Scholar]
- Xie Q. W., Whisnant R., Nathan C. Promoter of the mouse gene encoding calcium-independent nitric oxide synthase confers inducibility by interferon gamma and bacterial lipopolysaccharide. J Exp Med. 1993 Jun 1;177(6):1779–1784. doi: 10.1084/jem.177.6.1779. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Sugawara M., Ponath P. D., Wessendorf L., Banerji J., Li Y., Strominger J. L. Interferon gamma response region in the promoter of the human DPA gene. Proc Natl Acad Sci U S A. 1990 Dec;87(23):9226–9230. doi: 10.1073/pnas.87.23.9226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang D. E., Hetherington C. J., Chen H. M., Tenen D. G. The macrophage transcription factor PU.1 directs tissue-specific expression of the macrophage colony-stimulating factor receptor. Mol Cell Biol. 1994 Jan;14(1):373–381. doi: 10.1128/mcb.14.1.373. [DOI] [PMC free article] [PubMed] [Google Scholar]




