Abstract
BACKGROUND: Several immunotoxins in which antibodies are coupled to plant or bacterial toxins are now in clinical trials for the treatment of cancer. One of these is B3-LysPE38 in which MAb B3 which reacts with many human cancers, is coupled with a genetically modified form of Pseudomonas exotoxin (PE). MATERIALS AND METHODS: To investigate how cells can become resistant to PE-derived immunotoxins, we constructed an immunotoxin-sensitive MCF-7 breast cancer cell line that contains SV40 T antigen and allows episomal replication of SV40 origin containing plasmids. We transfected a pCDM8/HeLa cDNA expression library into these cells, thereby causing over-expression of the plasmid-encoded genes. The transfected cells were treated with immunotoxin to select for resistance-mediating plasmids, which were reisolated from these cells and amplified in Escherichia coli. The resulting plasmid pool was transfected into cells for two further rounds of selection and plasmid reisolation. RESULTS: Several plasmids that caused immunotoxin resistance were enriched by this selection procedure. Four plasmids were stably transfected into MCF-7 cells and found to increase their resistance to PE-derived immunotoxins by 5- to 20-fold. These plasmids also confer resistance to native PE and to diphtheria toxin but not to ricin or cycloheximide. Thus, they appear to specifically interfere with the action of ADP-ribosylating toxins. CONCLUSION: Cancer cells can become resistant to immunotoxins by deregulated expression of normal genes. The clinical significance of this type of resistance will be evaluated in clinical trials.
Full text
PDF


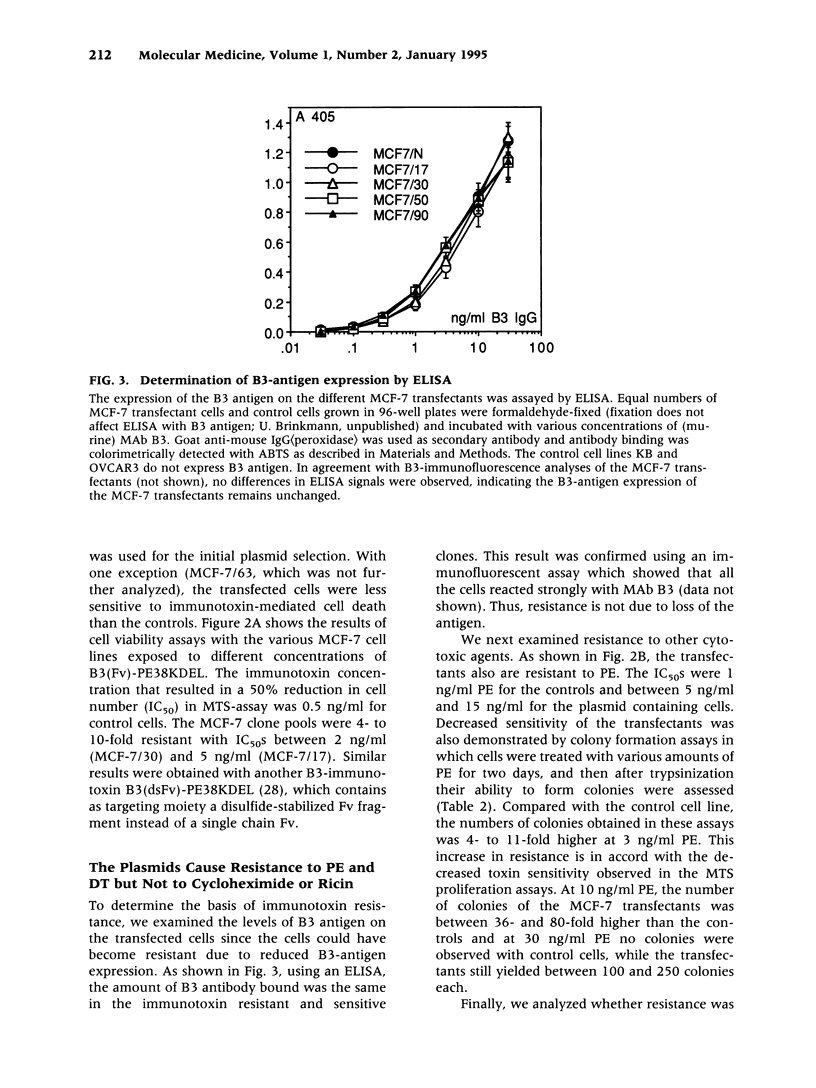
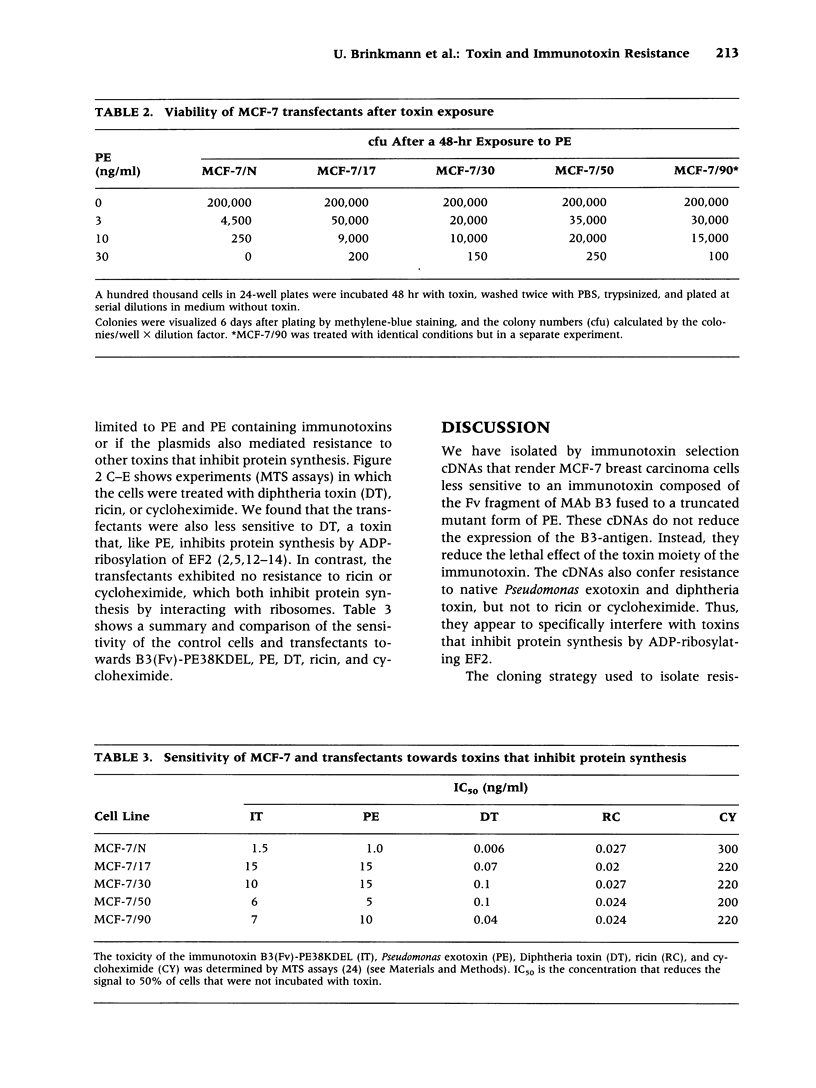



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brinkmann U., Pai L. H., FitzGerald D. J., Willingham M., Pastan I. B3(Fv)-PE38KDEL, a single-chain immunotoxin that causes complete regression of a human carcinoma in mice. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8616–8620. doi: 10.1073/pnas.88.19.8616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann U., Pastan I. Immunotoxins against cancer. Biochim Biophys Acta. 1994 May 27;1198(1):27–45. doi: 10.1016/0304-419x(94)90004-3. [DOI] [PubMed] [Google Scholar]
- Brinkmann U., Reiter Y., Jung S. H., Lee B., Pastan I. A recombinant immunotoxin containing a disulfide-stabilized Fv fragment. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7538–7542. doi: 10.1073/pnas.90.16.7538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buchner J., Pastan I., Brinkmann U. A method for increasing the yield of properly folded recombinant fusion proteins: single-chain immunotoxins from renaturation of bacterial inclusion bodies. Anal Biochem. 1992 Sep;205(2):263–270. doi: 10.1016/0003-2697(92)90433-8. [DOI] [PubMed] [Google Scholar]
- Chan A. M., Miki T., Meyers K. A., Aaronson S. A. A human oncogene of the RAS superfamily unmasked by expression cDNA cloning. Proc Natl Acad Sci U S A. 1994 Aug 2;91(16):7558–7562. doi: 10.1073/pnas.91.16.7558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaudhary V. K., Jinno Y., FitzGerald D., Pastan I. Pseudomonas exotoxin contains a specific sequence at the carboxyl terminus that is required for cytotoxicity. Proc Natl Acad Sci U S A. 1990 Jan;87(1):308–312. doi: 10.1073/pnas.87.1.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cory A. H., Owen T. C., Barltrop J. A., Cory J. G. Use of an aqueous soluble tetrazolium/formazan assay for cell growth assays in culture. Cancer Commun. 1991 Jul;3(7):207–212. doi: 10.3727/095535491820873191. [DOI] [PubMed] [Google Scholar]
- Endicott J. A., Ling V. The biochemistry of P-glycoprotein-mediated multidrug resistance. Annu Rev Biochem. 1989;58:137–171. doi: 10.1146/annurev.bi.58.070189.001033. [DOI] [PubMed] [Google Scholar]
- Fendrick J. L., Iglewski W. J., Moehring J. M., Moehring T. J. Characterization of the endogenous ADP-ribosylation of wild-type and mutant elongation factor 2 in eukaryotic cells. Eur J Biochem. 1992 Apr 1;205(1):25–31. doi: 10.1111/j.1432-1033.1992.tb16748.x. [DOI] [PubMed] [Google Scholar]
- Goldstein L. J., Galski H., Fojo A., Willingham M., Lai S. L., Gazdar A., Pirker R., Green A., Crist W., Brodeur G. M. Expression of a multidrug resistance gene in human cancers. J Natl Cancer Inst. 1989 Jan 18;81(2):116–124. doi: 10.1093/jnci/81.2.116. [DOI] [PubMed] [Google Scholar]
- Gottesman M. M., Pastan I. Biochemistry of multidrug resistance mediated by the multidrug transporter. Annu Rev Biochem. 1993;62:385–427. doi: 10.1146/annurev.bi.62.070193.002125. [DOI] [PubMed] [Google Scholar]
- Gudkov A. V., Kazarov A. R., Thimmapaya R., Axenovich S. A., Mazo I. A., Roninson I. B. Cloning mammalian genes by expression selection of genetic suppressor elements: association of kinesin with drug resistance and cell immortalization. Proc Natl Acad Sci U S A. 1994 Apr 26;91(9):3744–3748. doi: 10.1073/pnas.91.9.3744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gudkov A. V., Zelnick C. R., Kazarov A. R., Thimmapaya R., Suttle D. P., Beck W. T., Roninson I. B. Isolation of genetic suppressor elements, inducing resistance to topoisomerase II-interactive cytotoxic drugs, from human topoisomerase II cDNA. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3231–3235. doi: 10.1073/pnas.90.8.3231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirt B. Selective extraction of polyoma DNA from infected mouse cell cultures. J Mol Biol. 1967 Jun 14;26(2):365–369. doi: 10.1016/0022-2836(67)90307-5. [DOI] [PubMed] [Google Scholar]
- Hwang J., Richert N., Pastan I., Gottesman M. M. Mutant KB cells with decreased EGF receptor expression: biochemical characterization. J Cell Physiol. 1987 Oct;133(1):127–134. doi: 10.1002/jcp.1041330116. [DOI] [PubMed] [Google Scholar]
- Kido M., Miwatani H., Kohno K., Uchida T., Okada Y. Targeted introduction of a diphtheria toxin-resistant point mutation into the chromosomal EF-2 locus by in vivo homologous recombination. Cell Struct Funct. 1991 Dec;16(6):447–453. doi: 10.1247/csf.16.447. [DOI] [PubMed] [Google Scholar]
- Kimata Y., Kohno K. Elongation factor 2 mutants deficient in diphthamide formation show temperature-sensitive cell growth. J Biol Chem. 1994 May 6;269(18):13497–13501. [PubMed] [Google Scholar]
- Kondo T., FitzGerald D., Chaudhary V. K., Adhya S., Pastan I. Activity of immunotoxins constructed with modified Pseudomonas exotoxin A lacking the cell recognition domain. J Biol Chem. 1988 Jul 5;263(19):9470–9475. [PubMed] [Google Scholar]
- Kruh G. D., Chan A., Myers K., Gaughan K., Miki T., Aaronson S. A. Expression complementary DNA library transfer establishes mrp as a multidrug resistance gene. Cancer Res. 1994 Apr 1;54(7):1649–1652. [PubMed] [Google Scholar]
- Laurie S. M., Robbins A. R. A toxin-resistant mouse L-cell mutant defective in protein transport along the secretory pathway. J Cell Physiol. 1991 May;147(2):215–223. doi: 10.1002/jcp.1041470205. [DOI] [PubMed] [Google Scholar]
- Lyall R. M., Hwang J. L., Cardarelli C., FitzGerald D., Akiyama S., Gottesman M. M., Pastan I. Isolation of human KB cell lines resistant to epidermal growth factor-Pseudomonas exotoxin conjugates. Cancer Res. 1987 Jun 1;47(11):2961–2966. [PubMed] [Google Scholar]
- Martin R., Hoover C., Grimme S., Grogan C., Höltke J., Kessler C. A highly sensitive, nonradioactive DNA labeling and detection system. Biotechniques. 1990 Dec;9(6):762–768. [PubMed] [Google Scholar]
- Moehring J. M., Inocencio N. M., Robertson B. J., Moehring T. J. Expression of mouse furin in a Chinese hamster cell resistant to Pseudomonas exotoxin A and viruses complements the genetic lesion. J Biol Chem. 1993 Feb 5;268(4):2590–2594. [PubMed] [Google Scholar]
- Ogryzko V. V., Hirai T. H., Shih C. E., Howard B. H. Dissociation of retinoblastoma gene protein hyperphosphorylation and commitment to enter S phase. J Virol. 1994 Jun;68(6):3724–3732. doi: 10.1128/jvi.68.6.3724-3732.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okayama H., Berg P. High-efficiency cloning of full-length cDNA. Mol Cell Biol. 1982 Feb;2(2):161–170. doi: 10.1128/mcb.2.2.161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pai L. H., Batra J. K., FitzGerald D. J., Willingham M. C., Pastan I. Anti-tumor activities of immunotoxins made of monoclonal antibody B3 and various forms of Pseudomonas exotoxin. Proc Natl Acad Sci U S A. 1991 Apr 15;88(8):3358–3362. doi: 10.1073/pnas.88.8.3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastan I., Chaudhary V., FitzGerald D. J. Recombinant toxins as novel therapeutic agents. Annu Rev Biochem. 1992;61:331–354. doi: 10.1146/annurev.bi.61.070192.001555. [DOI] [PubMed] [Google Scholar]
- Pastan I., Lovelace E. T., Gallo M. G., Rutherford A. V., Magnani J. L., Willingham M. C. Characterization of monoclonal antibodies B1 and B3 that react with mucinous adenocarcinomas. Cancer Res. 1991 Jul 15;51(14):3781–3787. [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured animal cells. Cell. 1984 Jul;37(3):705–713. doi: 10.1016/0092-8674(84)90406-9. [DOI] [PubMed] [Google Scholar]
- Schimke R. T. Gene amplification in cultured cells. J Biol Chem. 1988 May 5;263(13):5989–5992. [PubMed] [Google Scholar]
- Suter W., Negro L., Barrera I., Schneider B. Resistance to Pseudomonas toxin A: a sensitive marker to screen for mutagenic substances using V79 cells. Mutagenesis. 1992 Mar;7(2):125–135. doi: 10.1093/mutage/7.2.125. [DOI] [PubMed] [Google Scholar]
- Tiah M., Ronen A. Dominant lethal cell mutants detected by the autoradiographic assay for exotoxin A resistance. Mutat Res. 1991 Jul;249(1):211–222. doi: 10.1016/0027-5107(91)90148-h. [DOI] [PubMed] [Google Scholar]
- Vitetta E. S. From the basic science of B cells to biological missiles at the bedside. J Immunol. 1994 Aug 15;153(4):1407–1420. [PubMed] [Google Scholar]
- Vitetta E. S., Thorpe P. E., Uhr J. W. Immunotoxins: magic bullets or misguided missiles? Trends Pharmacol Sci. 1993 May;14(5):148–154. doi: 10.1016/0165-6147(93)90199-t. [DOI] [PubMed] [Google Scholar]
- Zdanovsky A. G., Chiron M., Pastan I., FitzGerald D. J. Mechanism of action of Pseudomonas exotoxin. Identification of a rate-limiting step. J Biol Chem. 1993 Oct 15;268(29):21791–21799. [PubMed] [Google Scholar]



