Abstract
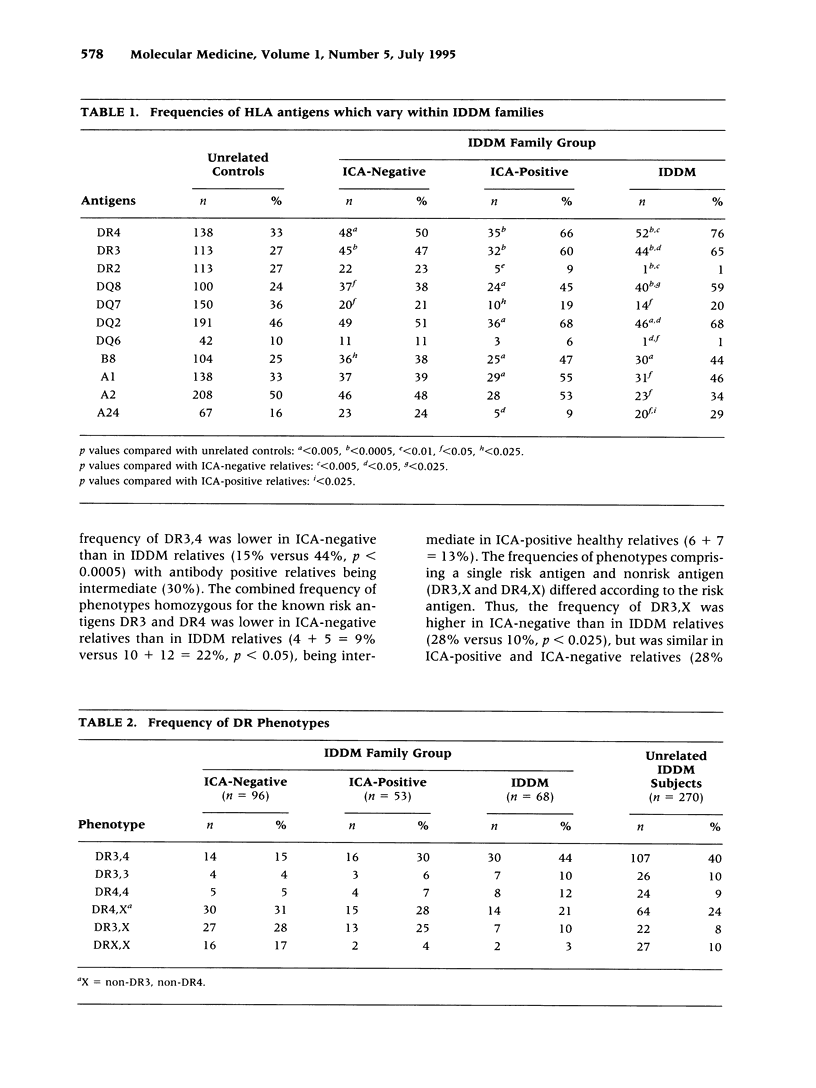
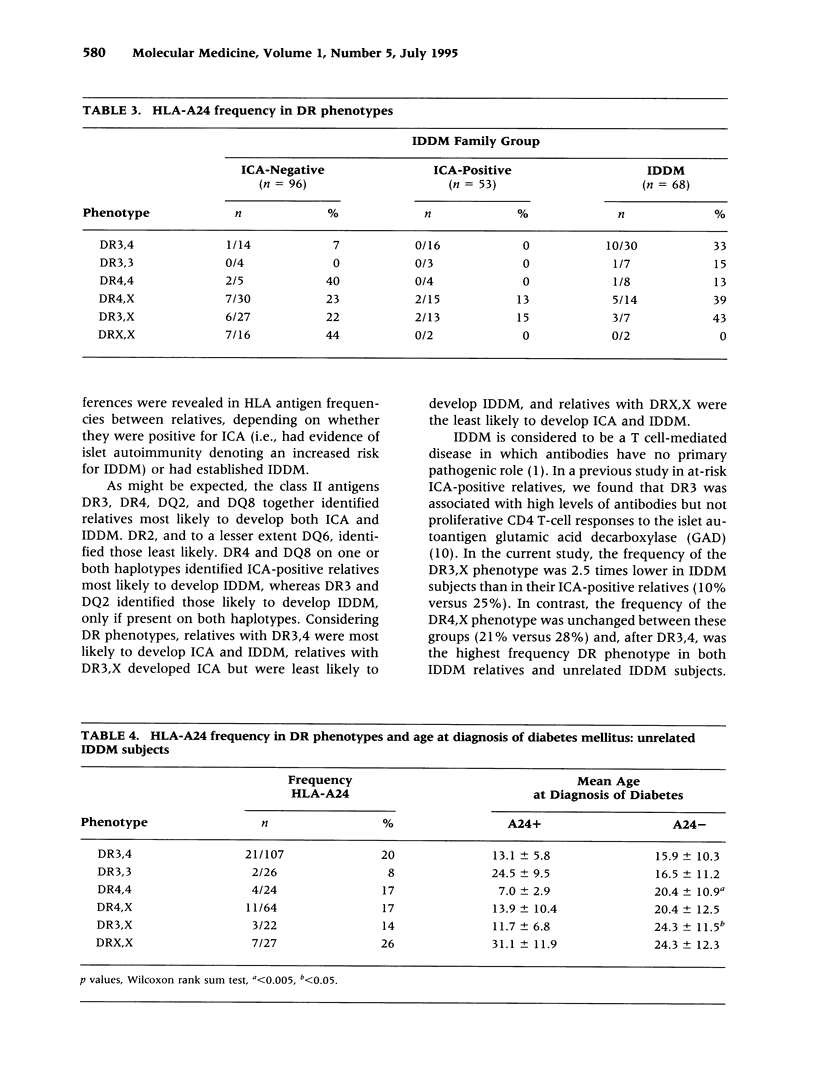
BACKGROUND: Individuals at risk for insulin-dependent diabetes mellitus (IDDM), with an affected first-degree relative, can be identified by the presence of islet cell antibodies (ICA). ICA-positive relatives progress at variable rates to IDDM and identification of those at highest risk is a prerequisite for possible preventative treatment. Those who develop IDDM may exhibit less genetic heterogeneity than their ICA-positive or ICA-negative relatives. Specific human leucocyte antigen (HLA) genes predispose to IDDM but could also influence the rate of progression of preclinical disease. Therefore, by comparing HLA antigen frequencies between first-degree relatives, we sought to identify HLA genes that influence progression to IDDM. MATERIALS AND METHODS: HLA antigen frequencies were compared in 68 IDDM, 53 ICA-positive, and 96 ICA-negative first-degree relatives from 40 Caucasoid families. Predictions were tested in a panel of 270 unrelated IDDM subjects. HLA typing was performed serologically (HLA class I and II) and by sequence-specific oligotyping (11th International Histocompatibility Workshop protocol) (HLA class II). ICA tests were measured by an internationally standardized indirect immunofluorescence assay. RESULTS: In general, known susceptibility class II HLA antigens increased in frequency and known protective class II HLA antigens decreased in frequency, from ICA-negative to ICA-positive to IDDM relatives. Thus, DR4 and DQ8 increased whereas DR2 and DQ6 decreased; DR3 and DQ2 increased from ICA-negative to ICA-positive relatives, but not further in IDDM relatives. The high-risk DR3, 4 phenotype increased across the three groups; DR4,X was unchanged, and DR3,X and DRX,X both decreased. The HLA class I antigen, A24, occurred more frequently in ICA-positive relatives who developed IDDM and, in 270 unrelated IDDM subjects, was associated with an earlier age at diagnosis of IDDM in those with the lower risk class II phenotypes DR4,4 and DR3,X. CONCLUSIONS: HLA-DR3 and DQ2 predispose to islet autoimmunity, but not development of clinical IDDM in the absence and DR4 and DQ8. DR4 and DQ8 predispose to the development of clinical IDDM in ICA-positive relatives, in combination with DR3-DQ2 and other haplotypes but not when homozygous. HLA-A24 is significantly associated with rapid progression to IDDM in ICA-positive relatives and with an earlier age at clinical diagnosis. Analysis of IDDM families reveals that HLA genes not only predispose to islet autoimmunity but influence progression to clinical disease. The findings have implications for identifying high-risk relatives as candidates for possible preventative therapy.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bingley P. J., Bonifacio E., Gale E. A. Can we really predict IDDM? Diabetes. 1993 Feb;42(2):213–220. doi: 10.2337/diab.42.2.213. [DOI] [PubMed] [Google Scholar]
- Caillat-Zucman S., Garchon H. J., Timsit J., Assan R., Boitard C., Djilali-Saiah I., Bougnères P., Bach J. F. Age-dependent HLA genetic heterogeneity of type 1 insulin-dependent diabetes mellitus. J Clin Invest. 1992 Dec;90(6):2242–2250. doi: 10.1172/JCI116110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fennessy M., Metcalfe K., Hitman G. A., Niven M., Biro P. A., Tuomilehto J., Tuomilehto-Wolf E. A gene in the HLA class I region contributes to susceptibility to IDDM in the Finnish population. Childhood Diabetes in Finland (DiMe) Study Group. Diabetologia. 1994 Sep;37(9):937–944. doi: 10.1007/BF00400951. [DOI] [PubMed] [Google Scholar]
- Ghosh S., Palmer S. M., Rodrigues N. R., Cordell H. J., Hearne C. M., Cornall R. J., Prins J. B., McShane P., Lathrop G. M., Peterson L. B. Polygenic control of autoimmune diabetes in nonobese diabetic mice. Nat Genet. 1993 Aug;4(4):404–409. doi: 10.1038/ng0893-404. [DOI] [PubMed] [Google Scholar]
- Hammer J., Belunis C., Bolin D., Papadopoulos J., Walsky R., Higelin J., Danho W., Sinigaglia F., Nagy Z. A. High-affinity binding of short peptides to major histocompatibility complex class II molecules by anchor combinations. Proc Natl Acad Sci U S A. 1994 May 10;91(10):4456–4460. doi: 10.1073/pnas.91.10.4456. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honeyman M. C., Harrison L. C. The immunologic insult in type 1 diabetes. Springer Semin Immunopathol. 1993;14(3):253–274. doi: 10.1007/BF00195977. [DOI] [PubMed] [Google Scholar]
- Kobayashi T., Tamemoto K., Nakanishi K., Kato N., Okubo M., Kajio H., Sugimoto T., Murase T., Kosaka K. Immunogenetic and clinical characterization of slowly progressive IDDM. Diabetes Care. 1993 May;16(5):780–788. doi: 10.2337/diacare.16.5.780. [DOI] [PubMed] [Google Scholar]
- Malcherek G., Gnau V., Stevanovic S., Rammensee H. G., Jung G., Melms A. Analysis of allele-specific contact sites of natural HLA-DR17 ligands. J Immunol. 1994 Aug 1;153(3):1141–1149. [PubMed] [Google Scholar]
- Nakanishi K., Kobayashi T., Murase T., Nakatsuji T., Inoko H., Tsuji K., Kosaka K. Association of HLA-A24 with complete beta-cell destruction in IDDM. Diabetes. 1993 Jul;42(7):1086–1093. doi: 10.2337/diab.42.7.1086. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C., Murray J., Madri J., Bottomly K. Selective activation of Th1- and Th2-like cells in vivo--response to human collagen IV. Immunol Rev. 1991 Oct;123:65–84. doi: 10.1111/j.1600-065x.1991.tb00606.x. [DOI] [PubMed] [Google Scholar]
- Pfeiffer C., Stein J., Southwood S., Ketelaar H., Sette A., Bottomly K. Altered peptide ligands can control CD4 T lymphocyte differentiation in vivo. J Exp Med. 1995 Apr 1;181(4):1569–1574. doi: 10.1084/jem.181.4.1569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sidney J., Oseroff C., Southwood S., Wall M., Ishioka G., Koning F., Sette A. DRB1*0301 molecules recognize a structural motif distinct from the one recognized by most DR beta 1 alleles. J Immunol. 1992 Oct 15;149(8):2634–2640. [PubMed] [Google Scholar]
- Tait B. D., Harrison L. C., Drummond B. P., Stewart V., Varney M. D., Honeyman M. C. HLA antigens and age at diagnosis of insulin-dependent diabetes mellitus. Hum Immunol. 1995 Feb;42(2):116–122. doi: 10.1016/0198-8859(94)00075-2. [DOI] [PubMed] [Google Scholar]
- Tait B. D., Harrison L. C. Overview: the major histocompatibility complex and insulin dependent diabetes mellitus. Baillieres Clin Endocrinol Metab. 1991 Jun;5(2):211–228. doi: 10.1016/s0950-351x(05)80124-7. [DOI] [PubMed] [Google Scholar]
- Tarn A. C., Thomas J. M., Dean B. M., Ingram D., Schwarz G., Bottazzo G. F., Gale E. A. Predicting insulin-dependent diabetes. Lancet. 1988 Apr 16;1(8590):845–850. doi: 10.1016/s0140-6736(88)91601-7. [DOI] [PubMed] [Google Scholar]


