Abstract
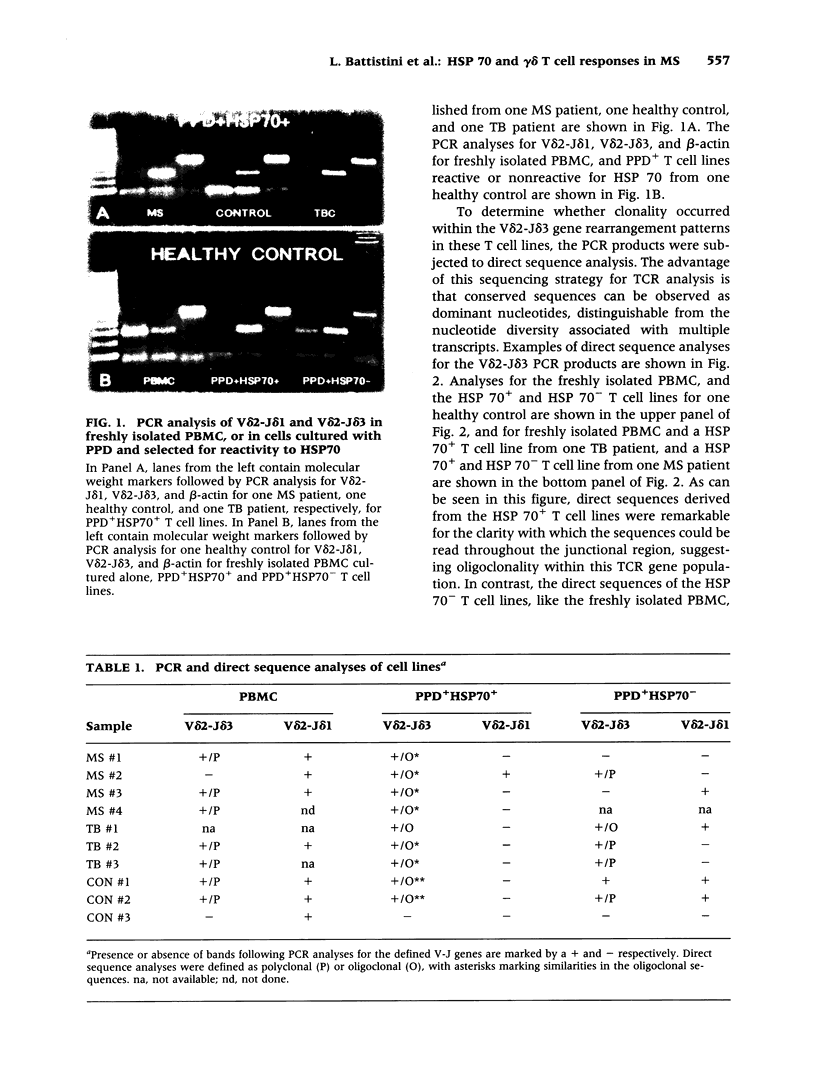
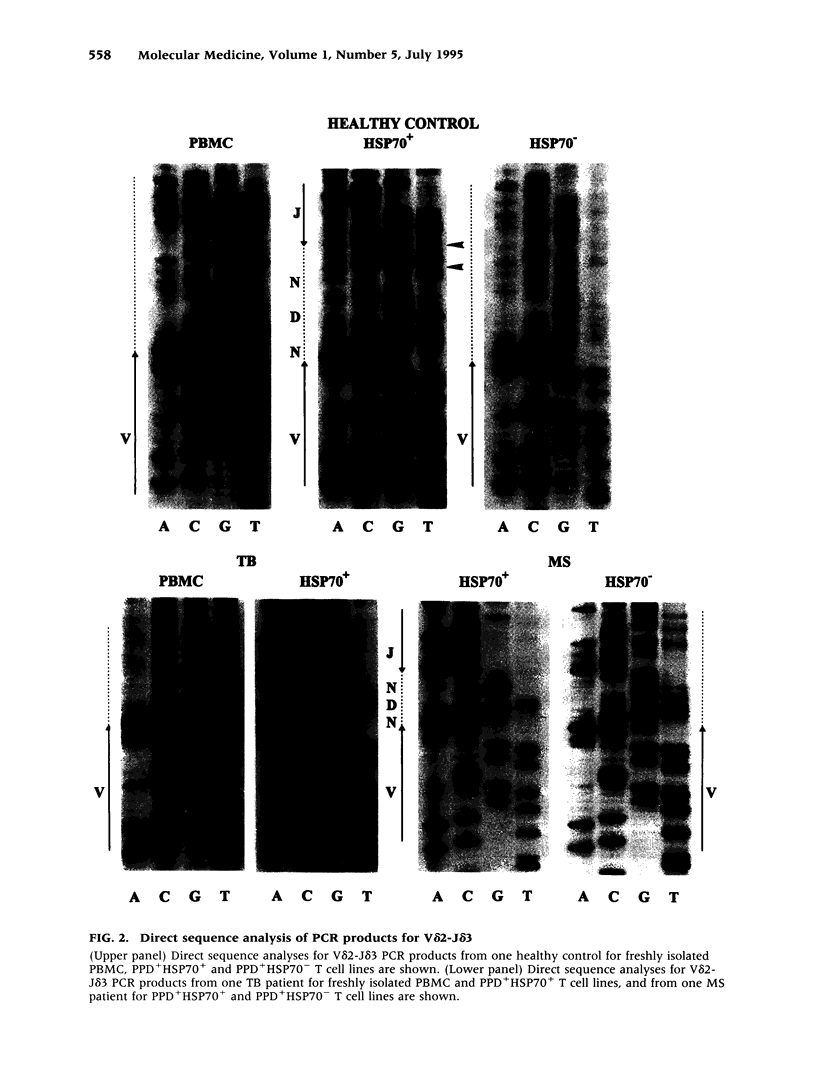
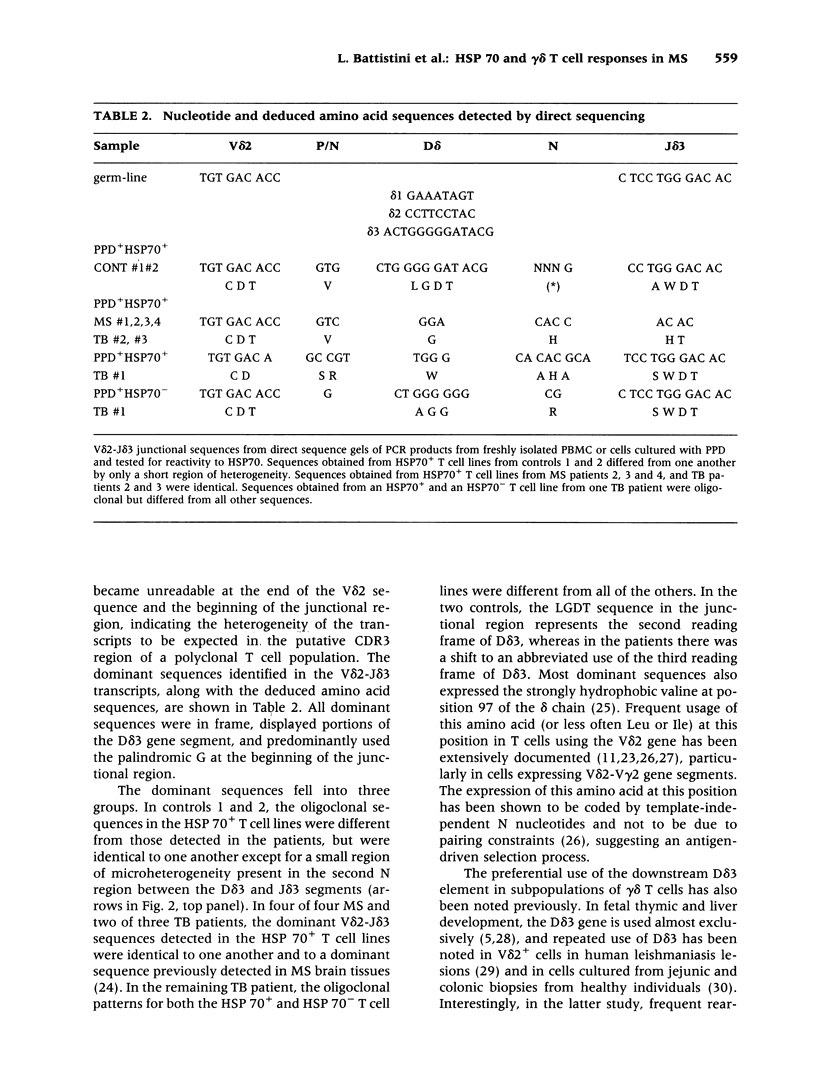
BACKGROUND: Interactions between gamma delta T cells and heat shock proteins (HSP) have been proposed as contributing factors in a number of diseases of possible autoimmune etiology but definitive evidence to support this hypothesis has been lacking. In multiple sclerosis (MS), a chronic inflammatory neurologic disease, HSP and gamma delta T cells are known to colocalize in brain lesions. Analysis of T cell receptor (TCR) gene usage in these lesions has detected evidence of clonality within both the V delta 2-J delta 1 and V delta 2-J delta 3 populations of gamma delta T cells. In our own studies, using direct sequence analysis, a dominant V delta 2-J delta 3 TCR sequence was found in 9 MS brain samples, suggesting a response to a common antigen. In this report, we have examined gamma delta T cell receptor gene usage in MS peripheral blood T cell lines selected for reactivity to HSP 70. MATERIALS AND METHODS: TCR rearrangement patterns for V delta 2-J delta 1 and V delta 2-J delta 3 were studied using the polymerase chain reaction (PCR) and a direct sequencing technique in populations of peripheral blood mononuclear cells (PBMC) cultured with Mycobacterium tuberculosis (M. tuberculosis) purified protein derivative (PPD) and then selected for reactivity to a 70-kD heat shock protein (HSP70). Cells were obtained from health donors, patients with MS, and patients with tuberculosis (TB). PCR products were subjected to direct sequence analysis to look for evidence for clonality within these T cell lines and to define the sequence of the V-D-J (CDR3) region of the TCR. RESULTS: In freshly isolated PBMC, both V delta 2-J delta 1 and V delta 2-J delta 3 gene rearrangement patterns were detected, whereas in HSP70+ T cell lines the predominant delta chain rearrangement pattern was V delta 2-J delta 3. Direct sequence analyses indicated that in cells reactive with HSP70 the V delta 2-J delta 3 sequences were usually oligoclonal and used D delta 3 exclusively. In four of four MS and two of three TB patients, the oligoclonal sequences in the HSP70+ T cell lines were identical to one another and to a dominant sequence previously detected in MS brain lesions. In two of three HSP70+ T cell lines from healthy controls, the oligoclonal sequences differed from those found in both groups of patients but were identical to one another except for a small region of heterogeneity in the second N region. In contrast, in freshly isolated PBMC or in PPD+HSP70- T cell lines, the V delta 2-J delta 3 gene rearrangement patterns were usually polyclonal and dominant sequences were rarely identified. CONCLUSIONS: These results support the conclusion that a subpopulation of gamma delta T cells in MS lesions are responding to HSP 70 and that non-CNS-specific antigens contribute to the pathogenesis of MS.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Battistini L., Selmaj K., Kowal C., Ohmen J., Modlin R. L., Raine C. S., Brosnan C. F. Multiple sclerosis: limited diversity of the V delta 2-J delta 3 T-cell receptor in chronic active lesions. Ann Neurol. 1995 Feb;37(2):198–203. doi: 10.1002/ana.410370210. [DOI] [PubMed] [Google Scholar]
- Birnbaum G., Kotilinek L., Albrecht L. Spinal fluid lymphocytes from a subgroup of multiple sclerosis patients respond to mycobacterial antigens. Ann Neurol. 1993 Jul;34(1):18–24. doi: 10.1002/ana.410340106. [DOI] [PubMed] [Google Scholar]
- D'Souza S. D., Antel J. P., Freedman M. S. Cytokine induction of heat shock protein expression in human oligodendrocytes: an interleukin-1-mediated mechanism. J Neuroimmunol. 1994 Feb;50(1):17–24. doi: 10.1016/0165-5728(94)90210-0. [DOI] [PubMed] [Google Scholar]
- Davodeau F., Peyrat M. A., Hallet M. M., Houde I., Vie H., Bonneville M. Peripheral selection of antigen receptor junctional features in a major human gamma delta subset. Eur J Immunol. 1993 Apr;23(4):804–808. doi: 10.1002/eji.1830230405. [DOI] [PubMed] [Google Scholar]
- De Libero G., Casorati G., Giachino C., Carbonara C., Migone N., Matzinger P., Lanzavecchia A. Selection by two powerful antigens may account for the presence of the major population of human peripheral gamma/delta T cells. J Exp Med. 1991 Jun 1;173(6):1311–1322. doi: 10.1084/jem.173.6.1311. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freedman M. S., Ruijs T. C., Selin L. K., Antel J. P. Peripheral blood gamma-delta T cells lyse fresh human brain-derived oligodendrocytes. Ann Neurol. 1991 Dec;30(6):794–800. doi: 10.1002/ana.410300608. [DOI] [PubMed] [Google Scholar]
- Georgopoulos C., McFarland H. Heat shock proteins in multiple sclerosis and other autoimmune diseases. Immunol Today. 1993 Aug;14(8):373–375. doi: 10.1016/0167-5699(93)90135-8. [DOI] [PubMed] [Google Scholar]
- Giachino C., Granziero L., Modena V., Maiocco V., Lomater C., Fantini F., Lanzavecchia A., Migone N. Clonal expansions of V delta 1+ and V delta 2+ cells increase with age and limit the repertoire of human gamma delta T cells. Eur J Immunol. 1994 Aug;24(8):1914–1918. doi: 10.1002/eji.1830240830. [DOI] [PubMed] [Google Scholar]
- Haas W., Pereira P., Tonegawa S. Gamma/delta cells. Annu Rev Immunol. 1993;11:637–685. doi: 10.1146/annurev.iy.11.040193.003225. [DOI] [PubMed] [Google Scholar]
- Haregewoin A., Soman G., Hom R. C., Finberg R. W. Human gamma delta+ T cells respond to mycobacterial heat-shock protein. Nature. 1989 Jul 27;340(6231):309–312. doi: 10.1038/340309a0. [DOI] [PubMed] [Google Scholar]
- Hvas J., Oksenberg J. R., Fernando R., Steinman L., Bernard C. C. Gamma delta T cell receptor repertoire in brain lesions of patients with multiple sclerosis. J Neuroimmunol. 1993 Jul;46(1-2):225–234. doi: 10.1016/0165-5728(93)90253-u. [DOI] [PubMed] [Google Scholar]
- Janeway C. A., Jr, Jones B., Hayday A. Specificity and function of T cells bearing gamma delta receptors. Immunol Today. 1988 Mar;9(3):73–76. doi: 10.1016/0167-5699(88)91267-4. [DOI] [PubMed] [Google Scholar]
- Janis E. M., Kaufmann S. H., Schwartz R. H., Pardoll D. M. Activation of gamma delta T cells in the primary immune response to Mycobacterium tuberculosis. Science. 1989 May 12;244(4905):713–716. doi: 10.1126/science.2524098. [DOI] [PubMed] [Google Scholar]
- Kabelitz D., Bender A., Prospero T., Wesselborg S., Janssen O., Pechhold K. The primary response of human gamma/delta + T cells to Mycobacterium tuberculosis is restricted to V gamma 9-bearing cells. J Exp Med. 1991 Jun 1;173(6):1331–1338. doi: 10.1084/jem.173.6.1331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Modlin R. L., Pirmez C., Hofman F. M., Torigian V., Uyemura K., Rea T. H., Bloom B. R., Brenner M. B. Lymphocytes bearing antigen-specific gamma delta T-cell receptors accumulate in human infectious disease lesions. Nature. 1989 Jun 15;339(6225):544–548. doi: 10.1038/339544a0. [DOI] [PubMed] [Google Scholar]
- O'Brien R. L., Happ M. P., Dallas A., Palmer E., Kubo R., Born W. K. Stimulation of a major subset of lymphocytes expressing T cell receptor gamma delta by an antigen derived from Mycobacterium tuberculosis. Cell. 1989 May 19;57(4):667–674. doi: 10.1016/0092-8674(89)90135-9. [DOI] [PubMed] [Google Scholar]
- Ohmen J. D., Barnes P. F., Uyemura K., Lu S. Z., Grisso C. L., Modlin R. L. The T cell receptors of human gamma delta T cells reactive to Mycobacterium tuberculosis are encoded by specific V genes but diverse V-J junctions. J Immunol. 1991 Nov 15;147(10):3353–3359. [PubMed] [Google Scholar]
- Olive C., Gatenby P. A., Serjeantson S. W. Variable gene usage of T cell receptor gamma- and delta-chain transcripts expressed in synovia and peripheral blood of patients with rheumatoid arthritis. Clin Exp Immunol. 1992 Feb;87(2):172–177. doi: 10.1111/j.1365-2249.1992.tb02970.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orsini D. L., van Gils M., Kooy Y. M., Struyk L., Klein G., van den Elsen P., Koning F. Functional and molecular characterization of B cell-responsive V delta 1+ gamma delta T cells. Eur J Immunol. 1994 Dec;24(12):3199–3204. doi: 10.1002/eji.1830241243. [DOI] [PubMed] [Google Scholar]
- Parker C. M., Groh V., Band H., Porcelli S. A., Morita C., Fabbi M., Glass D., Strominger J. L., Brenner M. B. Evidence for extrathymic changes in the T cell receptor gamma/delta repertoire. J Exp Med. 1990 May 1;171(5):1597–1612. doi: 10.1084/jem.171.5.1597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeffer K., Schoel B., Plesnila N., Lipford G. B., Kromer S., Deusch K., Wagner H. A lectin-binding, protease-resistant mycobacterial ligand specifically activates V gamma 9+ human gamma delta T cells. J Immunol. 1992 Jan 15;148(2):575–583. [PubMed] [Google Scholar]
- Pluschke G., Rüegg D., Hohlfeld R., Engel A. G. Autoaggressive myocytotoxic T lymphocytes expressing an unusual gamma/delta T cell receptor. J Exp Med. 1992 Dec 1;176(6):1785–1789. doi: 10.1084/jem.176.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porcelli S., Brenner M. B., Band H. Biology of the human gamma delta T-cell receptor. Immunol Rev. 1991 Apr;120:137–183. doi: 10.1111/j.1600-065x.1991.tb00591.x. [DOI] [PubMed] [Google Scholar]
- Rajasekar R., Sim G. K., Augustin A. Self heat shock and gamma delta T-cell reactivity. Proc Natl Acad Sci U S A. 1990 Mar;87(5):1767–1771. doi: 10.1073/pnas.87.5.1767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robijn R. J., Bloemendal H., Jainandunsing S., Wiegman L. J., VanBerge-Henegouwen G. P., Logtenberg T., Koningsberger J. C. Phenotypic and molecular characterization of human monoclonal TCR gamma/delta T-cell lines from jejunum and colon of healthy individuals. Scand J Immunol. 1993 Sep;38(3):247–253. doi: 10.1111/j.1365-3083.1993.tb01721.x. [DOI] [PubMed] [Google Scholar]
- Salvetti M., Buttinelli C., Ristori G., Carbonari M., Cherchi M., Fiorelli M., Grasso M. G., Toma L., Pozzilli C. T-lymphocyte reactivity to the recombinant mycobacterial 65- and 70-kDa heat shock proteins in multiple sclerosis. J Autoimmun. 1992 Dec;5(6):691–702. doi: 10.1016/0896-8411(92)90186-t. [DOI] [PubMed] [Google Scholar]
- Selmaj K., Brosnan C. F., Raine C. S. Colocalization of lymphocytes bearing gamma delta T-cell receptor and heat shock protein hsp65+ oligodendrocytes in multiple sclerosis. Proc Natl Acad Sci U S A. 1991 Aug 1;88(15):6452–6456. doi: 10.1073/pnas.88.15.6452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shimonkevitz R., Colburn C., Burnham J. A., Murray R. S., Kotzin B. L. Clonal expansions of activated gamma/delta T cells in recent-onset multiple sclerosis. Proc Natl Acad Sci U S A. 1993 Feb 1;90(3):923–927. doi: 10.1073/pnas.90.3.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stinissen P., Vandevyver C., Medaer R., Vandegaer L., Nies J., Tuyls L., Hafler D. A., Raus J., Zhang J. Increased frequency of gamma delta T cells in cerebrospinal fluid and peripheral blood of patients with multiple sclerosis. Reactivity, cytotoxicity, and T cell receptor V gene rearrangements. J Immunol. 1995 May 1;154(9):4883–4894. [PubMed] [Google Scholar]
- Tamura N., Holroyd K. J., Banks T., Kirby M., Okayama H., Crystal R. G. Diversity in junctional sequences associated with the common human V gamma 9 and V delta 2 gene segments in normal blood and lung compared with the limited diversity in a granulomatous disease. J Exp Med. 1990 Jul 1;172(1):169–181. doi: 10.1084/jem.172.1.169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanaka Y., Sano S., Nieves E., De Libero G., Rosa D., Modlin R. L., Brenner M. B., Bloom B. R., Morita C. T. Nonpeptide ligands for human gamma delta T cells. Proc Natl Acad Sci U S A. 1994 Aug 16;91(17):8175–8179. doi: 10.1073/pnas.91.17.8175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Triebel F., Faure F., Mami-Chouaib F., Jitsukawa S., Griscelli A., Genevée C., Roman-Roman S., Hercend T. A novel human V delta gene expressed predominantly in the Ti gamma A fraction of gamma/delta+ peripheral lymphocytes. Eur J Immunol. 1988 Dec;18(12):2021–2027. doi: 10.1002/eji.1830181223. [DOI] [PubMed] [Google Scholar]
- Uyemura K., Deans R. J., Band H., Ohmen J., Panchamoorthy G., Morita C. T., Rea T. H., Modlin R. L. Evidence for clonal selection of gamma/delta T cells in response to a human pathogen. J Exp Med. 1991 Sep 1;174(3):683–692. doi: 10.1084/jem.174.3.683. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uyemura K., Klotz J., Pirmez C., Ohmen J., Wang X. H., Ho C., Hoffman W. L., Modlin R. L. Microanatomic clonality of gamma delta T cells in human leishmaniasis lesions. J Immunol. 1992 Feb 15;148(4):1205–1211. [PubMed] [Google Scholar]
- Wucherpfennig K. W., Liao Y. J., Prendergast M., Prendergast J., Hafler D. A., Strominger J. L. Human fetal liver gamma/delta T cells predominantly use unusual rearrangements of the T cell receptor delta and gamma loci expressed on both CD4+CD8- and CD4-CD8- gamma/delta T cells. J Exp Med. 1993 Feb 1;177(2):425–432. doi: 10.1084/jem.177.2.425. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Newcombe J., Li H., Keddy C., Cuzner M. L., Hafler D. A. Gamma delta T-cell receptor repertoire in acute multiple sclerosis lesions. Proc Natl Acad Sci U S A. 1992 May 15;89(10):4588–4592. doi: 10.1073/pnas.89.10.4588. [DOI] [PMC free article] [PubMed] [Google Scholar]