Abstract
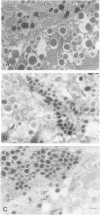
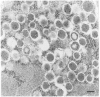
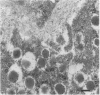
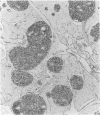
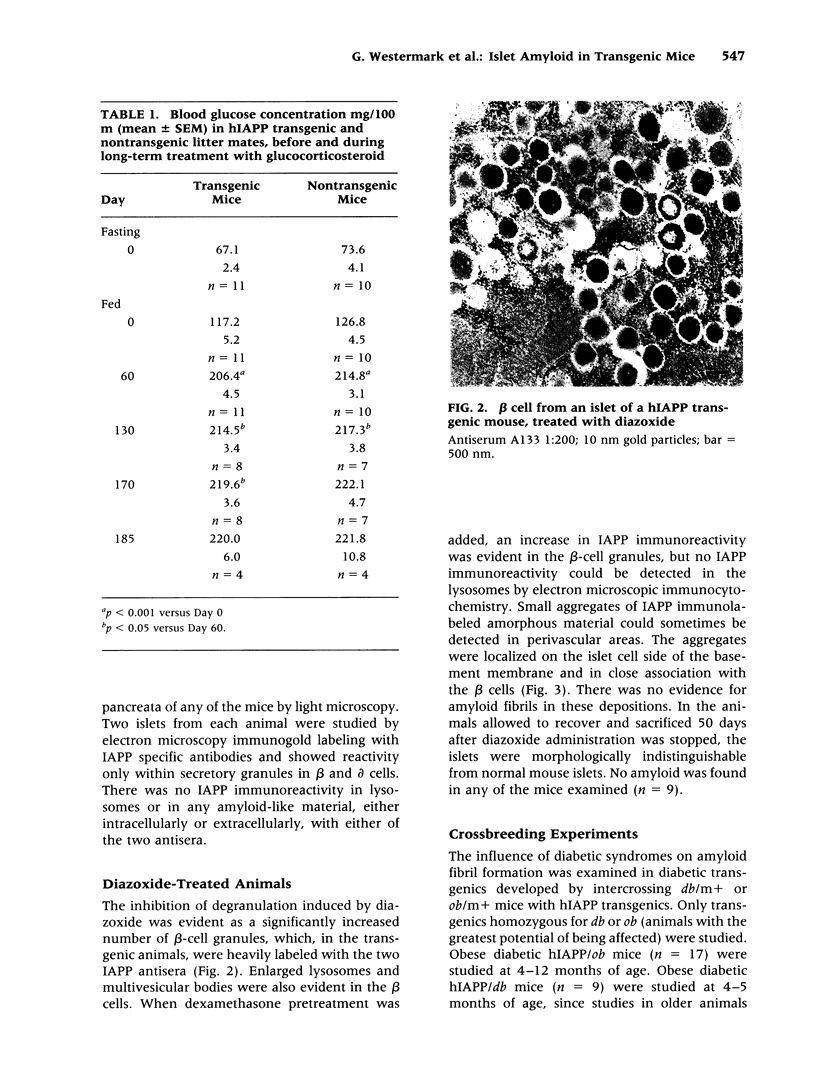
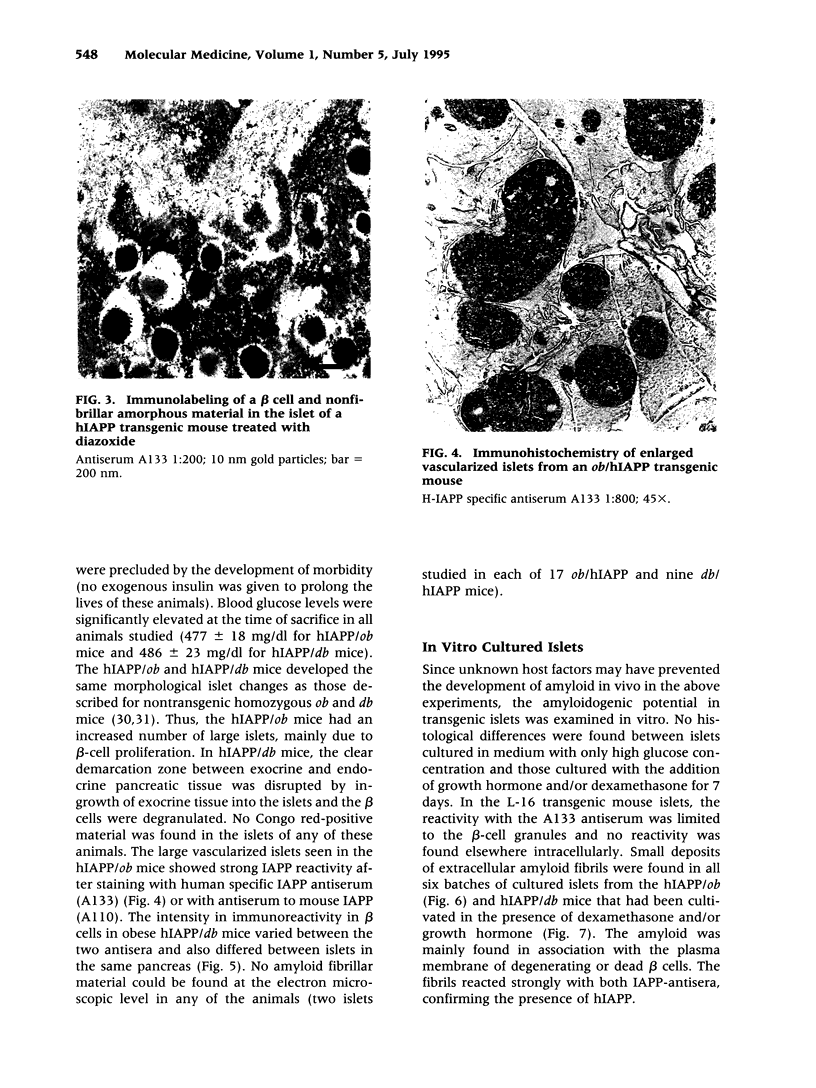
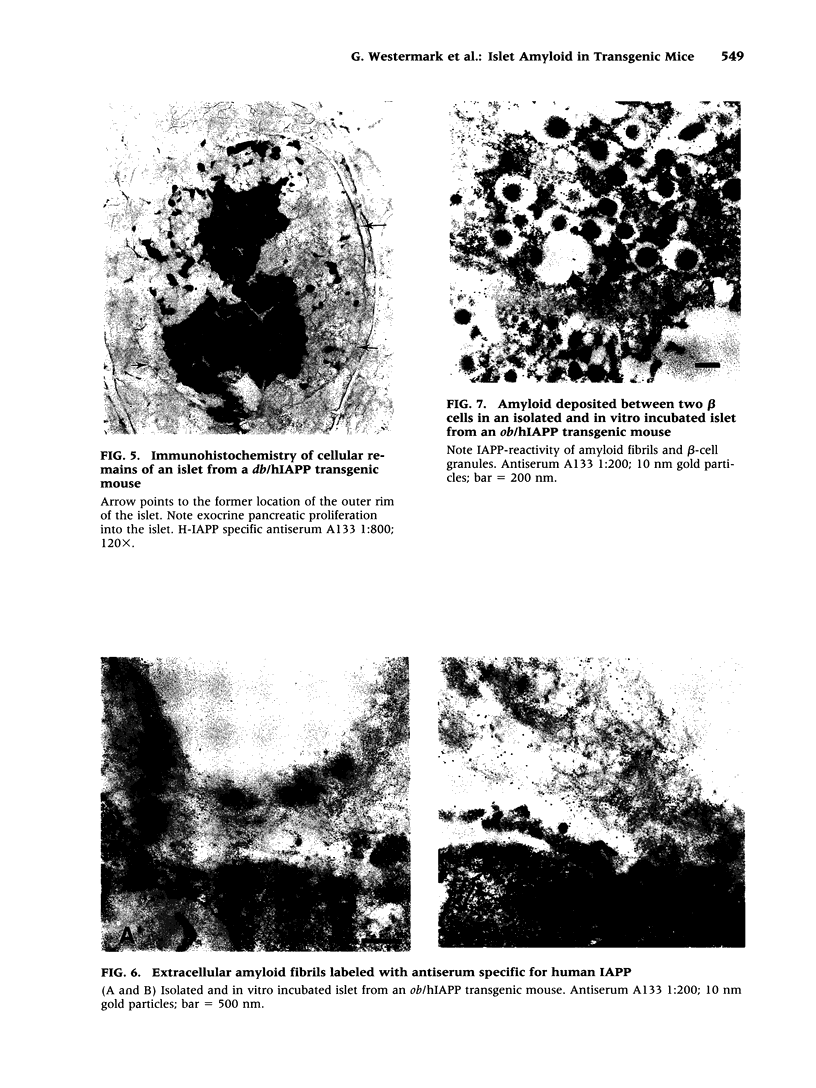
BACKGROUND: Human, but not mouse, islet amyloid polypeptide (IAPP) is amyloidogenic. Transgenic mice overexpressing human IAPP in the beta cells of the islets of Langerhans should be useful in identifying factors important for the deposition of IAPP as insoluble amyloid fibrils. MATERIALS AND METHODS: Transgenic mice expressing human IAPP were examined using several experimental models for the production of persistent hyperglycemia, as well as for the overstimulation and/or inhibition of beta cell secretion. Obesity was induced by aurothioglucose. Persistent hyperglycemia was produced by long-term administration of glucocorticosteroids or by partial pancreatectomy. Inhibition of normal beta cell exocytosis by diazoxide administration, with or without concurrent dexamethasone injections, was carried out to increase crinophagy of secretory granules. The human IAPP gene was also introduced into the ab and ob mouse models for diabetes. Finally, isolated islets cultivated in vitro at high glucose concentration were also examined. RESULTS: No amyloid deposits were found in the pancreata of any of the animals, either by light microscopy after Congo red staining or by electron microscopy after immunogold labeling with antibodies specific for human IAPP. Aurothioglucose treatment resulted in increased numbers of granules in the beta cell and the appearance of large lysosomal bodies without amyloid. However, islets from db and ob mice expressing human IAPP cultivated in vitro in the presence of glucocorticosteroid and/or growth hormone, were found to contain extracellular amyloid deposits reacting with antibodies to human IAPP. CONCLUSIONS: Oversecretion of human IAPP or increased crinophagy are not sufficient for amyloid formation. This indicates that other factors must influence amyloid deposition; one such factor may be the local clearance of IAPP.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BELL E. T. Hyalinization of the islet of Langerhans in diabetes mellitus. Diabetes. 1952 Sep-Oct;1(5):341–344. doi: 10.2337/diab.1.5.341. [DOI] [PubMed] [Google Scholar]
- BELL E. T. Hyalinization of the islets of Langerhans in nondiabetic individuals. Am J Pathol. 1959 Jul-Aug;35(4):801–805. [PMC free article] [PubMed] [Google Scholar]
- Beaumont K., Kenney M. A., Young A. A., Rink T. J. High affinity amylin binding sites in rat brain. Mol Pharmacol. 1993 Sep;44(3):493–497. [PubMed] [Google Scholar]
- Betsholtz C., Christmanson L., Engström U., Rorsman F., Jordan K., O'Brien T. D., Murtaugh M., Johnson K. H., Westermark P. Structure of cat islet amyloid polypeptide and identification of amino acid residues of potential significance for islet amyloid formation. Diabetes. 1990 Jan;39(1):118–122. doi: 10.2337/diacare.39.1.118. [DOI] [PubMed] [Google Scholar]
- Betsholtz C., Svensson V., Rorsman F., Engström U., Westermark G. T., Wilander E., Johnson K., Westermark P. Islet amyloid polypeptide (IAPP):cDNA cloning and identification of an amyloidogenic region associated with the species-specific occurrence of age-related diabetes mellitus. Exp Cell Res. 1989 Aug;183(2):484–493. doi: 10.1016/0014-4827(89)90407-2. [DOI] [PubMed] [Google Scholar]
- Bhogal R., Smith D. M., Purkiss P., Bloom S. R. Molecular identification of binding sites for calcitonin gene-related peptide (CGRP) and islet amyloid polypeptide (IAPP) in mammalian lung: species variation and binding of truncated CGRP and IAPP. Endocrinology. 1993 Nov;133(5):2351–2361. doi: 10.1210/endo.133.5.8404688. [DOI] [PubMed] [Google Scholar]
- Clark A., Edwards C. A., Ostle L. R., Sutton R., Rothbard J. B., Morris J. F., Turner R. C. Localisation of islet amyloid peptide in lipofuscin bodies and secretory granules of human B-cells and in islets of type-2 diabetic subjects. Cell Tissue Res. 1989 Jul;257(1):179–185. doi: 10.1007/BF00221649. [DOI] [PubMed] [Google Scholar]
- Clark A. Islet amyloid: an enigma of type 2 diabetes. Diabetes Metab Rev. 1992 Jul;8(2):117–132. doi: 10.1002/dmr.5610080204. [DOI] [PubMed] [Google Scholar]
- Coleman D. L., Hummel K. P. Studies with the mutation, diabetes, in the mouse. Diabetologia. 1967 Apr;3(2):238–248. doi: 10.1007/BF01222201. [DOI] [PubMed] [Google Scholar]
- Coleman D. L. Obese and diabetes: two mutant genes causing diabetes-obesity syndromes in mice. Diabetologia. 1978 Mar;14(3):141–148. doi: 10.1007/BF00429772. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Day A. J., Willis A. C., Roberts A. N., Reid K. B., Leighton B. Amylin and the amylin gene: structure, function and relationship to islet amyloid and to diabetes mellitus. Biochim Biophys Acta. 1989 Dec 14;1014(3):247–258. doi: 10.1016/0167-4889(89)90220-6. [DOI] [PubMed] [Google Scholar]
- Cooper G. J., Leighton B., Dimitriadis G. D., Parry-Billings M., Kowalchuk J. M., Howland K., Rothbard J. B., Willis A. C., Reid K. B. Amylin found in amyloid deposits in human type 2 diabetes mellitus may be a hormone that regulates glycogen metabolism in skeletal muscle. Proc Natl Acad Sci U S A. 1988 Oct;85(20):7763–7766. doi: 10.1073/pnas.85.20.7763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cooper G. J., Willis A. C., Clark A., Turner R. C., Sim R. B., Reid K. B. Purification and characterization of a peptide from amyloid-rich pancreases of type 2 diabetic patients. Proc Natl Acad Sci U S A. 1987 Dec;84(23):8628–8632. doi: 10.1073/pnas.84.23.8628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Creutzfeldt W., Creutzfeldt C., Frerichs H., Perings E., Sicklinger K. The morphological substrate of the inhibition of insulin secretion by diazoxide. Horm Metab Res. 1969 Mar;1(2):53–64. doi: 10.1055/s-0028-1095173. [DOI] [PubMed] [Google Scholar]
- DRACHMAN R. H., TEPPERMAN J. Aurothioglucose obesity in the mouse. Yale J Biol Med. 1954 Apr;26(5):394–409. [PMC free article] [PubMed] [Google Scholar]
- Dubuc P. U. The development of obesity, hyperinsulinemia, and hyperglycemia in ob/ob mice. Metabolism. 1976 Dec;25(12):1567–1574. doi: 10.1016/0026-0495(76)90109-8. [DOI] [PubMed] [Google Scholar]
- Fox N., Schrementi J., Nishi M., Ohagi S., Chan S. J., Heisserman J. A., Westermark G. T., Leckström A., Westermark P., Steiner D. F. Human islet amyloid polypeptide transgenic mice as a model of non-insulin-dependent diabetes mellitus (NIDDM). FEBS Lett. 1993 May 24;323(1-2):40–44. doi: 10.1016/0014-5793(93)81444-5. [DOI] [PubMed] [Google Scholar]
- Höppener J. W., Verbeek J. S., de Koning E. J., Oosterwijk C., van Hulst K. L., Visser-Vernooy H. J., Hofhuis F. M., van Gaalen S., Berends M. J., Hackeng W. H. Chronic overproduction of islet amyloid polypeptide/amylin in transgenic mice: lysosomal localization of human islet amyloid polypeptide and lack of marked hyperglycaemia or hyperinsulinaemia. Diabetologia. 1993 Dec;36(12):1258–1265. doi: 10.1007/BF00400803. [DOI] [PubMed] [Google Scholar]
- Jansen G. R., Hutchison C. F., Zanetti M. E. Effect of diazoxide on glucose U-C-14 utilization in mice. Diabetes. 1967 Nov;16(11):777–783. doi: 10.2337/diab.16.11.777. [DOI] [PubMed] [Google Scholar]
- Lernmark A., Nathans A., Steiner D. F. Preparation and characterization of plasma membrane-enriched fractions from rat pancreatic islets. J Cell Biol. 1976 Nov;71(2):606–623. doi: 10.1083/jcb.71.2.606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzo A., Razzaboni B., Weir G. C., Yankner B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994 Apr 21;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Lukinius A., Wilander E., Westermark G. T., Engström U., Westermark P. Co-localization of islet amyloid polypeptide and insulin in the B cell secretory granules of the human pancreatic islets. Diabetologia. 1989 Apr;32(4):240–244. doi: 10.1007/BF00285291. [DOI] [PubMed] [Google Scholar]
- Nagamatsu S., Nishi M., Steiner D. F. Biosynthesis of islet amyloid polypeptide. Elevated expression in mouse beta TC3 cells. J Biol Chem. 1991 Jul 25;266(21):13737–13741. [PubMed] [Google Scholar]
- Nishi M., Bell G. I., Steiner D. F. Sequence of a cDNA encoding Syrian hamster islet amyloid polypeptide precursor. Nucleic Acids Res. 1990 Nov 25;18(22):6726–6726. doi: 10.1093/nar/18.22.6726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nishi M., Chan S. J., Nagamatsu S., Bell G. I., Steiner D. F. Conservation of the sequence of islet amyloid polypeptide in five mammals is consistent with its putative role as an islet hormone. Proc Natl Acad Sci U S A. 1989 Aug;86(15):5738–5742. doi: 10.1073/pnas.86.15.5738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novials A., Sarri Y., Casamitjana R., Rivera F., Gomis R. Regulation of islet amyloid polypeptide in human pancreatic islets. Diabetes. 1993 Oct;42(10):1514–1519. doi: 10.2337/diab.42.10.1514. [DOI] [PubMed] [Google Scholar]
- O'Brien T. D., Westermark P., Johnson K. H. Islet amyloid polypeptide and calcitonin gene-related peptide immunoreactivity in amyloid and tumor cells of canine pancreatic endocrine tumors. Vet Pathol. 1990 May;27(3):194–198. doi: 10.1177/030098589002700307. [DOI] [PubMed] [Google Scholar]
- Ohagi S., Nishi M., Bell G. I., Ensinck J. W., Steiner D. F. Sequences of islet amyloid polypeptide precursors of an Old World monkey, the pig-tailed macaque (Macaca nemestrina), and the dog (Canis familiaris). Diabetologia. 1991 Aug;34(8):555–558. doi: 10.1007/BF00400272. [DOI] [PubMed] [Google Scholar]
- Roden M., Liener K., Fürnsinn C., Nowotny P., Hollenstein U., Vierhapper H., Waldhäusl W. Effects of islet amyloid polypeptide on hepatic insulin resistance and glucose production in the isolated perfused rat liver. Diabetologia. 1992 Feb;35(2):116–120. doi: 10.1007/BF00402542. [DOI] [PubMed] [Google Scholar]
- Sanke T., Bell G. I., Sample C., Rubenstein A. H., Steiner D. F. An islet amyloid peptide is derived from an 89-amino acid precursor by proteolytic processing. J Biol Chem. 1988 Nov 25;263(33):17243–17246. [PubMed] [Google Scholar]
- Shirahama T., Cohen A. S. Intralysosomal formation of amyloid fibrils. Am J Pathol. 1975 Oct;81(1):101–116. [PMC free article] [PubMed] [Google Scholar]
- Verchere C. B., D'Alessio D. A., Palmiter R. D., Kahn S. E. Transgenic mice overproducing islet amyloid polypeptide have increased insulin storage and secretion in vitro. Diabetologia. 1994 Jul;37(7):725–728. doi: 10.1007/BF00417699. [DOI] [PubMed] [Google Scholar]
- Westermark P., Eizirik D. L., Pipeleers D. G., Hellerström C., Andersson A. Rapid deposition of amyloid in human islets transplanted into nude mice. Diabetologia. 1995 May;38(5):543–549. doi: 10.1007/BF00400722. [DOI] [PubMed] [Google Scholar]
- Westermark P., Engström U., Johnson K. H., Westermark G. T., Betsholtz C. Islet amyloid polypeptide: pinpointing amino acid residues linked to amyloid fibril formation. Proc Natl Acad Sci U S A. 1990 Jul;87(13):5036–5040. doi: 10.1073/pnas.87.13.5036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P. Fine structure of islets of Langerhans in insular amyloidosis. Virchows Arch A Pathol Pathol Anat. 1973 Mar 20;359(1):1–18. doi: 10.1007/BF00549079. [DOI] [PubMed] [Google Scholar]
- Westermark P., Grimelius L. The pancreatic islet cells in insular amyloidosis in human diabetic and non-diabetic adults. Acta Pathol Microbiol Scand A. 1973 May;81(3):291–300. doi: 10.1111/j.1699-0463.1973.tb03538.x. [DOI] [PubMed] [Google Scholar]
- Westermark P., Johnson K. H., O'Brien T. D., Betsholtz C. Islet amyloid polypeptide--a novel controversy in diabetes research. Diabetologia. 1992 Apr;35(4):297–303. doi: 10.1007/BF00401195. [DOI] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Hayden D. W., O'Brien T. D., Johnson K. H. Amyloid fibrils in human insulinoma and islets of Langerhans of the diabetic cat are derived from a neuropeptide-like protein also present in normal islet cells. Proc Natl Acad Sci U S A. 1987 Jun;84(11):3881–3885. doi: 10.1073/pnas.84.11.3881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westermark P., Wernstedt C., Wilander E., Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochem Biophys Res Commun. 1986 Nov 14;140(3):827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- Wisniewski T., Frangione B. Apolipoprotein E: a pathological chaperone protein in patients with cerebral and systemic amyloid. Neurosci Lett. 1992 Feb 3;135(2):235–238. doi: 10.1016/0304-3940(92)90444-c. [DOI] [PubMed] [Google Scholar]
- Young I. D., Ailles L., Narindrasorasak S., Tan R., Kisilevsky R. Localization of the basement membrane heparan sulfate proteoglycan in islet amyloid deposits in type II diabetes mellitus. Arch Pathol Lab Med. 1992 Sep;116(9):951–954. [PubMed] [Google Scholar]
- de Koning E. J., Morris E. R., Hofhuis F. M., Posthuma G., Höppener J. W., Morris J. F., Capel P. J., Clark A., Verbeek J. S. Intra- and extracellular amyloid fibrils are formed in cultured pancreatic islets of transgenic mice expressing human islet amyloid polypeptide. Proc Natl Acad Sci U S A. 1994 Aug 30;91(18):8467–8471. doi: 10.1073/pnas.91.18.8467. [DOI] [PMC free article] [PubMed] [Google Scholar]