Abstract
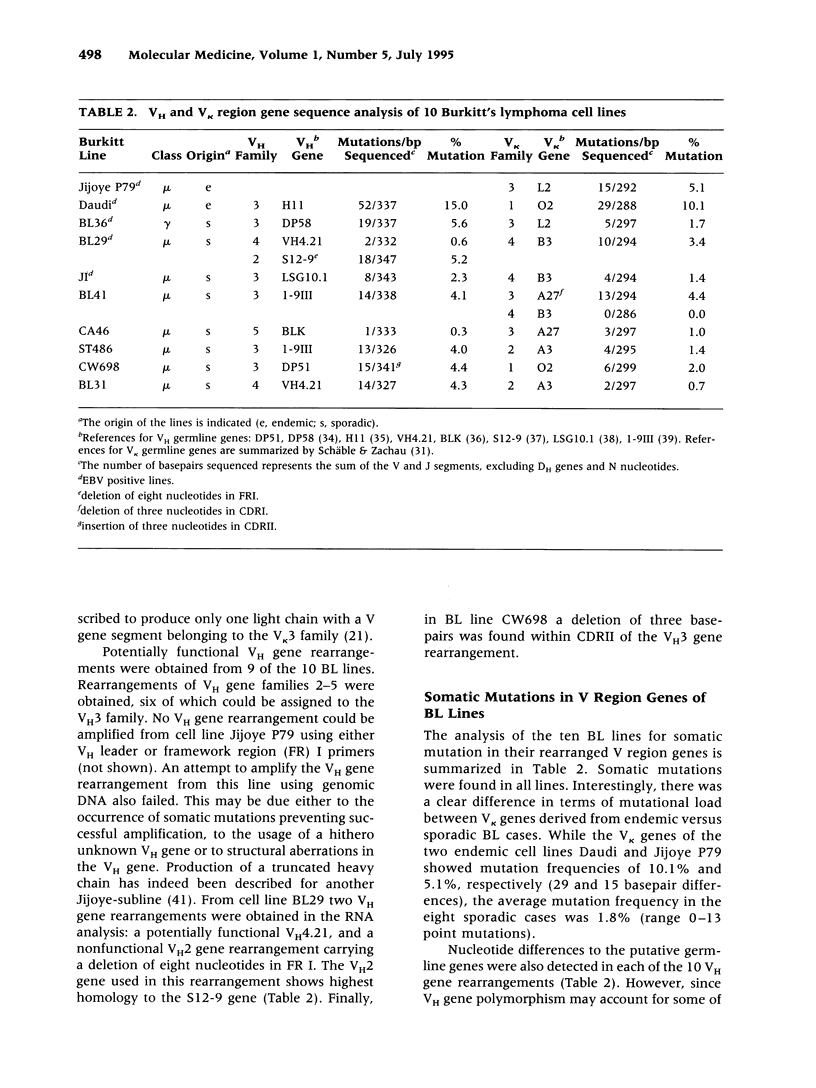
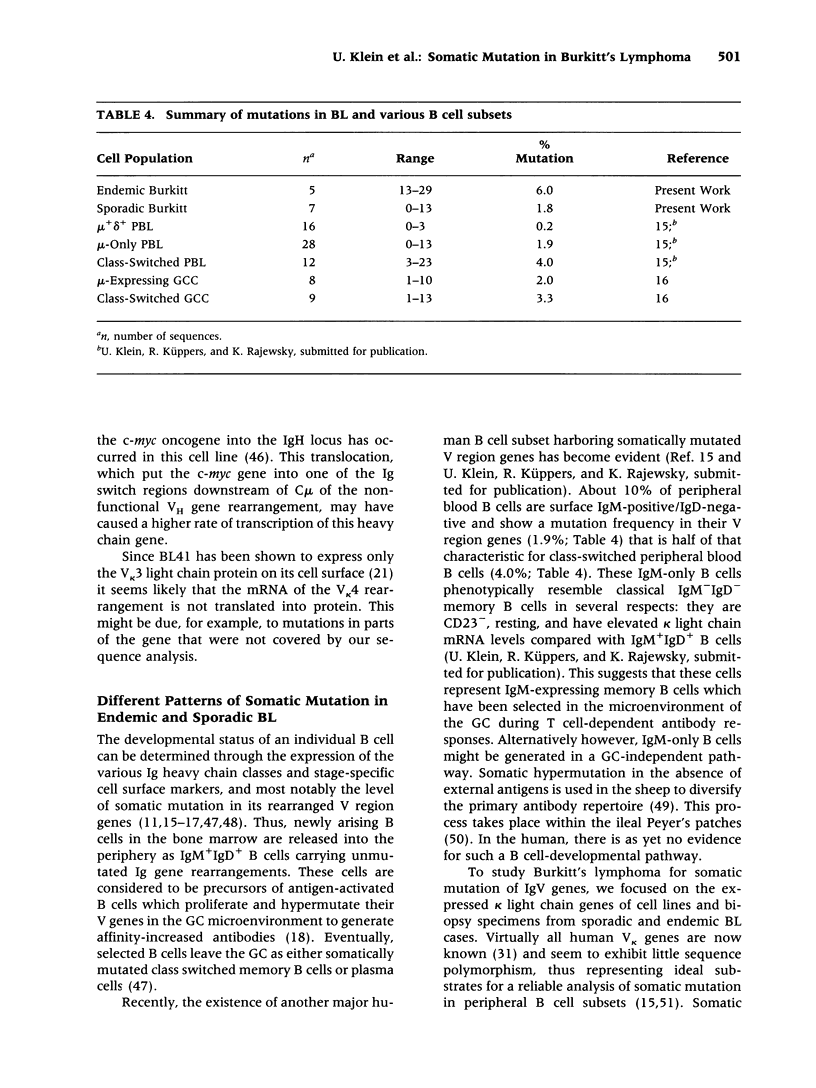
BACKGROUND: The developmental stage from which stems the malignant B cell population in Burkitt's lymphoma (BL) is unclear. An approach to answering this question is provided by the sequence analysis of rear-ranged immunoglobulin (Ig) variable region (V) genes from BL for evidence of somatic mutations, together with a phenotypic characterization. As somatic hypermutation of Ig V region genes occurs in germinal center B cells, somatically mutated Ig genes are found in germinal center B cells and their descendents. MATERIALS AND METHODS: Rearranged V kappa region genes from 10 kappa-expressing sporadic and endemic BL-derived cell lines (9 IgM and 1 IgG positive) and three kappa-expressing endemic BL biopsy specimens were amplified by polymerase chain reaction and sequenced. In addition, VH region gene sequences from these cell lines were determined. RESULTS: All BL cell lines and the three biopsy specimens carried somatically mutated V region genes. The average mutation frequency of rearranged V kappa genes from eight BL cell lines established from sporadic BL was 1.8%. A higher frequency (6%) was found in five endemic cases (three biopsy specimens and two BL cell lines). CONCLUSIONS: The detection of somatic mutations in the rearranged V region genes suggests that both sporadic and endemic BL represent a B-cell malignancy originating from germinal center B cells or their descendants. Interestingly, the mutation frequency detected in sporadic BL is in a range similar to that characteristic for IgM-expressing B cells in the human peripheral blood and for mu chain-expressing germinal center B cells, whereas the mutation frequency found in endemic BL is significantly higher.
Full text
PDF








Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adderson E. E., Azmi F. H., Wilson P. M., Shackelford P. G., Carroll W. L. The human VH3b gene subfamily is highly polymorphic. J Immunol. 1993 Jul 15;151(2):800–809. [PubMed] [Google Scholar]
- Anderson M. L., Brown L., McKenzie E., Kellow J. E., Young B. D. Cloning and sequence analysis of an Ig lambda light chain mRNA expressed in the Burkitt's lymphoma cell line EB4. Nucleic Acids Res. 1985 Apr 25;13(8):2931–2941. doi: 10.1093/nar/13.8.2931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andris J. S., Brodeur B. R., Capra J. D. Molecular characterization of human antibodies to bacterial antigens: utilization of the less frequently expressed VH2 and VH6 heavy chain variable region gene families. Mol Immunol. 1993 Dec;30(17):1601–1616. doi: 10.1016/0161-5890(93)90452-h. [DOI] [PubMed] [Google Scholar]
- Anker R., Caldwell J., Brokaw J., Pollok B. A. Characterization of immunoglobulin mRNA expression in Burkitt lymphoma cell lines. Int J Cancer. 1989 May 15;43(5):930–935. doi: 10.1002/ijc.2910430534. [DOI] [PubMed] [Google Scholar]
- Bahler D. W., Levy R. Clonal evolution of a follicular lymphoma: evidence for antigen selection. Proc Natl Acad Sci U S A. 1992 Aug 1;89(15):6770–6774. doi: 10.1073/pnas.89.15.6770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berman J. E., Mellis S. J., Pollock R., Smith C. L., Suh H., Heinke B., Kowal C., Surti U., Chess L., Cantor C. R. Content and organization of the human Ig VH locus: definition of three new VH families and linkage to the Ig CH locus. EMBO J. 1988 Mar;7(3):727–738. doi: 10.1002/j.1460-2075.1988.tb02869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carroll W. L., Yu M., Link M. P., Korsmeyer S. J. Absence of Ig V region gene somatic hypermutation in advanced Burkitt's lymphoma. J Immunol. 1989 Jul 15;143(2):692–698. [PubMed] [Google Scholar]
- Chapman C. J., Mockridge C. I., Rowe M., Rickinson A. B., Stevenson F. K. Analysis of VH genes used by neoplastic B cells in endemic Burkitt's lymphoma shows somatic hypermutation and intraclonal heterogeneity. Blood. 1995 Apr 15;85(8):2176–2181. [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleary M. L., Meeker T. C., Levy S., Lee E., Trela M., Sklar J., Levy R. Clustering of extensive somatic mutations in the variable region of an immunoglobulin heavy chain gene from a human B cell lymphoma. Cell. 1986 Jan 17;44(1):97–106. doi: 10.1016/0092-8674(86)90488-5. [DOI] [PubMed] [Google Scholar]
- Cogné M., Mounir S., Mahdi T., Preud'homme J. L., Nau F., Guglielmi P. Production of an abnormal mu chain with a shortened VHIV subgroup variable region in a Burkitt's lymphoma cell line. Mol Immunol. 1990 Sep;27(9):929–934. doi: 10.1016/0161-5890(90)90160-2. [DOI] [PubMed] [Google Scholar]
- Feuillard J., Taylor D., Casamayor-Palleja M., Johnson G. D., MacLennan I. C. Isolation and characteristics of tonsil centroblasts with reference to Ig class switching. Int Immunol. 1995 Jan;7(1):121–130. doi: 10.1093/intimm/7.1.121. [DOI] [PubMed] [Google Scholar]
- Greaves M. F., Hariri G., Newman R. A., Sutherland D. R., Ritter M. A., Ritz J. Selective expression of the common acute lymphoblastic leukemia (gp 100) antigen on immature lymphoid cells and their malignant counterparts. Blood. 1983 Apr;61(4):628–639. [PubMed] [Google Scholar]
- Gregory C. D., Tursz T., Edwards C. F., Tetaud C., Talbot M., Caillou B., Rickinson A. B., Lipinski M. Identification of a subset of normal B cells with a Burkitt's lymphoma (BL)-like phenotype. J Immunol. 1987 Jul 1;139(1):313–318. [PubMed] [Google Scholar]
- Harris N. L., Jaffe E. S., Stein H., Banks P. M., Chan J. K., Cleary M. L., Delsol G., De Wolf-Peeters C., Falini B., Gatter K. C. A revised European-American classification of lymphoid neoplasms: a proposal from the International Lymphoma Study Group. Blood. 1994 Sep 1;84(5):1361–1392. [PubMed] [Google Scholar]
- Hieter P. A., Maizel J. V., Jr, Leder P. Evolution of human immunoglobulin kappa J region genes. J Biol Chem. 1982 Feb 10;257(3):1516–1522. [PubMed] [Google Scholar]
- Jain R., Roncella S., Hashimoto S., Carbone A., Francia di Celle P., Foa R., Ferrarini M., Chiorazzi N. A potential role for antigen selection in the clonal evolution of Burkitt's lymphoma. J Immunol. 1994 Jul 1;153(1):45–52. [PubMed] [Google Scholar]
- Jäck H. M., Berg J., Wabl M. Translation affects immunoglobulin mRNA stability. Eur J Immunol. 1989 May;19(5):843–847. doi: 10.1002/eji.1830190510. [DOI] [PubMed] [Google Scholar]
- Kato S., Tachibana K., Takayama N., Kataoka H., Yoshida M. C., Takano T. Genetic recombination in a chromosomal translocation t(2;8)(p11;q24) of a Burkitt's lymphoma cell line, KOBK101. Gene. 1991 Jan 15;97(2):239–244. doi: 10.1016/0378-1119(91)90057-i. [DOI] [PubMed] [Google Scholar]
- Klein G., Klein E. Evolution of tumours and the impact of molecular oncology. Nature. 1985 May 16;315(6016):190–195. doi: 10.1038/315190a0. [DOI] [PubMed] [Google Scholar]
- Klein R., Jaenichen R., Zachau H. G. Expressed human immunoglobulin kappa genes and their hypermutation. Eur J Immunol. 1993 Dec;23(12):3248–3262. doi: 10.1002/eji.1830231231. [DOI] [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Human IgM+IgD+ B cells, the major B cell subset in the peripheral blood, express V kappa genes with no or little somatic mutation throughout life. Eur J Immunol. 1993 Dec;23(12):3272–3277. doi: 10.1002/eji.1830231232. [DOI] [PubMed] [Google Scholar]
- Klein U., Küppers R., Rajewsky K. Variable region gene analysis of B cell subsets derived from a 4-year-old child: somatically mutated memory B cells accumulate in the peripheral blood already at young age. J Exp Med. 1994 Oct 1;180(4):1383–1393. doi: 10.1084/jem.180.4.1383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Bornkamm G. W., Combriato G., Mocikat R., Pohlenz H. D., Zachau H. G. Subgroup IV of human immunoglobulin K light chains is encoded by a single germline gene. Nucleic Acids Res. 1985 Sep 25;13(18):6515–6529. doi: 10.1093/nar/13.18.6515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klobeck H. G., Meindl A., Combriato G., Solomon A., Zachau H. G. Human immunoglobulin kappa light chain genes of subgroups II and III. Nucleic Acids Res. 1985 Sep 25;13(18):6499–6513. doi: 10.1093/nar/13.18.6499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kraal G., Weissman I. L., Butcher E. C. Germinal centre B cells: antigen specificity and changes in heavy chain class expression. Nature. 1982 Jul 22;298(5872):377–379. doi: 10.1038/298377a0. [DOI] [PubMed] [Google Scholar]
- Küppers R., Zhao M., Hansmann M. L., Rajewsky K. Tracing B cell development in human germinal centres by molecular analysis of single cells picked from histological sections. EMBO J. 1993 Dec 15;12(13):4955–4967. doi: 10.1002/j.1460-2075.1993.tb06189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ling N. R., Hardie D., Lowe J., Johnson G. D., Khan M., MacLennan I. C. A phenotypic study of cells from Burkitt lymphoma and EBV-B-lymphoblastoid lines and their relationship to cells in normal lymphoid tissues. Int J Cancer. 1989 Jan 15;43(1):112–118. doi: 10.1002/ijc.2910430122. [DOI] [PubMed] [Google Scholar]
- Liu Y. J., Johnson G. D., Gordon J., MacLennan I. C. Germinal centres in T-cell-dependent antibody responses. Immunol Today. 1992 Jan;13(1):17–21. doi: 10.1016/0167-5699(92)90199-H. [DOI] [PubMed] [Google Scholar]
- Logtenberg T., Schutte M. E., Ebeling S. B., Gmelig-Meyling F. H., van Es J. H. Molecular approaches to the study of human B-cell and (auto)antibody repertoire generation and selection. Immunol Rev. 1992 Aug;128:23–47. doi: 10.1111/j.1600-065x.1992.tb00831.x. [DOI] [PubMed] [Google Scholar]
- Magrath I. The pathogenesis of Burkitt's lymphoma. Adv Cancer Res. 1990;55:133–270. doi: 10.1016/s0065-230x(08)60470-4. [DOI] [PubMed] [Google Scholar]
- Mounir S., Guglielmi P., Preud'homme J., Nau F., Cogné M. Alternate splice sites within the human VH gene coding sequences lead to truncated Ig mu-chains. J Immunol. 1990 Jan 1;144(1):342–347. [PubMed] [Google Scholar]
- Ng V. L., Hurt M. H., Fein C. L., Khayam-Bashi F., Marsh J., Nunes W. M., McPhaul L. W., Feigal E., Nelson P., Herndier B. G. IgMs produced by two acquired immune deficiency syndrome lymphoma cell lines: Ig binding specificity and VH-gene putative somatic mutation analysis. Blood. 1994 Feb 15;83(4):1067–1078. [PubMed] [Google Scholar]
- Pascual V., Liu Y. J., Magalski A., de Bouteiller O., Banchereau J., Capra J. D. Analysis of somatic mutation in five B cell subsets of human tonsil. J Exp Med. 1994 Jul 1;180(1):329–339. doi: 10.1084/jem.180.1.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rechavi G., Bienz B., Ram D., Ben-Neriah Y., Cohen J. B., Zakut R., Givol D. Organization and evolution of immunoglobulin VH gene subgroups. Proc Natl Acad Sci U S A. 1982 Jul;79(14):4405–4409. doi: 10.1073/pnas.79.14.4405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynaud C. A., Garcia C., Hein W. R., Weill J. C. Hypermutation generating the sheep immunoglobulin repertoire is an antigen-independent process. Cell. 1995 Jan 13;80(1):115–125. doi: 10.1016/0092-8674(95)90456-5. [DOI] [PubMed] [Google Scholar]
- Reynaud C. A., Mackay C. R., Müller R. G., Weill J. C. Somatic generation of diversity in a mammalian primary lymphoid organ: the sheep ileal Peyer's patches. Cell. 1991 Mar 8;64(5):995–1005. doi: 10.1016/0092-8674(91)90323-q. [DOI] [PubMed] [Google Scholar]
- Riboldi P., Gaidano G., Schettino E. W., Steger T. G., Knowles D. M., Dalla-Favera R., Casali P. Two acquired immunodeficiency syndrome-associated Burkitt's lymphomas produce specific anti-i IgM cold agglutinins using somatically mutated VH4-21 segments. Blood. 1994 May 15;83(10):2952–2961. [PMC free article] [PubMed] [Google Scholar]
- Rowe M., Rooney C. M., Edwards C. F., Lenoir G. M., Rickinson A. B. Epstein-Barr virus status and tumour cell phenotype in sporadic Burkitt's lymphoma. Int J Cancer. 1986 Mar 15;37(3):367–373. doi: 10.1002/ijc.2910370307. [DOI] [PubMed] [Google Scholar]
- Sanz I., Kelly P., Williams C., Scholl S., Tucker P., Capra J. D. The smaller human VH gene families display remarkably little polymorphism. EMBO J. 1989 Dec 1;8(12):3741–3748. doi: 10.1002/j.1460-2075.1989.tb08550.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schittek B., Rajewsky K. Natural occurrence and origin of somatically mutated memory B cells in mice. J Exp Med. 1992 Aug 1;176(2):427–438. doi: 10.1084/jem.176.2.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäble K. F., Zachau H. G. The variable genes of the human immunoglobulin kappa locus. Biol Chem Hoppe Seyler. 1993 Nov;374(11):1001–1022. [PubMed] [Google Scholar]
- Shiramizu B., Barriga F., Neequaye J., Jafri A., Dalla-Favera R., Neri A., Guttierez M., Levine P., Magrath I. Patterns of chromosomal breakpoint locations in Burkitt's lymphoma: relevance to geography and Epstein-Barr virus association. Blood. 1991 Apr 1;77(7):1516–1526. [PubMed] [Google Scholar]
- Snapper C. M., Mond J. J. Towards a comprehensive view of immunoglobulin class switching. Immunol Today. 1993 Jan;14(1):15–17. doi: 10.1016/0167-5699(93)90318-F. [DOI] [PubMed] [Google Scholar]
- Stewart A. K., Schwartz R. S. Immunoglobulin V regions and the B cell. Blood. 1994 Apr 1;83(7):1717–1730. [PubMed] [Google Scholar]
- Sun L. H., Croce C. M., Showe L. C. Cloning and sequencing of a rearranged V lambda gene from a Burkitt's lymphoma cell line expressing kappa light chains. Nucleic Acids Res. 1985 Jul 11;13(13):4921–4934. doi: 10.1093/nar/13.13.4921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tedder T. F., Clement L. T., Cooper M. D. Discontinuous expression of a membrane antigen (HB-7) during B lymphocyte differentiation. Tissue Antigens. 1984 Sep;24(3):140–149. doi: 10.1111/j.1399-0039.1984.tb02118.x. [DOI] [PubMed] [Google Scholar]
- Tomlinson I. M., Walter G., Marks J. D., Llewelyn M. B., Winter G. The repertoire of human germline VH sequences reveals about fifty groups of VH segments with different hypervariable loops. J Mol Biol. 1992 Oct 5;227(3):776–798. doi: 10.1016/0022-2836(92)90223-7. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y., Croce C. M. Molecular cloning of a human immunoglobulin lambda chain variable sequence. Nucleic Acids Res. 1984 Nov 26;12(22):8407–8414. doi: 10.1093/nar/12.22.8407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weber J. C., Blaison G., Martin T., Knapp A. M., Pasquali J. L. Evidence that the V kappa III gene usage is nonstochastic in both adult and newborn peripheral B cells and that peripheral CD5+ adult B cells are oligoclonal. J Clin Invest. 1994 May;93(5):2093–2105. doi: 10.1172/JCI117204. [DOI] [PMC free article] [PubMed] [Google Scholar]



