Abstract
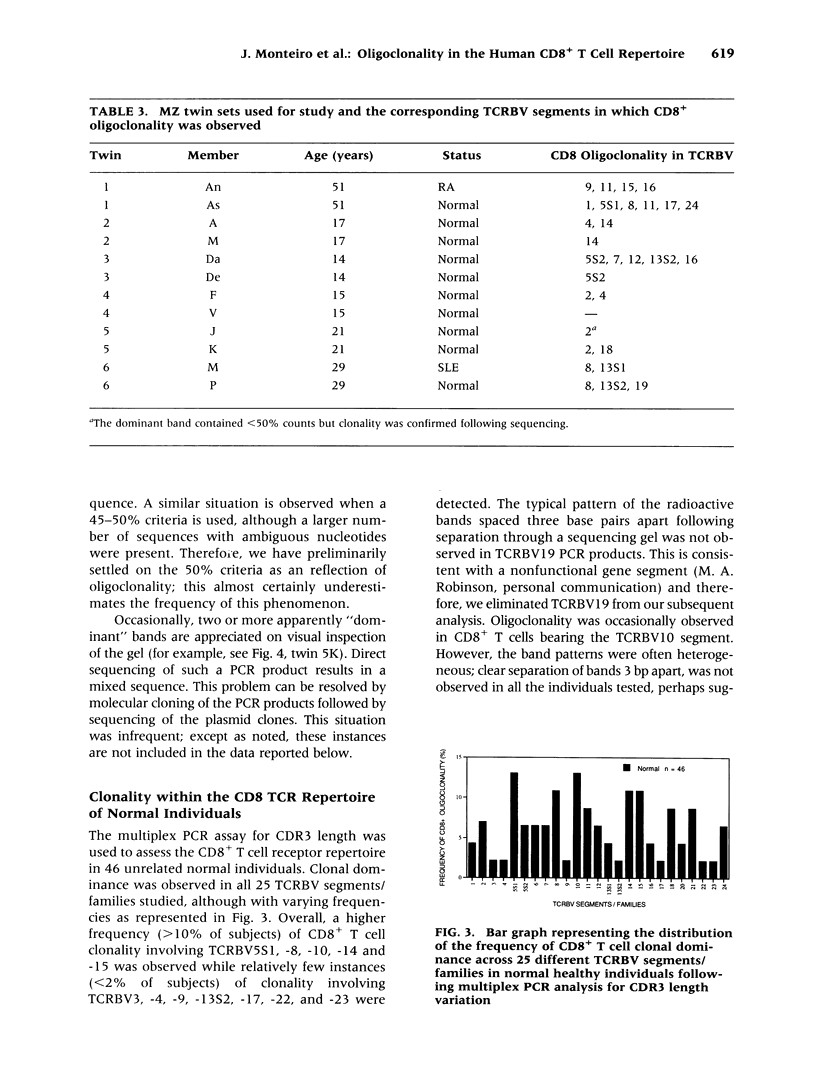
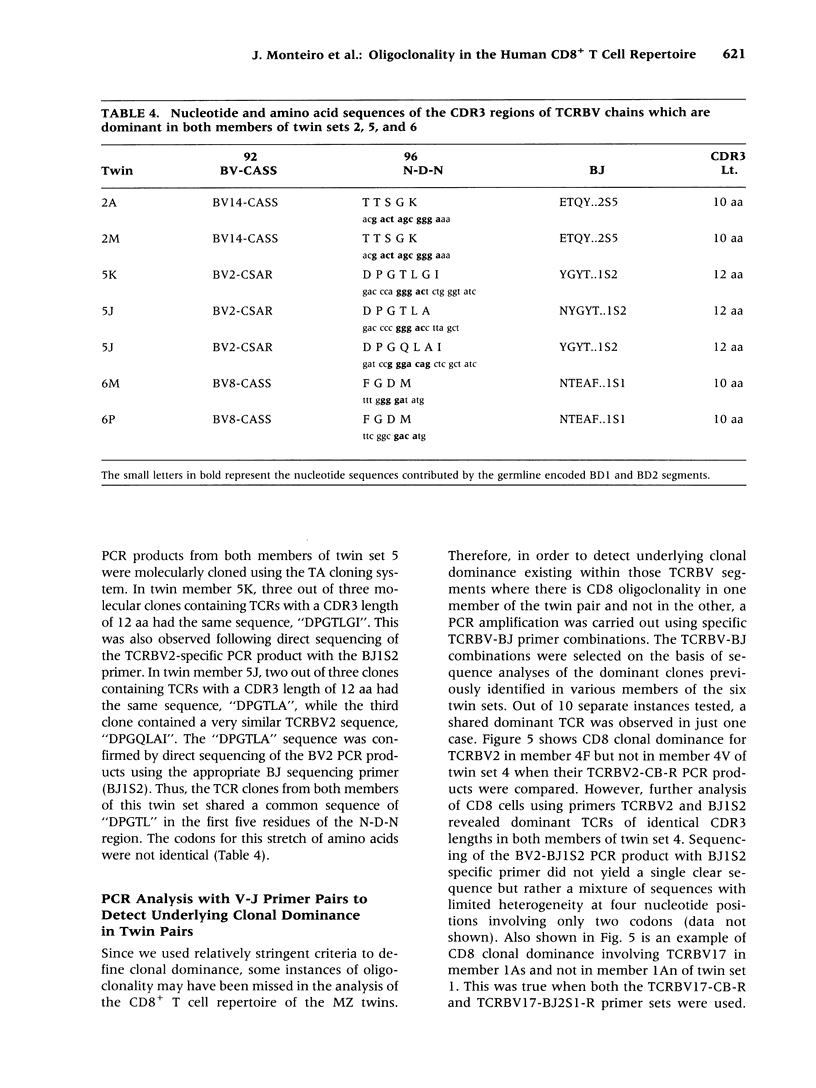
BACKGROUND: We have previously demonstrated CD8+ T cell clonal dominance using a PCR assay for the CDR3 length of T cell receptors belonging to a limited number of TCRBV segments/families. In this study, we have modified this approach in order to analyze more comprehensively the frequency of oligoclonality in the CD8+ T cell subset in 25 known TCRBV segments/families. In order to assess the relative roles of genes and environment in the shaping of a clonally restricted CD8+ T cell repertoire, we have analyzed clonal dominance in the CD8+ T cell population of monozygotic twins, related siblings, and adoptees. MATERIALS AND METHODS: Oligoclonality was assessed in the CD8+ T cell subsets using a multiplex PCR approach to assay for CDR3 length variation across 25 different TCRBV segments/families. Specific criteria for oligoclonality were established, and confirmed by direct sequence analysis of the PCR products. This assay was used to investigate the CD8+ T cell repertoire of 56 normal subjects, as well as six sets of monozygotic (MZ) twins. RESULTS: Seventy-two percent of normal subjects (n = 56) had evidence of oligoclonality in the CD8+ T cell subset, using well-defined criteria. Although MZ twins frequently displayed CD8+ T cell clonal dominance, the overall pattern of oligoclonality was very diverse within each twin pair. However, we occasionally observed dominant CD8+ T cell clones that were highly similar in sequence in both members of some twin pairs. Not a single example of such similarity was observed in normal controls or siblings. CONCLUSIONS: Oligoclonality of circulating CD8+ T cells is a characteristic feature of the human immune system; both host genetic factors and environment shape the pattern of oligoclonality in this T cell subset. The high frequency of this phenomenon in normal subjects provides a background with which to evaluate CD8+ T cell oligoclonality in the setting of infection or autoimmune disease. Further phenotypic and functional characterization of these clonally expanded T cells should provide insight into normal immune homeostasis.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Candéias S., Waltzinger C., Benoist C., Mathis D. The V beta 17+ T cell repertoire: skewed J beta usage after thymic selection; dissimilar CDR3s in CD4+ versus CD8+ cells. J Exp Med. 1991 Nov 1;174(5):989–1000. doi: 10.1084/jem.174.5.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casanova J. L., Cerottini J. C., Matthes M., Necker A., Gournier H., Barra C., Widmann C., MacDonald H. R., Lemonnier F., Malissen B. H-2-restricted cytolytic T lymphocytes specific for HLA display T cell receptors of limited diversity. J Exp Med. 1992 Aug 1;176(2):439–447. doi: 10.1084/jem.176.2.439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis M. M., Bjorkman P. J. T-cell antigen receptor genes and T-cell recognition. Nature. 1988 Aug 4;334(6181):395–402. doi: 10.1038/334395a0. [DOI] [PubMed] [Google Scholar]
- Deapen D., Escalante A., Weinrib L., Horwitz D., Bachman B., Roy-Burman P., Walker A., Mack T. M. A revised estimate of twin concordance in systemic lupus erythematosus. Arthritis Rheum. 1992 Mar;35(3):311–318. doi: 10.1002/art.1780350310. [DOI] [PubMed] [Google Scholar]
- DerSimonian H., Sugita M., Glass D. N., Maier A. L., Weinblatt M. E., Rème T., Brenner M. B. Clonal V alpha 12.1+ T cell expansions in the peripheral blood of rheumatoid arthritis patients. J Exp Med. 1993 Jun 1;177(6):1623–1631. doi: 10.1084/jem.177.6.1623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fitzgerald J. E., Ricalton N. S., Meyer A. C., West S. G., Kaplan H., Behrendt C., Kotzin B. L. Analysis of clonal CD8+ T cell expansions in normal individuals and patients with rheumatoid arthritis. J Immunol. 1995 Apr 1;154(7):3538–3547. [PubMed] [Google Scholar]
- Gorski J., Yassai M., Zhu X., Kissela B., Kissella B [corrected to Kissela B. ]., Keever C., Flomenberg N. Circulating T cell repertoire complexity in normal individuals and bone marrow recipients analyzed by CDR3 size spectratyping. Correlation with immune status. J Immunol. 1994 May 15;152(10):5109–5119. [PubMed] [Google Scholar]
- Grunewald J., Jeddi-Tehrani M., Dersimonian H., Andersson R., Wigzell H. A persistent T cell expansion in the peripheral blood of a normal adult male: a new clinical entity? Clin Exp Immunol. 1992 Aug;89(2):279–284. doi: 10.1111/j.1365-2249.1992.tb06945.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hingorani R., Choi I. H., Akolkar P., Gulwani-Akolkar B., Pergolizzi R., Silver J., Gregersen P. K. Clonal predominance of T cell receptors within the CD8+ CD45RO+ subset in normal human subjects. J Immunol. 1993 Nov 15;151(10):5762–5769. [PubMed] [Google Scholar]
- Jorgensen J. L., Reay P. A., Ehrich E. W., Davis M. M. Molecular components of T-cell recognition. Annu Rev Immunol. 1992;10:835–873. doi: 10.1146/annurev.iy.10.040192.004155. [DOI] [PubMed] [Google Scholar]
- Morley J. K., Batliwalla F. M., Hingorani R., Gregersen P. K. Oligoclonal CD8+ T cells are preferentially expanded in the CD57+ subset. J Immunol. 1995 Jun 1;154(11):6182–6190. [PubMed] [Google Scholar]
- Oksenberg J. R., Panzara M. A., Begovich A. B., Mitchell D., Erlich H. A., Murray R. S., Shimonkevitz R., Sherritt M., Rothbard J., Bernard C. C. Selection for T-cell receptor V beta-D beta-J beta gene rearrangements with specificity for a myelin basic protein peptide in brain lesions of multiple sclerosis. Nature. 1993 Mar 4;362(6415):68–70. doi: 10.1038/362068a0. [DOI] [PubMed] [Google Scholar]
- Paliard X., West S. G., Lafferty J. A., Clements J. R., Kappler J. W., Marrack P., Kotzin B. L. Evidence for the effects of a superantigen in rheumatoid arthritis. Science. 1991 Jul 19;253(5017):325–329. doi: 10.1126/science.1857971. [DOI] [PubMed] [Google Scholar]
- Pannetier C., Cochet M., Darche S., Casrouge A., Zöller M., Kourilsky P. The sizes of the CDR3 hypervariable regions of the murine T-cell receptor beta chains vary as a function of the recombined germ-line segments. Proc Natl Acad Sci U S A. 1993 May 1;90(9):4319–4323. doi: 10.1073/pnas.90.9.4319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pantaleo G., Demarest J. F., Soudeyns H., Graziosi C., Denis F., Adelsberger J. W., Borrow P., Saag M. S., Shaw G. M., Sekaly R. P. Major expansion of CD8+ T cells with a predominant V beta usage during the primary immune response to HIV. Nature. 1994 Aug 11;370(6489):463–467. doi: 10.1038/370463a0. [DOI] [PubMed] [Google Scholar]
- Posnett D. N., Sinha R., Kabak S., Russo C. Clonal populations of T cells in normal elderly humans: the T cell equivalent to "benign monoclonal gammapathy". J Exp Med. 1994 Feb 1;179(2):609–618. doi: 10.1084/jem.179.2.609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Puisieux I., Even J., Pannetier C., Jotereau F., Favrot M., Kourilsky P. Oligoclonality of tumor-infiltrating lymphocytes from human melanomas. J Immunol. 1994 Sep 15;153(6):2807–2818. [PubMed] [Google Scholar]
- Shanmugam A., Copie-Bergman C., Hashim G., Rebibo D., Jais J. P., Bach J. F., Bach M. A., Tournier-Lasserve E. Healthy monozygous twins do not recognize identical T cell epitopes on the myelin basic protein autoantigen. Eur J Immunol. 1994 Oct;24(10):2299–2303. doi: 10.1002/eji.1830241006. [DOI] [PubMed] [Google Scholar]
- Trejo V., Derom C., Vlietinck R., Ollier W., Silman A., Ebers G., Derom R., Gregersen P. K. X chromosome inactivation patterns correlate with fetal-placental anatomy in monozygotic twin pairs: implications for immune relatedness and concordance for autoimmunity. Mol Med. 1994 Nov;1(1):62–70. [PMC free article] [PubMed] [Google Scholar]
- Wang E. C., Taylor-Wiedeman J., Perera P., Fisher J., Borysiewicz L. K. Subsets of CD8+, CD57+ cells in normal, healthy individuals: correlations with human cytomegalovirus (HCMV) carrier status, phenotypic and functional analyses. Clin Exp Immunol. 1993 Nov;94(2):297–305. doi: 10.1111/j.1365-2249.1993.tb03447.x. [DOI] [PMC free article] [PubMed] [Google Scholar]