Abstract
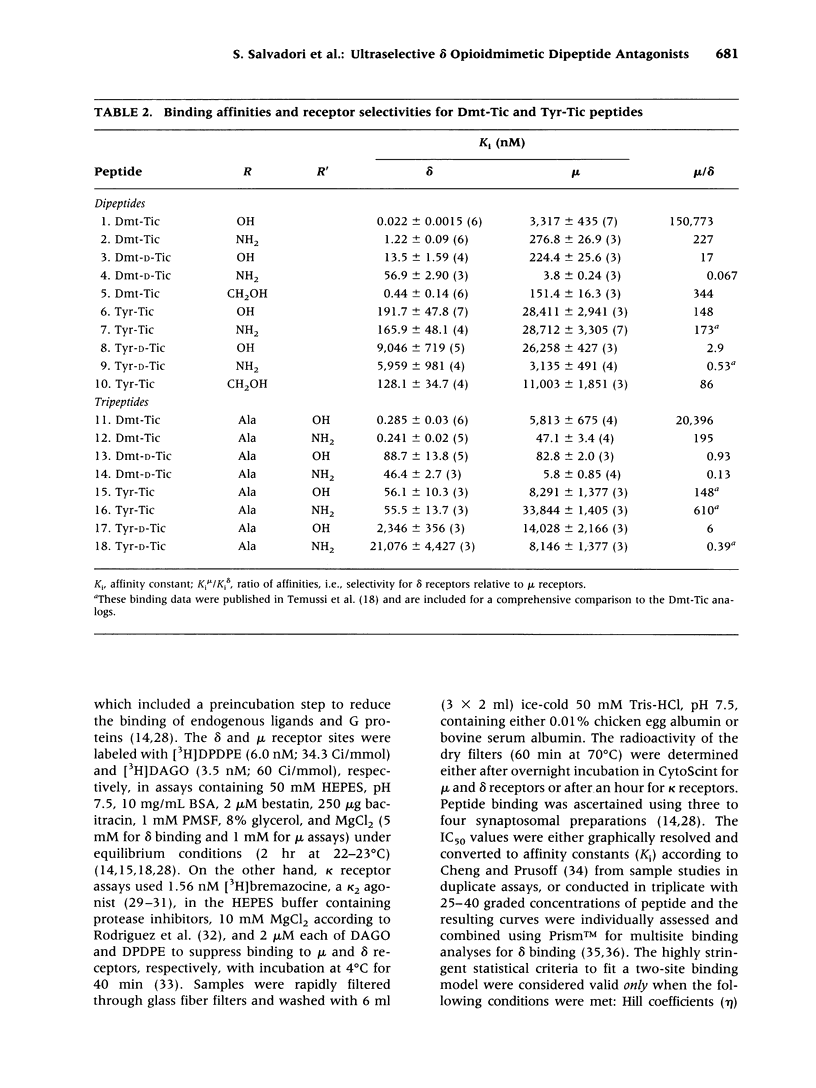
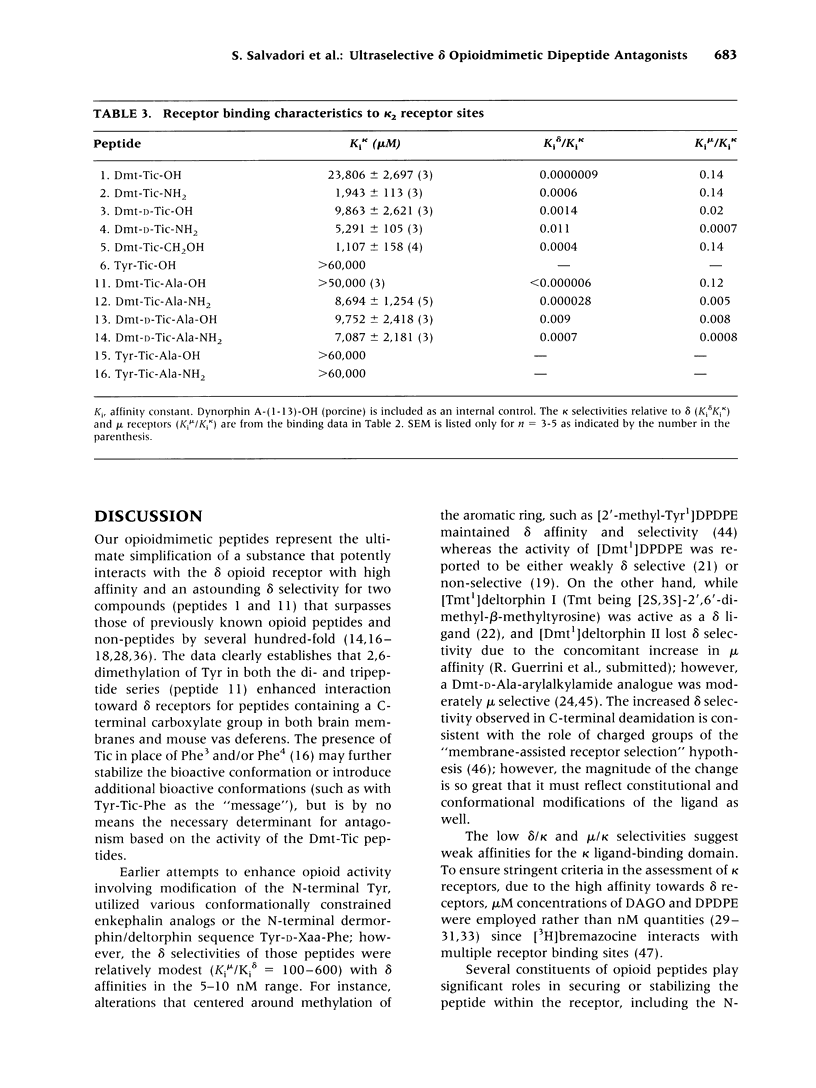
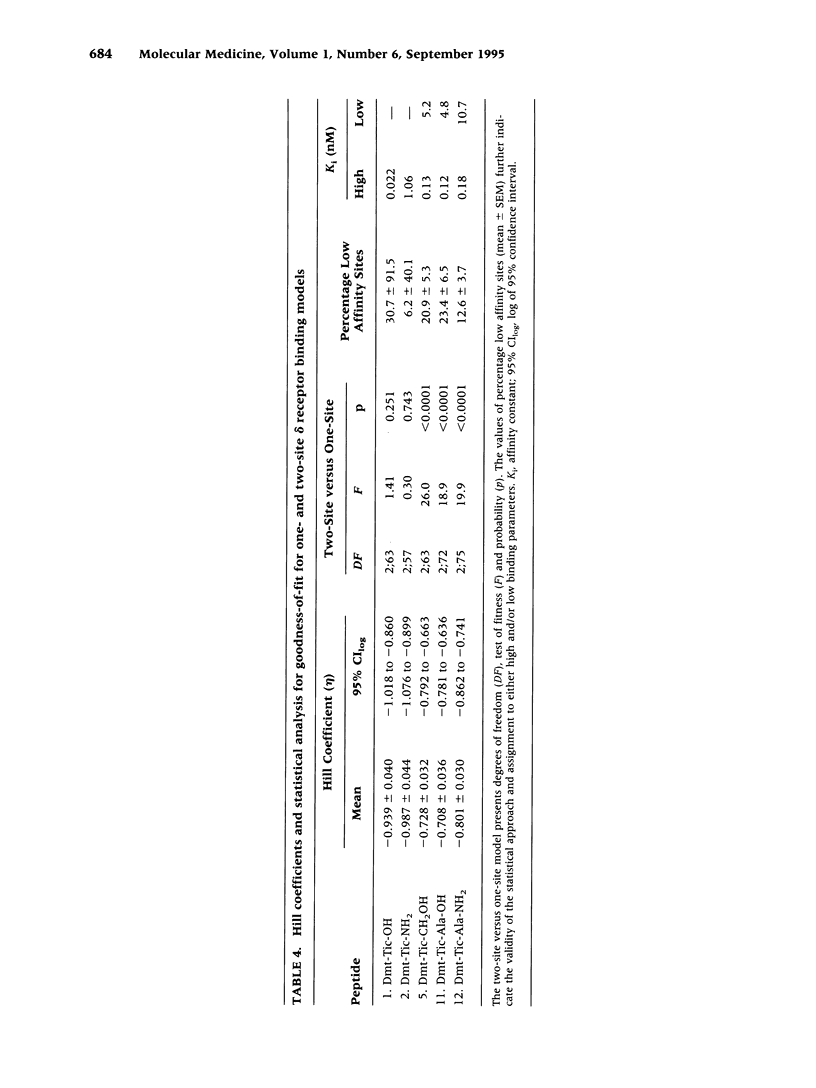
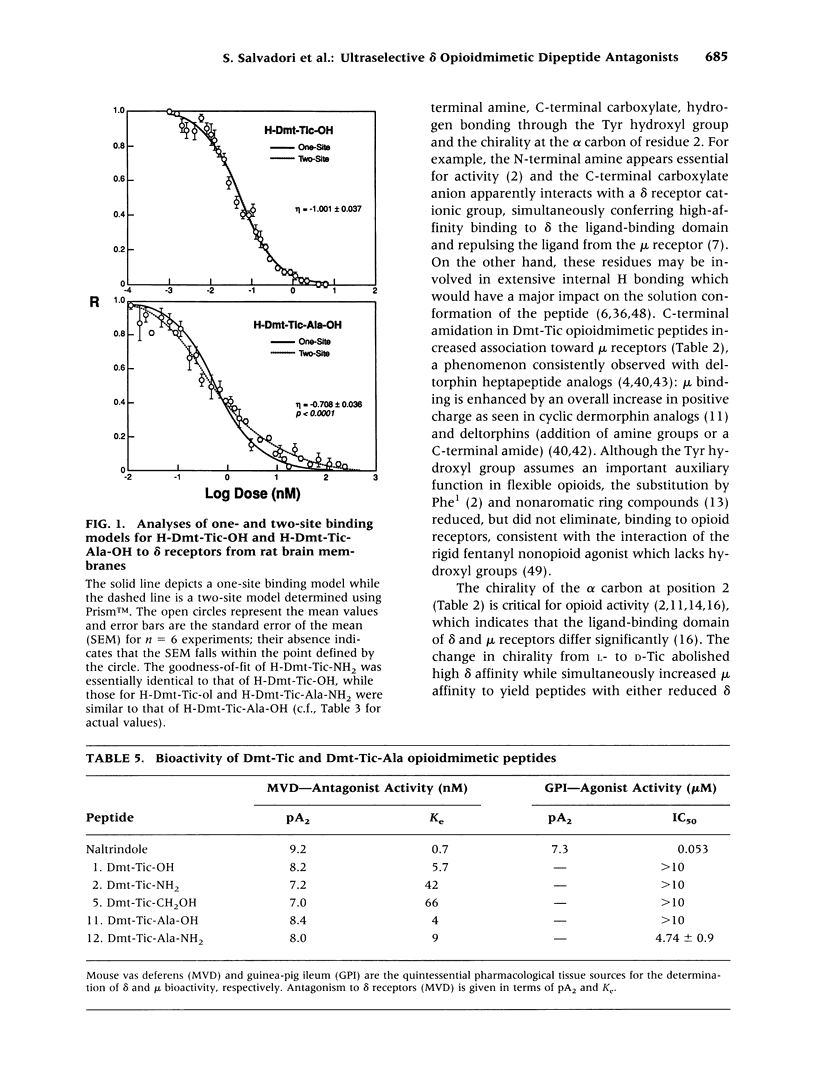
BACKGROUND: Tyr-Tic (1,2,3,4-tetrahydroisoquinoline-3-carboxylic acid) and Tyr-Tic-Ala were the first peptides with delta opioid antagonist activity lacking Phe, considered essential for opioid activity based on the N-terminal tripeptide sequence (Tyr-D-Xaa-Phe) of amphibian skin opioids. Analogs were then designed to restrain the rotational flexibility of Tyr by the substitution of 2,6-dimethyl-L-tyrosine (Dmt). MATERIALS AND METHODS: Tyr and Dmt peptides were synthesized by solid phase and solution methods using Fmoc technology or condensing Boc-Dmt-OH or Boc-Tyr(But)-OH with H-L-Tic-OBut or H-D-Tic-OBut, respectively. Peptides were purified (> 99%) by HPLC and characteristics determined by 1H-NMR, FAB-MS, melting point, TLC, and amino acid analyses. RESULTS: H-Dmt-Tic-OH had high affinity (Ki delta = 0.022 nM) and extraordinary selectivity (Ki mu/Ki delta = 150,000); H-Dmt-Tic-Ala-OH had a Ki delta = 0.29 nM and delta selectivity = 20,000. Affinity and selectivity increased 8700- and 1000-fold relative to H-Tyr-Tic-OH, respectively. H-Dmt-Tic-OH and H-Dmt-Tic-NH2 fitted one-site receptor binding models (eta = 0.939-0.987), while H-Dmt-Tic-ol, H-Dmt-Tic-Ala-OH and H-Dmt-Tic-Ala-NH2 best fitted two-site models (eta = 0.708-0.801, F 18.9-26.0, p < 0.0001). Amidation increased mu affinity by 10- to 100-fold and acted synergistically with D-Tic2 to reverse selectivity (delta-->mu). Dmt-Tic di- and tripeptides exhibited delta antagonist bioactivity (Ke = 4-66 nM) with mouse vas deferens and lacked agonist mu activity (> 10 microM) in guinea-pig ileum preparations. Dmt-Tic analogs weakly interacted with kappa receptors in the 1 to > 20 microM range. CONCLUSIONS: Dmt-Tic opioidmimetic peptides represent a highly potent class of opioid peptide antagonists with greater potency than the nonopioid delta antagonist naltrindole and have potential application as clinical and therapeutic compounds.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Amodeo P., Motta A., Tancredi T., Salvadori S., Tomatis R., Picone D., Saviano G., Temussi P. A. Solution structure of deltorphin I at 265 K: a quantitative NMR study. Pept Res. 1992 Jan-Feb;5(1):48–55. [PubMed] [Google Scholar]
- Attila M., Salvadori S., Balboni G., Bryant S. D., Lazarus L. H. Synthesis and receptor binding analysis of dermorphin hepta-, hexa- and pentapeptide analogues. Evidence for one- and two-side binding models for the mu-opioid receptor. Int J Pept Protein Res. 1993 Dec;42(6):550–559. doi: 10.1111/j.1399-3011.1993.tb00363.x. [DOI] [PubMed] [Google Scholar]
- Balboni G., Marastoni M., Picone D., Salvadori S., Tancredi T., Temussi P. A., Tomatis R. New features of the delta opioid receptor: conformational properties of deltorphin I analogues. Biochem Biophys Res Commun. 1990 Jun 15;169(2):617–622. doi: 10.1016/0006-291x(90)90375-w. [DOI] [PubMed] [Google Scholar]
- Bilsky E. J., Bernstein R. N., Pasternak G. W., Hruby V. J., Patel D., Porreca F., Lai J. Selective inhibition of [D-Ala2, Glu4]deltorphin antinociception by supraspinal, but not spinal, administration of an antisense oligodeoxynucleotide to an opioid delta receptor. Life Sci. 1994;55(2):PL37–PL43. doi: 10.1016/0024-3205(94)90110-4. [DOI] [PubMed] [Google Scholar]
- Bryant S. D., Attila M., Salvadori S., Guerrini R., Lazarus L. H. Molecular dynamics conformations of deltorphin analogues advocate delta opioid binding site models. Pept Res. 1994 Jul-Aug;7(4):175–184. [PubMed] [Google Scholar]
- Castiglione-Morelli M. A., Lelj F., Pastore A., Salvadori S., Tancredi T., Tomatis R., Trivellone E., Temussi P. A. A 500-MHz proton nuclear magnetic resonance study of mu opioid peptides in a simulated receptor environment. J Med Chem. 1987 Nov;30(11):2067–2073. doi: 10.1021/jm00394a023. [DOI] [PubMed] [Google Scholar]
- Chandrakumar N. S., Stapelfeld A., Beardsley P. M., Lopez O. T., Drury B., Anthony E., Savage M. A., Williamson L. N., Reichman M. Analogs of the delta opioid receptor selective cyclic peptide [2-D-penicillamine,5-D-penicillamine]-enkephalin: 2',6'-dimethyltyrosine and Gly3-Phe4 amide bond isostere substitutions. J Med Chem. 1992 Aug 7;35(16):2928–2938. doi: 10.1021/jm00094a002. [DOI] [PubMed] [Google Scholar]
- Chandrakumar N. S., Yonan P. K., Stapelfeld A., Savage M., Rorbacher E., Contreras P. C., Hammond D. Preparation and opioid activity of analogues of the analgesic dipeptide 2,6-dimethyl-L-tyrosyl-N-(3-phenylpropyl)-D-alaninamide. J Med Chem. 1992 Jan 24;35(2):223–233. doi: 10.1021/jm00080a005. [DOI] [PubMed] [Google Scholar]
- Charpentier S., Sagan S., Delfour A., Nicolas P. Dermenkephalin and deltorphin I reveal similarities within ligand-binding domains of mu- and delta-opioid receptors and an additional address subsite on the delta-receptor. Biochem Biophys Res Commun. 1991 Sep 30;179(3):1161–1168. doi: 10.1016/0006-291x(91)91693-7. [DOI] [PubMed] [Google Scholar]
- Cheng Y., Prusoff W. H. Relationship between the inhibition constant (K1) and the concentration of inhibitor which causes 50 per cent inhibition (I50) of an enzymatic reaction. Biochem Pharmacol. 1973 Dec 1;22(23):3099–3108. doi: 10.1016/0006-2952(73)90196-2. [DOI] [PubMed] [Google Scholar]
- Collin E., Cesselin F. Neurobiological mechanisms of opioid tolerance and dependence. Clin Neuropharmacol. 1991 Dec;14(6):465–488. doi: 10.1097/00002826-199112000-00001. [DOI] [PubMed] [Google Scholar]
- Corbett A. D., Paterson S. J., McKnight A. T., Magnan J., Kosterlitz H. W. Dynorphin and dynorphin are ligands for the kappa-subtype of opiate receptor. Nature. 1982 Sep 2;299(5878):79–81. doi: 10.1038/299079a0. [DOI] [PubMed] [Google Scholar]
- Costopanagiotis A. A., Preston J., Weinstein B. Amino acids and peptides. V. Synthesis of the C-terminal tripeptide sequence (A27-A29) of glucagon. J Org Chem. 1966 Oct;31(10):3398–3400. doi: 10.1021/jo01348a503. [DOI] [PubMed] [Google Scholar]
- Erspamer V. The opioid peptides of the amphibian skin. Int J Dev Neurosci. 1992;10(1):3–30. doi: 10.1016/0736-5748(92)90003-i. [DOI] [PubMed] [Google Scholar]
- Hansen D. W., Jr, Stapelfeld A., Savage M. A., Reichman M., Hammond D. L., Haaseth R. C., Mosberg H. I. Systemic analgesic activity and delta-opioid selectivity in [2,6-dimethyl-Tyr1,D-Pen2,D-Pen5]enkephalin. J Med Chem. 1992 Feb 21;35(4):684–687. doi: 10.1021/jm00082a008. [DOI] [PubMed] [Google Scholar]
- Hruby V. J., Gehrig C. A. Recent developments in the design of receptor specific opioid peptides. Med Res Rev. 1989 Jul-Sep;9(3):343–401. doi: 10.1002/med.2610090306. [DOI] [PubMed] [Google Scholar]
- Kosterlitz H. W., Lees G. M., Wallis D. I., Watt A. J. Non-specific inhibitory effects of morphine-like drugs on transmission in the superior cervical ganglion and guinea-pig isolated ileum. Br J Pharmacol. 1968 Nov;34(3):691P–692P. [PMC free article] [PubMed] [Google Scholar]
- Lazarus L. H., Salvadori S., Attila M., Grieco P., Bundy D. M., Wilson W. E., Tomatis R. Interaction of deltorphin with opioid receptors: molecular determinants for affinity and selectivity. Peptides. 1993 Jan-Feb;14(1):21–28. doi: 10.1016/0196-9781(93)90006-3. [DOI] [PubMed] [Google Scholar]
- Lazarus L. H., Salvadori S., Balboni G., Tomatis R., Wilson W. E. Stereospecificity of amino acid side chains in deltorphin defines binding to opioid receptors. J Med Chem. 1992 Apr 3;35(7):1222–1227. doi: 10.1021/jm00085a009. [DOI] [PubMed] [Google Scholar]
- Lazarus L. H., Salvadori S., Santagada V., Tomatis R., Wilson W. E. Function of negative charge in the "address domain" of deltorphins. J Med Chem. 1991 Apr;34(4):1350–1355. doi: 10.1021/jm00108a017. [DOI] [PubMed] [Google Scholar]
- Lazarus L. H., Salvadori S., Tomatis R., Wilson W. E. Opioid receptor selectivity reversal in deltorphin tetrapeptide analogues. Biochem Biophys Res Commun. 1991 Jul 15;178(1):110–115. doi: 10.1016/0006-291x(91)91786-c. [DOI] [PubMed] [Google Scholar]
- Lazarus L. H., Wilson W. E., de Castiglione R., Guglietta A. Dermorphin gene sequence peptide with high affinity and selectivity for delta-opioid receptors. J Biol Chem. 1989 Feb 25;264(6):3047–3050. [PubMed] [Google Scholar]
- Marastoni M., Tomatis R., Balboni G., Salvadori S., Lazarus L. H. On the degradation of the deltorphin peptides by plasma and brain homogenate. Farmaco. 1991 Nov;46(11):1273–1279. [PubMed] [Google Scholar]
- Marsden B. J., Nguyen T. M., Schiller P. W. Spontaneous degradation via diketopiperazine formation of peptides containing a tetrahydroisoquinoline-3-carboxylic acid residue in the 2-position of the peptide sequence. Int J Pept Protein Res. 1993 Mar;41(3):313–316. doi: 10.1111/j.1399-3011.1993.tb00340.x. [DOI] [PubMed] [Google Scholar]
- Melchiorri P., Negri L., Falconieri-Erspamer G., Severini C., Corsi R., Soaje M., Erspamer V., Barra D. Structure-activity relationships of the delta-opioid-selective agonists, deltorphins. Eur J Pharmacol. 1991 Mar 26;195(2):201–207. doi: 10.1016/0014-2999(91)90536-y. [DOI] [PubMed] [Google Scholar]
- Mosberg H. I., Kroona H. B. Incorporation of a novel conformationally restricted tyrosine analog into a cyclic, delta opioid receptor selective tetrapeptide (JOM-13) enhances delta receptor binding affinity and selectivity. J Med Chem. 1992 Nov 13;35(23):4498–4500. doi: 10.1021/jm00101a030. [DOI] [PubMed] [Google Scholar]
- Pitzele B. S., Hamilton R. W., Kudla K. D., Tsymbalov S., Stapelfeld A., Savage M. A., Clare M., Hammond D. L., Hansen D. W., Jr Enkephalin analogs as systemically active antinociceptive agents: O- and N-alkylated derivatives of the dipeptide amide L-2,6-dimethyltyrosyl-N-(3-phenylpropyl)-D-alaninamide. J Med Chem. 1994 Apr 1;37(7):888–896. doi: 10.1021/jm00033a005. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S. Bivalent ligands and the message-address concept in the design of selective opioid receptor antagonists. Trends Pharmacol Sci. 1989 Jun;10(6):230–235. doi: 10.1016/0165-6147(89)90267-8. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S. Edward E. Smissman-Bristol-Myers Squibb Award Address. The role of concepts in structure-activity relationship studies of opioid ligands. J Med Chem. 1992 May 29;35(11):1927–1937. doi: 10.1021/jm00089a001. [DOI] [PubMed] [Google Scholar]
- Portoghese P. S., Sultana M., Takemori A. E. Naltrindole, a highly selective and potent non-peptide delta opioid receptor antagonist. Eur J Pharmacol. 1988 Jan 27;146(1):185–186. doi: 10.1016/0014-2999(88)90502-x. [DOI] [PubMed] [Google Scholar]
- Qian X., Kövér K. E., Shenderovich M. D., Lou B. S., Misicka A., Zalewska T., Horváth R., Davis P., Bilsky E. J., Porreca F. Newly discovered stereochemical requirements in the side-chain conformation of delta opioid agonists for recognizing opioid delta receptors. J Med Chem. 1994 Jun 10;37(12):1746–1757. doi: 10.1021/jm00038a004. [DOI] [PubMed] [Google Scholar]
- Rodriguez F. D., Bardaji E., Traynor J. R. Differential effects of Mg2+ and other divalent cations on the binding of tritiated opioid ligands. J Neurochem. 1992 Aug;59(2):467–472. doi: 10.1111/j.1471-4159.1992.tb09393.x. [DOI] [PubMed] [Google Scholar]
- Sagan S., Amiche M., Delfour A., Camus A., Mor A., Nicolas P. Differential contribution of C-terminal regions of dermorphin and dermenkephalin to opioid-sites selection and binding potency. Biochem Biophys Res Commun. 1989 Sep 15;163(2):726–732. doi: 10.1016/0006-291x(89)92283-3. [DOI] [PubMed] [Google Scholar]
- Sagan S., Amiche M., Delfour A., Mor A., Camus A., Nicolas P. Molecular determinants of receptor affinity and selectivity of the natural delta-opioid agonist, dermenkephalin. J Biol Chem. 1989 Oct 15;264(29):17100–17106. [PubMed] [Google Scholar]
- Sagan S., Charpentier S., Delfour A., Amiche M., Nicolas P. The aspartic acid in deltorphin I and dermenkephalin promotes targeting to delta-opioid receptor independently of receptor binding. Biochem Biophys Res Commun. 1992 Sep 30;187(3):1203–1210. doi: 10.1016/0006-291x(92)90431-j. [DOI] [PubMed] [Google Scholar]
- Salvadori S., Bryant S. D., Bianchi C., Balboni G., Scaranari V., Attila M., Lazarus L. H. Phe3-substituted analogues of deltorphin C. Spatial conformation and topography of the aromatic ring in peptide recognition by delta opioid receptors. J Med Chem. 1993 Nov 26;36(24):3748–3756. doi: 10.1021/jm00076a001. [DOI] [PubMed] [Google Scholar]
- Schild H. O. pA, a new scale for the measurement of drug antagonism. 1947. Br J Pharmacol. 1997 Feb;120(4 Suppl):29–28. doi: 10.1111/j.1476-5381.1997.tb06773.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller P. W., Nguyen T. M., Chung N. N., Lemieux C. Dermorphin analogues carrying an increased positive net charge in their "message" domain display extremely high mu opioid receptor selectivity. J Med Chem. 1989 Mar;32(3):698–703. doi: 10.1021/jm00123a035. [DOI] [PubMed] [Google Scholar]
- Schiller P. W., Nguyen T. M., Weltrowska G., Wilkes B. C., Marsden B. J., Lemieux C., Chung N. N. Differential stereochemical requirements of mu vs. delta opioid receptors for ligand binding and signal transduction: development of a class of potent and highly delta-selective peptide antagonists. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11871–11875. doi: 10.1073/pnas.89.24.11871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller P. W., Weltrowska G., Nguyen T. M., Lemieux C., Chung N. N., Marsden B. J., Wilkes B. C. Conformational restriction of the phenylalanine residue in a cyclic opioid peptide analogue: effects on receptor selectivity and stereospecificity. J Med Chem. 1991 Oct;34(10):3125–3132. doi: 10.1021/jm00114a023. [DOI] [PubMed] [Google Scholar]
- Schiller P. W., Weltrowska G., Nguyen T. M., Wilkes B. C., Chung N. N., Lemieux C. Conformationally restricted deltorphin analogues. J Med Chem. 1992 Oct 16;35(21):3956–3961. doi: 10.1021/jm00099a025. [DOI] [PubMed] [Google Scholar]
- Schiller P. W., Weltrowska G., Nguyen T. M., Wilkes B. C., Chung N. N., Lemieux C. TIPP[psi]: a highly potent and stable pseudopeptide delta opioid receptor antagonist with extraordinary delta selectivity. J Med Chem. 1993 Oct 15;36(21):3182–3187. doi: 10.1021/jm00073a020. [DOI] [PubMed] [Google Scholar]
- Schwyzer R. Molecular mechanism of opioid receptor selection. Biochemistry. 1986 Oct 7;25(20):6335–6342. doi: 10.1021/bi00368a075. [DOI] [PubMed] [Google Scholar]
- Snyder K. R., Story S. C., Heidt M. E., Murray T. F., DeLander G. E., Aldrich J. V. Effect of modification of the basic residues of dynorphin A-(1-13) amide on kappa opioid receptor selectivity and opioid activity. J Med Chem. 1992 Nov 13;35(23):4330–4333. doi: 10.1021/jm00101a010. [DOI] [PubMed] [Google Scholar]
- Standifer K. M., Cheng J., Brooks A. I., Honrado C. P., Su W., Visconti L. M., Biedler J. L., Pasternak G. W. Biochemical and pharmacological characterization of mu, delta and kappa 3 opioid receptors expressed in BE(2)-C neuroblastoma cells. J Pharmacol Exp Ther. 1994 Sep;270(3):1246–1255. [PubMed] [Google Scholar]
- Standifer K. M., Chien C. C., Wahlestedt C., Brown G. P., Pasternak G. W. Selective loss of delta opioid analgesia and binding by antisense oligodeoxynucleotides to a delta opioid receptor. Neuron. 1994 Apr;12(4):805–810. doi: 10.1016/0896-6273(94)90333-6. [DOI] [PubMed] [Google Scholar]
- Szabadi E. A model of two functionally antagonistic receptor populations activated by the same agonist. J Theor Biol. 1977 Nov 7;69(1):101–112. doi: 10.1016/0022-5193(77)90390-3. [DOI] [PubMed] [Google Scholar]
- Takemori A. E., Sultana M., Nagase H., Portoghese P. S. Agonist and antagonist activities of ligands derived from naltrexone and oxymorphone. Life Sci. 1992;50(20):1491–1495. doi: 10.1016/0024-3205(92)90138-f. [DOI] [PubMed] [Google Scholar]
- Tancredi T., Salvadori S., Amodeo P., Picone D., Lazarus L. H., Bryant S. D., Guerrini R., Marzola G., Temussi P. A. Conversion of enkephalin and dermorphin into delta-selective opioid antagonists by single-residue substitution. Eur J Biochem. 1994 Aug 15;224(1):241–247. doi: 10.1111/j.1432-1033.1994.tb20017.x. [DOI] [PubMed] [Google Scholar]
- Temussi P. A., Salvadori S., Amodeo P., Bianchi C., Guerrini R., Tomatis R., Lazarus L. H., Picone D., Tancredi T. Selective opioid dipeptides. Biochem Biophys Res Commun. 1994 Feb 15;198(3):933–939. doi: 10.1006/bbrc.1994.1133. [DOI] [PubMed] [Google Scholar]
- Terenius L. Somatostatin and ACTH are peptides with partial antagonist-like selectivity for opiate receptors. Eur J Pharmacol. 1976 Jul;38(1):211–213. doi: 10.1016/0014-2999(76)90221-1. [DOI] [PubMed] [Google Scholar]
- Webster J. L., Polgar W. E., Brandt S. R., Berzetei-Gurske I. P., Toll L. Comparison of kappa 2-opioid receptors in guinea pig brain and guinea pig ileum membranes. Eur J Pharmacol. 1993 Feb 9;231(2):251–258. doi: 10.1016/0014-2999(93)90457-s. [DOI] [PubMed] [Google Scholar]
- Wollemann M., Benyhe S., Simon J. The kappa-opioid receptor: evidence for the different subtypes. Life Sci. 1993;52(7):599–611. doi: 10.1016/0024-3205(93)90451-8. [DOI] [PubMed] [Google Scholar]


