Abstract
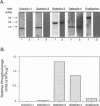
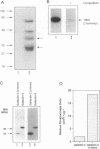
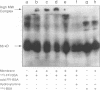
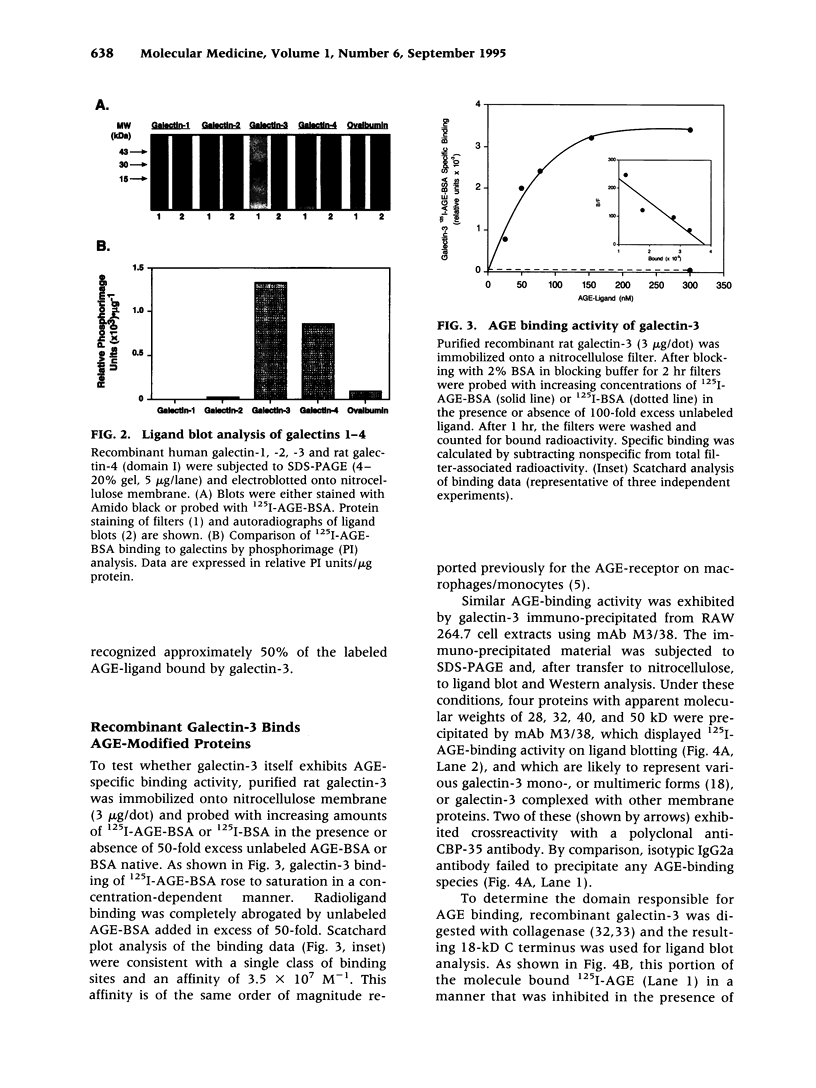
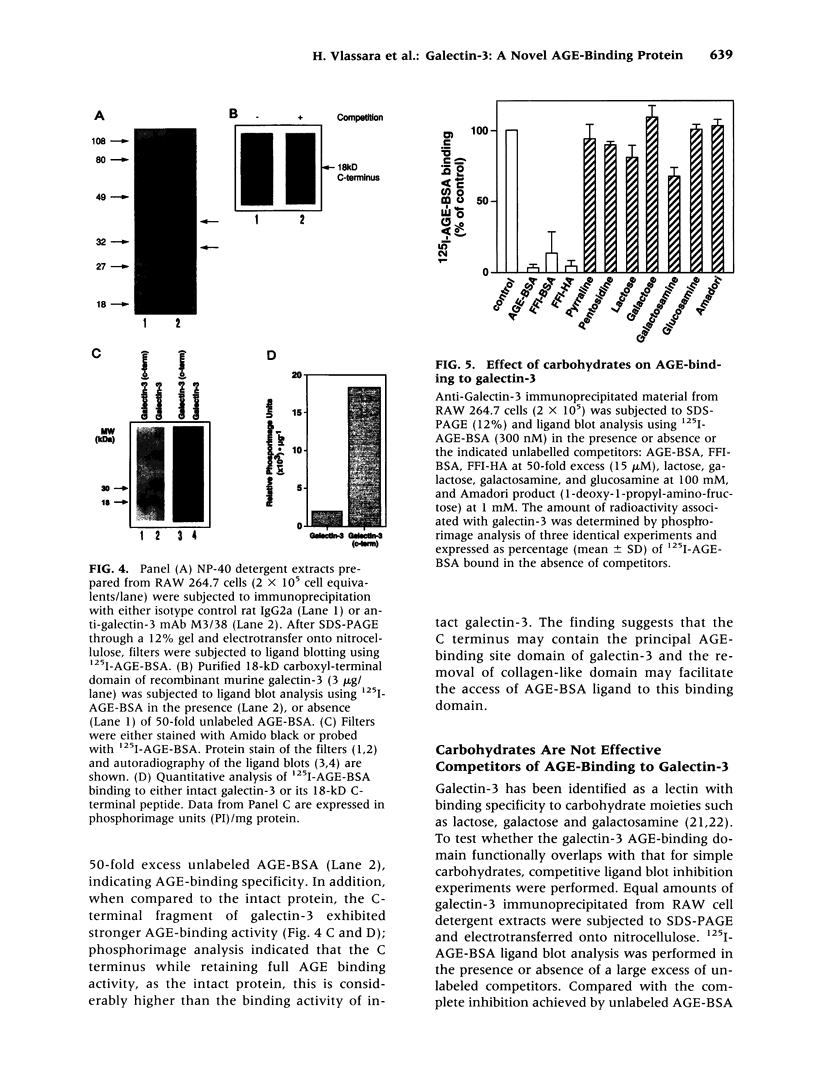
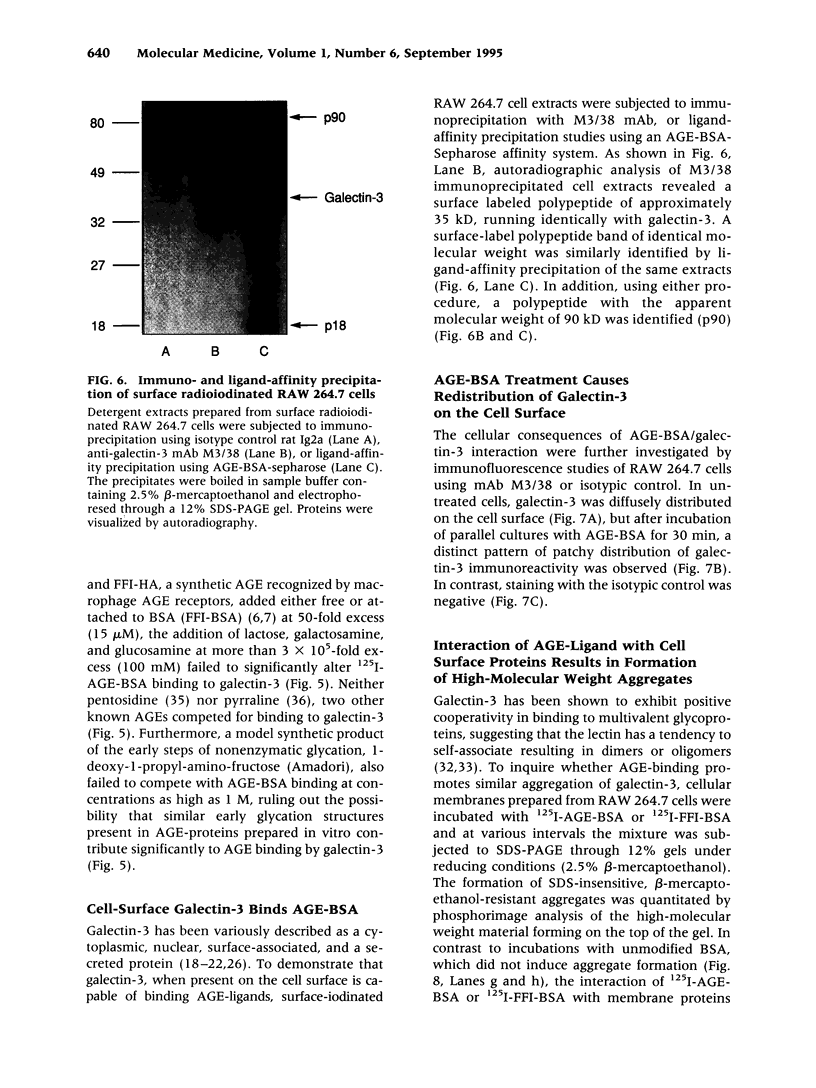
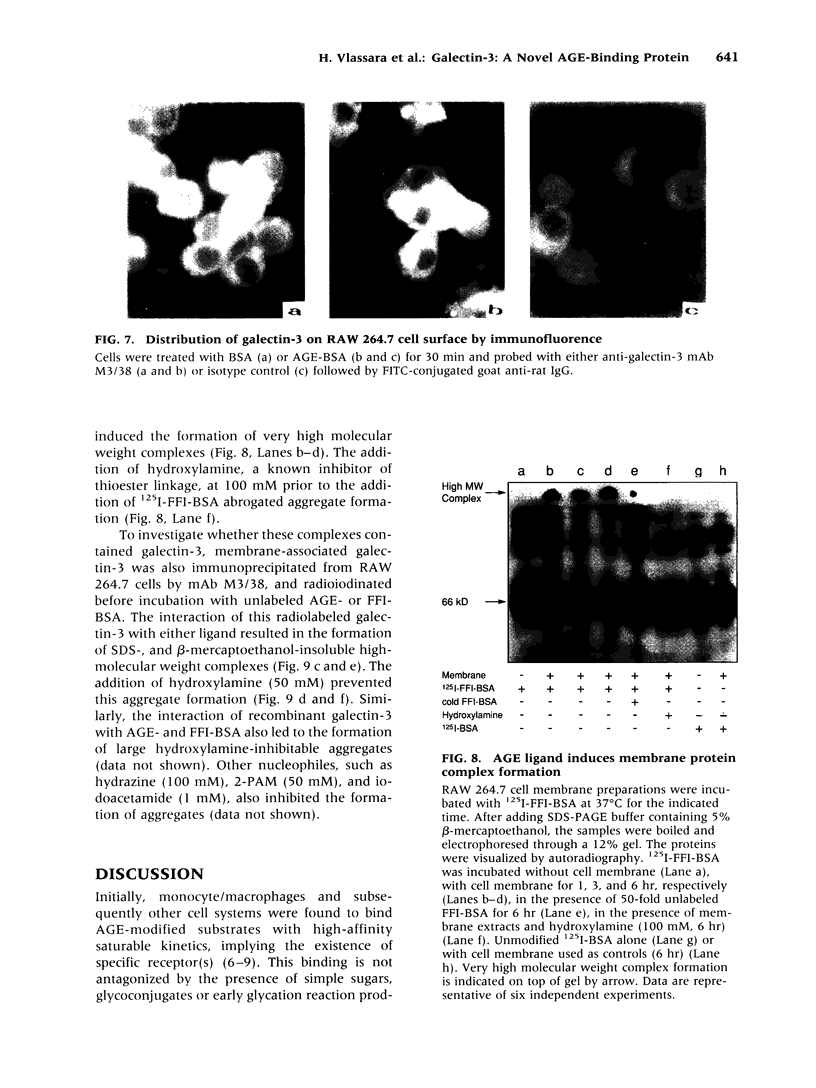
BACKGROUND: Advanced glycation end products (AGE), the reactive derivatives of nonenzymatic glucose-protein condensation reactions, are implicated in the multiorgan complications of diabetes and aging. An AGE-specific cellular receptor complex (AGE-R) mediating AGE removal as well as multiple biological responses has been identified. By screening an expression library using antibody against a previously identified component of the AGE-R complex p90, a known partial cDNA clone was isolated with homology to galectin-3, a protein of diverse identity, and member of the galectin family. MATERIALS AND METHODS: To explore this unexpected finding, the nature of the interactions between galectin-3 and AGE was studied using intact macrophage-like RAW 264.7 cells, membrane-associated and recombinant galectin-1 through -4, and model AGE-ligands (AGE-BSA, FFI-BSA). RESULTS: Among the members of this family (galectin-1 through 4), recombinant rat galectin-3 was found to exhibit high-affinity 125I-AGE-BSA binding with saturable kinetics (kD 3.5 x 10(7) M-1) that was fully blocked by excess unlabeled naturally formed AGE-BSA or synthetic FFI-BSA, but only weakly inhibited by several known galectin-3 ligands, such as lactose. In addition to the p90, immunoprecipitation with anti-galectin-3, followed by 125I-AGE-BSA ligand blot analysis of RAW 264.7 cell extracts, revealed galectin-3 (28 and 32 kD), as well as galectin-3-associated proteins (40 and 50 kD) with AGE-binding activity. Interaction of galectin-3 with AGE-BSA or FFI-BSA resulted in formation of SDS-, and beta-mercaptoethanol-insoluble, but hydroxylamine-sensitive high-molecular weight complexes between AGE-ligand, galectin-3, and other membrane components. CONCLUSIONS: The findings point toward a mechanism by which galectin-3 may serve in the assembly of AGE-R components and in the efficient cell surface attachment and endocytosis by macrophages of a heterogenous pool of AGE moieties with diverse affinities, thus contributing to the elimination of these pathogenic substances.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Barondes S. H., Castronovo V., Cooper D. N., Cummings R. D., Drickamer K., Feizi T., Gitt M. A., Hirabayashi J., Hughes C., Kasai K. Galectins: a family of animal beta-galactoside-binding lectins. Cell. 1994 Feb 25;76(4):597–598. doi: 10.1016/0092-8674(94)90498-7. [DOI] [PubMed] [Google Scholar]
- Barondes S. H., Cooper D. N., Gitt M. A., Leffler H. Galectins. Structure and function of a large family of animal lectins. J Biol Chem. 1994 Aug 19;269(33):20807–20810. [PubMed] [Google Scholar]
- Brownlee M., Cerami A., Vlassara H. Advanced glycosylation end products in tissue and the biochemical basis of diabetic complications. N Engl J Med. 1988 May 19;318(20):1315–1321. doi: 10.1056/NEJM198805193182007. [DOI] [PubMed] [Google Scholar]
- Cooper D. N., Barondes S. H. Evidence for export of a muscle lectin from cytosol to extracellular matrix and for a novel secretory mechanism. J Cell Biol. 1990 May;110(5):1681–1691. doi: 10.1083/jcb.110.5.1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doi T., Vlassara H., Kirstein M., Yamada Y., Striker G. E., Striker L. J. Receptor-specific increase in extracellular matrix production in mouse mesangial cells by advanced glycosylation end products is mediated via platelet-derived growth factor. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):2873–2877. doi: 10.1073/pnas.89.7.2873. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drickamer K., Taylor M. E. Biology of animal lectins. Annu Rev Cell Biol. 1993;9:237–264. doi: 10.1146/annurev.cb.09.110193.001321. [DOI] [PubMed] [Google Scholar]
- Dyer D. G., Dunn J. A., Thorpe S. R., Bailie K. E., Lyons T. J., McCance D. R., Baynes J. W. Accumulation of Maillard reaction products in skin collagen in diabetes and aging. J Clin Invest. 1993 Jun;91(6):2463–2469. doi: 10.1172/JCI116481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito C., Gerlach H., Brett J., Stern D., Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989 Oct 1;170(4):1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frigeri L. G., Robertson M. W., Liu F. T. Expression of biologically active recombinant rat IgE-binding protein in Escherichia coli. J Biol Chem. 1990 Dec 5;265(34):20763–20769. [PubMed] [Google Scholar]
- Hatfield P. M., Vierstra R. D. Multiple forms of ubiquitin-activating enzyme E1 from wheat. Identification of an essential cysteine by in vitro mutagenesis. J Biol Chem. 1992 Jul 25;267(21):14799–14803. [PubMed] [Google Scholar]
- Ho M. K., Springer T. A. Mac-2, a novel 32,000 Mr mouse macrophage subpopulation-specific antigen defined by monoclonal antibodies. J Immunol. 1982 Mar;128(3):1221–1228. [PubMed] [Google Scholar]
- Hsu D. K., Zuberi R. I., Liu F. T. Biochemical and biophysical characterization of human recombinant IgE-binding protein, an S-type animal lectin. J Biol Chem. 1992 Jul 15;267(20):14167–14174. [PubMed] [Google Scholar]
- Imani F., Horii Y., Suthanthiran M., Skolnik E. Y., Makita Z., Sharma V., Sehajpal P., Vlassara H. Advanced glycosylation endproduct-specific receptors on human and rat T-lymphocytes mediate synthesis of interferon gamma: role in tissue remodeling. J Exp Med. 1993 Dec 1;178(6):2165–2172. doi: 10.1084/jem.178.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia S., Wang J. L. Carbohydrate binding protein 35. Complementary DNA sequence reveals homology with proteins of the heterogeneous nuclear RNP. J Biol Chem. 1988 May 5;263(13):6009–6011. [PubMed] [Google Scholar]
- Knibbs R. N., Agrwal N., Wang J. L., Goldstein I. J. Carbohydrate-binding protein 35. II. Analysis of the interaction of the recombinant polypeptide with saccharides. J Biol Chem. 1993 Jul 15;268(20):14940–14947. [PubMed] [Google Scholar]
- Law S. K., Levine R. P. Interaction between the third complement protein and cell surface macromolecules. Proc Natl Acad Sci U S A. 1977 Jul;74(7):2701–2705. doi: 10.1073/pnas.74.7.2701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S. K., Lichtenberg N. A., Levine R. P. Evidence for an ester linkage between the labile binding site of C3b and receptive surfaces. J Immunol. 1979 Sep;123(3):1388–1394. [PubMed] [Google Scholar]
- Lindstedt R., Apodaca G., Barondes S. H., Mostov K. E., Leffler H. Apical secretion of a cytosolic protein by Madin-Darby canine kidney cells. Evidence for polarized release of an endogenous lectin by a nonclassical secretory pathway. J Biol Chem. 1993 Jun 5;268(16):11750–11757. [PubMed] [Google Scholar]
- Lotz M. M., Andrews C. W., Jr, Korzelius C. A., Lee E. C., Steele G. D., Jr, Clarke A., Mercurio A. M. Decreased expression of Mac-2 (carbohydrate binding protein 35) and loss of its nuclear localization are associated with the neoplastic progression of colon carcinoma. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3466–3470. doi: 10.1073/pnas.90.8.3466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Massa S. M., Cooper D. N., Leffler H., Barondes S. H. L-29, an endogenous lectin, binds to glycoconjugate ligands with positive cooperativity. Biochemistry. 1993 Jan 12;32(1):260–267. doi: 10.1021/bi00052a033. [DOI] [PubMed] [Google Scholar]
- Miyata S., Monnier V. Immunohistochemical detection of advanced glycosylation end products in diabetic tissues using monoclonal antibody to pyrraline. J Clin Invest. 1992 Apr;89(4):1102–1112. doi: 10.1172/JCI115690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992 Jul 25;267(21):14998–15004. [PubMed] [Google Scholar]
- Palinski W., Koschinsky T., Butler S. W., Miller E., Vlassara H., Cerami A., Witztum J. L. Immunological evidence for the presence of advanced glycosylation end products in atherosclerotic lesions of euglycemic rabbits. Arterioscler Thromb Vasc Biol. 1995 May;15(5):571–582. doi: 10.1161/01.atv.15.5.571. [DOI] [PubMed] [Google Scholar]
- Pangburn M. K. Spontaneous reformation of the intramolecular thioester in complement protein C3 and low temperature capture of a conformational intermediate capable of reformation. J Biol Chem. 1992 Apr 25;267(12):8584–8590. [PubMed] [Google Scholar]
- Pongor S., Ulrich P. C., Bencsath F. A., Cerami A. Aging of proteins: isolation and identification of a fluorescent chromophore from the reaction of polypeptides with glucose. Proc Natl Acad Sci U S A. 1984 May;81(9):2684–2688. doi: 10.1073/pnas.81.9.2684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radoff S., Cerami A., Vlassara H. Isolation of surface binding protein specific for advanced glycosylation end products from mouse macrophage-derived cell line RAW 264.7. Diabetes. 1990 Dec;39(12):1510–1518. doi: 10.2337/diab.39.12.1510. [DOI] [PubMed] [Google Scholar]
- Raz A., Pazerini G., Carmi P. Identification of the metastasis-associated, galactoside-binding lectin as a chimeric gene product with homology to an IgE-binding protein. Cancer Res. 1989 Jul 1;49(13):3489–3493. [PubMed] [Google Scholar]
- Schmidt A. M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992 Jul 25;267(21):14987–14997. [PubMed] [Google Scholar]
- Sell D. R., Nagaraj R. H., Grandhee S. K., Odetti P., Lapolla A., Fogarty J., Monnier V. M. Pentosidine: a molecular marker for the cumulative damage to proteins in diabetes, aging, and uremia. Diabetes Metab Rev. 1991 Dec;7(4):239–251. doi: 10.1002/dmr.5610070404. [DOI] [PubMed] [Google Scholar]
- Skolnik E. Y., Yang Z., Makita Z., Radoff S., Kirstein M., Vlassara H. Human and rat mesangial cell receptors for glucose-modified proteins: potential role in kidney tissue remodelling and diabetic nephropathy. J Exp Med. 1991 Oct 1;174(4):931–939. doi: 10.1084/jem.174.4.931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sparrow C. P., Leffler H., Barondes S. H. Multiple soluble beta-galactoside-binding lectins from human lung. J Biol Chem. 1987 May 25;262(15):7383–7390. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. High-affinity-receptor-mediated uptake and degradation of glucose-modified proteins: a potential mechanism for the removal of senescent macromolecules. Proc Natl Acad Sci U S A. 1985 Sep;82(17):5588–5592. doi: 10.1073/pnas.82.17.5588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Cerami A. Novel macrophage receptor for glucose-modified proteins is distinct from previously described scavenger receptors. J Exp Med. 1986 Oct 1;164(4):1301–1309. doi: 10.1084/jem.164.4.1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Manogue K. R., Dinarello C. A., Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988 Jun 10;240(4858):1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Bucala R., Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994 Feb;70(2):138–151. [PubMed] [Google Scholar]
- Vlassara H., Moldawer L., Chan B. Macrophage/monocyte receptor for nonenzymatically glycosylated protein is upregulated by cachectin/tumor necrosis factor. J Clin Invest. 1989 Dec;84(6):1813–1820. doi: 10.1172/JCI114366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Woo H. J., Shaw L. M., Messier J. M., Mercurio A. M. The major non-integrin laminin binding protein of macrophages is identical to carbohydrate binding protein 35 (Mac-2). J Biol Chem. 1990 May 5;265(13):7097–7099. [PubMed] [Google Scholar]
- Yang Z., Makita Z., Horii Y., Brunelle S., Cerami A., Sehajpal P., Suthanthiran M., Vlassara H. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991 Sep 1;174(3):515–524. doi: 10.1084/jem.174.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]