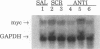
Abstract
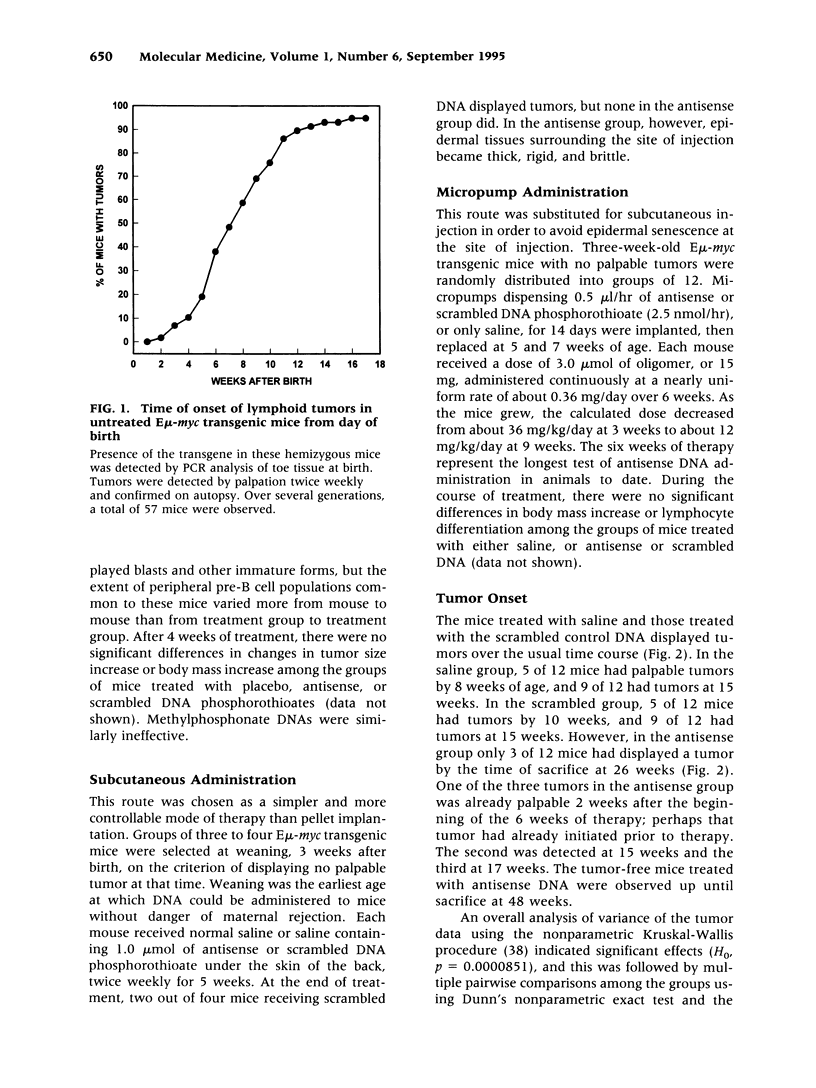
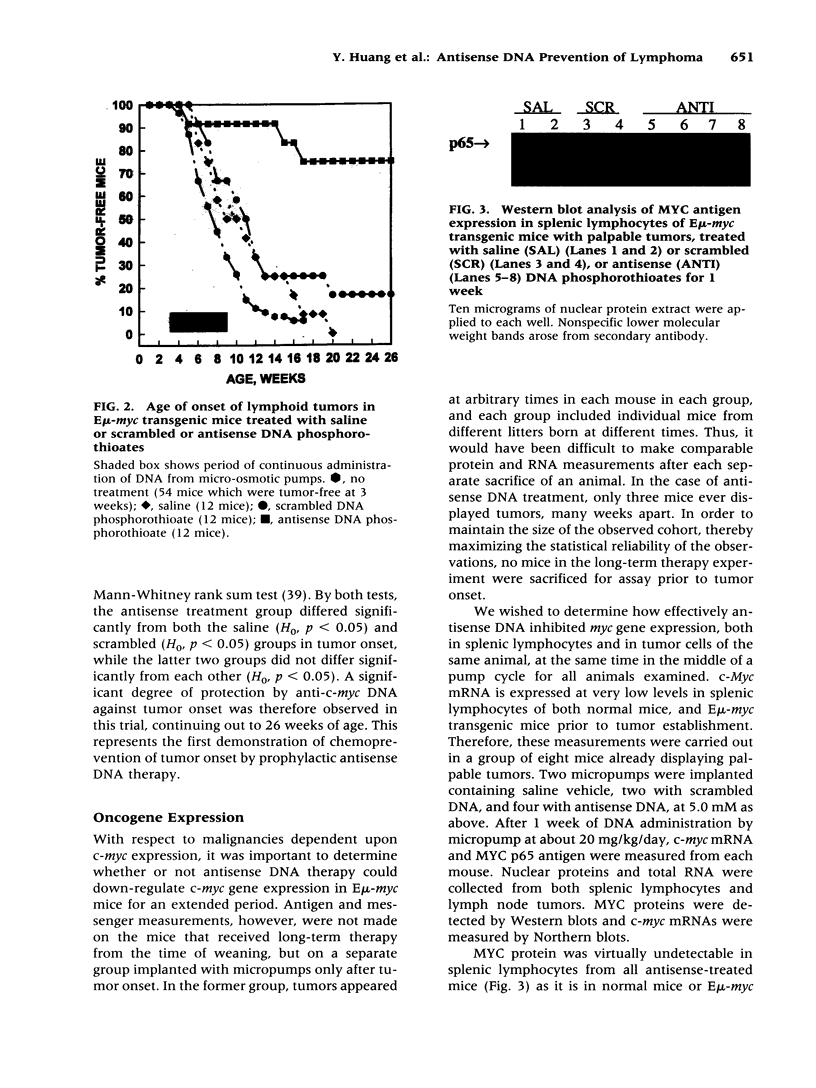
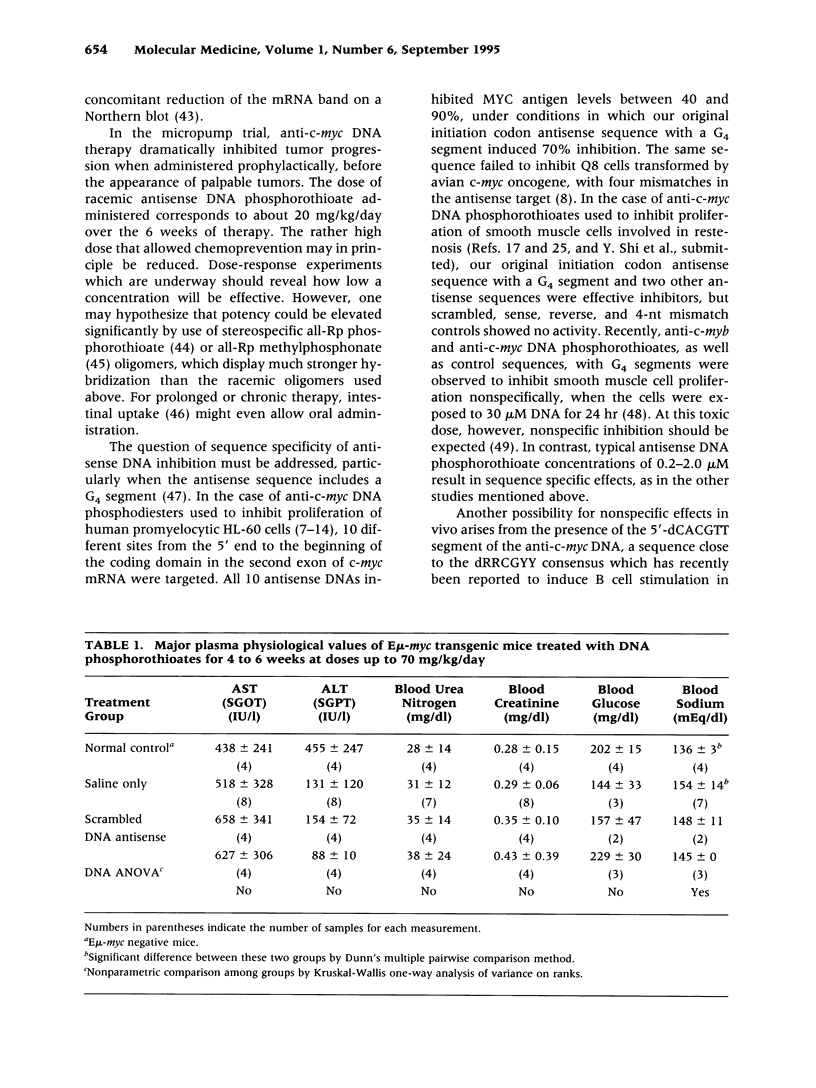
BACKGROUND: Transgenic mice bearing a murine immunoglobulin enhancer/c-myc fusion transgene (Emu-myc) provide a useful model for Burkitt's lymphoma. MATERIALS AND METHODS: Groups of 12 Emu-myc mice were treated prophylactically for 6 weeks after weaning with anti-c-myc DNA phosphorothioate (20 mg/kg/day), scrambled control DNA, or saline, delivered by micro-osmotic pumps. RESULTS: Half of the mice treated with saline or scrambled control DNA displayed palpable tumors by 8-9 weeks after birth, and 95% of them did so by 16 weeks, but 75% of the mice treated with antisense DNA were still free of tumors at the age of 26 weeks. Antisense therapy ablated MYC antigen in the spleens of tumor-bearing mice. Plasma physiological parameters indicated no acute toxicity. CONCLUSIONS: Long-term tumor resistance after anti-c-myc DNA therapy implies induction of a host response. Prophylactic anti-c-myc DNA therapy might prevent lymphoma in asymptomatic individuals displaying c-myc translocations.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bacon T. A., Wickstrom E. Daily addition of an anti-c-myc DNA oligomer induces granulocytic differentiation of human promyelocytic leukemia HL-60 cells in both serum-containing and serum-free media. Oncogene Res. 1991;6(1):21–32. [PubMed] [Google Scholar]
- Bacon T. A., Wickstrom E. Walking along human c-myc mRNA with antisense oligodeoxynucleotides: maximum efficacy at the 5' cap region. Oncogene Res. 1991;6(1):13–19. [PubMed] [Google Scholar]
- Biro S., Fu Y. M., Yu Z. X., Epstein S. E. Inhibitory effects of antisense oligodeoxynucleotides targeting c-myc mRNA on smooth muscle cell proliferation and migration. Proc Natl Acad Sci U S A. 1993 Jan 15;90(2):654–658. doi: 10.1073/pnas.90.2.654. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bishop J. M. Molecular themes in oncogenesis. Cell. 1991 Jan 25;64(2):235–248. doi: 10.1016/0092-8674(91)90636-d. [DOI] [PubMed] [Google Scholar]
- Branda R. F., Moore A. L., Mathews L., McCormack J. J., Zon G. Immune stimulation by an antisense oligomer complementary to the rev gene of HIV-1. Biochem Pharmacol. 1993 May 25;45(10):2037–2043. doi: 10.1016/0006-2952(93)90014-n. [DOI] [PubMed] [Google Scholar]
- Burgess T. L., Fisher E. F., Ross S. L., Bready J. V., Qian Y. X., Bayewitch L. A., Cohen A. M., Herrera C. J., Hu S. S., Kramer T. B. The antiproliferative activity of c-myb and c-myc antisense oligonucleotides in smooth muscle cells is caused by a nonantisense mechanism. Proc Natl Acad Sci U S A. 1995 Apr 25;92(9):4051–4055. doi: 10.1073/pnas.92.9.4051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherney B. W., Bhatia K., Tosato G. A role for deregulated c-Myc expression in apoptosis of Epstein-Barr virus-immortalized B cells. Proc Natl Acad Sci U S A. 1994 Dec 20;91(26):12967–12971. doi: 10.1073/pnas.91.26.12967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Corcoran L. M., Cory S., Adams J. M. Transposition of the immunoglobulin heavy chain enhancer to the myc oncogene in a murine plasmacytoma. Cell. 1985 Jan;40(1):71–79. doi: 10.1016/0092-8674(85)90310-1. [DOI] [PubMed] [Google Scholar]
- Gaidano G., Parsa N. Z., Tassi V., Della-Latta P., Chaganti R. S., Knowles D. M., Dalla-Favera R. In vitro establishment of AIDS-related lymphoma cell lines: phenotypic characterization, oncogene and tumor suppressor gene lesions, and heterogeneity in Epstein-Barr virus infection. Leukemia. 1993 Oct;7(10):1621–1629. [PubMed] [Google Scholar]
- Gray G. D., Hernandez O. M., Hebel D., Root M., Pow-Sang J. M., Wickstrom E. Antisense DNA inhibition of tumor growth induced by c-Ha-ras oncogene in nude mice. Cancer Res. 1993 Feb 1;53(3):577–580. [PubMed] [Google Scholar]
- Harel-Bellan A., Ferris D. K., Vinocour M., Holt J. T., Farrar W. L. Specific inhibition of c-myc protein biosynthesis using an antisense synthetic deoxy-oligonucleotide in human T lymphocytes. J Immunol. 1988 Apr 1;140(7):2431–2435. [PubMed] [Google Scholar]
- Harris A. W., Pinkert C. A., Crawford M., Langdon W. Y., Brinster R. L., Adams J. M. The E mu-myc transgenic mouse. A model for high-incidence spontaneous lymphoma and leukemia of early B cells. J Exp Med. 1988 Feb 1;167(2):353–371. doi: 10.1084/jem.167.2.353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heikkila R., Schwab G., Wickstrom E., Loke S. L., Pluznik D. H., Watt R., Neckers L. M. A c-myc antisense oligodeoxynucleotide inhibits entry into S phase but not progress from G0 to G1. 1987 Jul 30-Aug 5Nature. 328(6129):445–449. doi: 10.1038/328445a0. [DOI] [PubMed] [Google Scholar]
- Holt J. T., Redner R. L., Nienhuis A. W. An oligomer complementary to c-myc mRNA inhibits proliferation of HL-60 promyelocytic cells and induces differentiation. Mol Cell Biol. 1988 Feb;8(2):963–973. doi: 10.1128/mcb.8.2.963. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes J. A., Avrutskaya A. V., Brouwer K. L., Wickstrom E., Juliano R. L. Radiolabeling of methylphosphonate and phosphorothioate oligonucleotides and evaluation of their transport in everted rat jejunum sacs. Pharm Res. 1995 Jun;12(6):817–824. doi: 10.1023/a:1016296617434. [DOI] [PubMed] [Google Scholar]
- Iversen P. L., Crouse D., Zon G., Perry G. Binding of antisense phosphorothioate oligonucleotides to murine lymphocytes is lineage specific and inducible. Antisense Res Dev. 1992 Fall;2(3):223–233. doi: 10.1089/ard.1992.2.223. [DOI] [PubMed] [Google Scholar]
- Kimura S., Maekawa T., Hirakawa K., Murakami A., Abe T. Alterations of c-myc expression by antisense oligodeoxynucleotides enhance the induction of apoptosis in HL-60 cells. Cancer Res. 1995 Mar 15;55(6):1379–1384. [PubMed] [Google Scholar]
- Klempnauer K. H., Sippel A. E. Subnuclear localization of proteins encoded by the oncogene v-myb and its cellular homolog c-myb. Mol Cell Biol. 1986 Jan;6(1):62–69. doi: 10.1128/mcb.6.1.62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg A. M., Yi A. K., Matson S., Waldschmidt T. J., Bishop G. A., Teasdale R., Koretzky G. A., Klinman D. M. CpG motifs in bacterial DNA trigger direct B-cell activation. Nature. 1995 Apr 6;374(6522):546–549. doi: 10.1038/374546a0. [DOI] [PubMed] [Google Scholar]
- Magrath I. The pathogenesis of Burkitt's lymphoma. Adv Cancer Res. 1990;55:133–270. doi: 10.1016/s0065-230x(08)60470-4. [DOI] [PubMed] [Google Scholar]
- McManaway M. E., Neckers L. M., Loke S. L., al-Nasser A. A., Redner R. L., Shiramizu B. T., Goldschmidts W. L., Huber B. E., Bhatia K., Magrath I. T. Tumour-specific inhibition of lymphoma growth by an antisense oligodeoxynucleotide. Lancet. 1990 Apr 7;335(8693):808–811. doi: 10.1016/0140-6736(90)90934-w. [DOI] [PubMed] [Google Scholar]
- Noonberg S. B., Garovoy M. R., Hunt C. A. Characteristics of oligonucleotide uptake in human keratinocyte cultures. J Invest Dermatol. 1993 Nov;101(5):727–731. doi: 10.1111/1523-1747.ep12371683. [DOI] [PubMed] [Google Scholar]
- Paria B. C., Dey S. K., Andrews G. K. Antisense c-myc effects on preimplantation mouse embryo development. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10051–10055. doi: 10.1073/pnas.89.21.10051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peña A., Reddy C. D., Wu S., Hickok N. J., Reddy E. P., Yumet G., Soprano D. R., Soprano K. J. Regulation of human ornithine decarboxylase expression by the c-Myc.Max protein complex. J Biol Chem. 1993 Dec 25;268(36):27277–27285. [PubMed] [Google Scholar]
- Pieles U., Zürcher W., Schär M., Moser H. E. Matrix-assisted laser desorption ionization time-of-flight mass spectrometry: a powerful tool for the mass and sequence analysis of natural and modified oligonucleotides. Nucleic Acids Res. 1993 Jul 11;21(14):3191–3196. doi: 10.1093/nar/21.14.3191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Polakowska R. R., Haake A. R. Apoptosis: the skin from a new perspective. Cell Death Differ. 1994 Jul;1(1):19–31. [PubMed] [Google Scholar]
- Prevot S., Raphael M., Fournier J. G., Diebold J. Detection by in situ hybridization of HIV and c-myc RNA in tumour cells of AIDS-related B-cell lymphomas. Histopathology. 1993 Feb;22(2):151–156. doi: 10.1111/j.1365-2559.1993.tb00094.x. [DOI] [PubMed] [Google Scholar]
- Ratajczak M. Z., Kant J. A., Luger S. M., Hijiya N., Zhang J., Zon G., Gewirtz A. M. In vivo treatment of human leukemia in a scid mouse model with c-myb antisense oligodeoxynucleotides. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11823–11827. doi: 10.1073/pnas.89.24.11823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Resnicoff M., Sell C., Rubini M., Coppola D., Ambrose D., Baserga R., Rubin R. Rat glioblastoma cells expressing an antisense RNA to the insulin-like growth factor-1 (IGF-1) receptor are nontumorigenic and induce regression of wild-type tumors. Cancer Res. 1994 Apr 15;54(8):2218–2222. [PubMed] [Google Scholar]
- Sarmiento U. M., Perez J. R., Becker J. M., Narayanan R. In vivo toxicological effects of rel A antisense phosphorothioates in CD-1 mice. Antisense Res Dev. 1994 Summer;4(2):99–107. doi: 10.1089/ard.1994.4.99. [DOI] [PubMed] [Google Scholar]
- Sawadogo M., Van Dyke M. W. A rapid method for the purification of deprotected oligodeoxynucleotides. Nucleic Acids Res. 1991 Feb 11;19(3):674–674. doi: 10.1093/nar/19.3.674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Fard A., Galeo A., Hutchinson H. G., Vermani P., Dodge G. R., Hall D. J., Shaheen F., Zalewski A. Transcatheter delivery of c-myc antisense oligomers reduces neointimal formation in a porcine model of coronary artery balloon injury. Circulation. 1994 Aug;90(2):944–951. doi: 10.1161/01.cir.90.2.944. [DOI] [PubMed] [Google Scholar]
- Shi Y., Hutchinson H. G., Hall D. J., Zalewski A. Downregulation of c-myc expression by antisense oligonucleotides inhibits proliferation of human smooth muscle cells. Circulation. 1993 Sep;88(3):1190–1195. doi: 10.1161/01.cir.88.3.1190. [DOI] [PubMed] [Google Scholar]
- Sinha N. D., Biernat J., McManus J., Köster H. Polymer support oligonucleotide synthesis XVIII: use of beta-cyanoethyl-N,N-dialkylamino-/N-morpholino phosphoramidite of deoxynucleosides for the synthesis of DNA fragments simplifying deprotection and isolation of the final product. Nucleic Acids Res. 1984 Jun 11;12(11):4539–4557. doi: 10.1093/nar/12.11.4539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stec W. J., Grajkowski A., Koziolkiewicz M., Uznanski B. Novel route to oligo(deoxyribonucleoside phosphorothioates). Stereocontrolled synthesis of P-chiral oligo(deoxyribonucleoside phosphorothioates). Nucleic Acids Res. 1991 Nov 11;19(21):5883–5888. doi: 10.1093/nar/19.21.5883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun L. K., Showe L. C., Croce C. M. Analysis of the 3' flanking region of the human c-myc gene in lymphomas with the t(8;22) and t(2;8) chromosomal translocations. Nucleic Acids Res. 1986 May 27;14(10):4037–4050. doi: 10.1093/nar/14.10.4037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Townsend S. E., Su F. W., Atherton J. M., Allison J. P. Specificity and longevity of antitumor immune responses induced by B7-transfected tumors. Cancer Res. 1994 Dec 15;54(24):6477–6483. [PubMed] [Google Scholar]
- Trojan J., Johnson T. R., Rudin S. D., Ilan J., Tykocinski M. L., Ilan J. Treatment and prevention of rat glioblastoma by immunogenic C6 cells expressing antisense insulin-like growth factor I RNA. Science. 1993 Jan 1;259(5091):94–97. doi: 10.1126/science.8418502. [DOI] [PubMed] [Google Scholar]
- Vile R. G., Nelson J. A., Castleden S., Chong H., Hart I. R. Systemic gene therapy of murine melanoma using tissue specific expression of the HSVtk gene involves an immune component. Cancer Res. 1994 Dec 1;54(23):6228–6234. [PubMed] [Google Scholar]
- Vyazovkina E. V., Savchenko E. V., Lokhov S. G., Engels J. W., Wickstrom E., Lebedev A. V. Synthesis of specific diastereomers of a DNA methylphosphonate heptamer, d(CpCpApApApCpA), and stability of base pairing with the normal DNA octamer d(TPGPTPTPTPGPGPC). Nucleic Acids Res. 1994 Jun 25;22(12):2404–2409. doi: 10.1093/nar/22.12.2404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watson P. H., Pon R. T., Shiu R. P. Inhibition of c-myc expression by phosphorothioate antisense oligonucleotide identifies a critical role for c-myc in the growth of human breast cancer. Cancer Res. 1991 Aug 1;51(15):3996–4000. [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Freeman D. L., Lyman G. H., Wickstrom E. Human promyelocytic leukemia HL-60 cell proliferation and c-myc protein expression are inhibited by an antisense pentadecadeoxynucleotide targeted against c-myc mRNA. Proc Natl Acad Sci U S A. 1988 Feb;85(4):1028–1032. doi: 10.1073/pnas.85.4.1028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wickstrom E. L., Bacon T. A., Gonzalez A., Lyman G. H., Wickstrom E. Anti-c-myc DNA increases differentiation and decreases colony formation by HL-60 cells. In Vitro Cell Dev Biol. 1989 Mar;25(3 Pt 1):297–302. doi: 10.1007/BF02628470. [DOI] [PubMed] [Google Scholar]
- Wickstrom E., Bacon T. A., Wickstrom E. L. Down-regulation of c-MYC antigen expression in lymphocytes of Emu-c-myc transgenic mice treated with anti-c-myc DNA methylphosphonates. Cancer Res. 1992 Dec 15;52(24):6741–6745. [PubMed] [Google Scholar]
- Yaswen P., Stampfer M. R., Ghosh K., Cohen J. S. Effects of sequence of thioated oligonucleotides on cultured human mammary epithelial cells. Antisense Res Dev. 1993 Spring;3(1):67–77. doi: 10.1089/ard.1993.3.67. [DOI] [PubMed] [Google Scholar]