Abstract
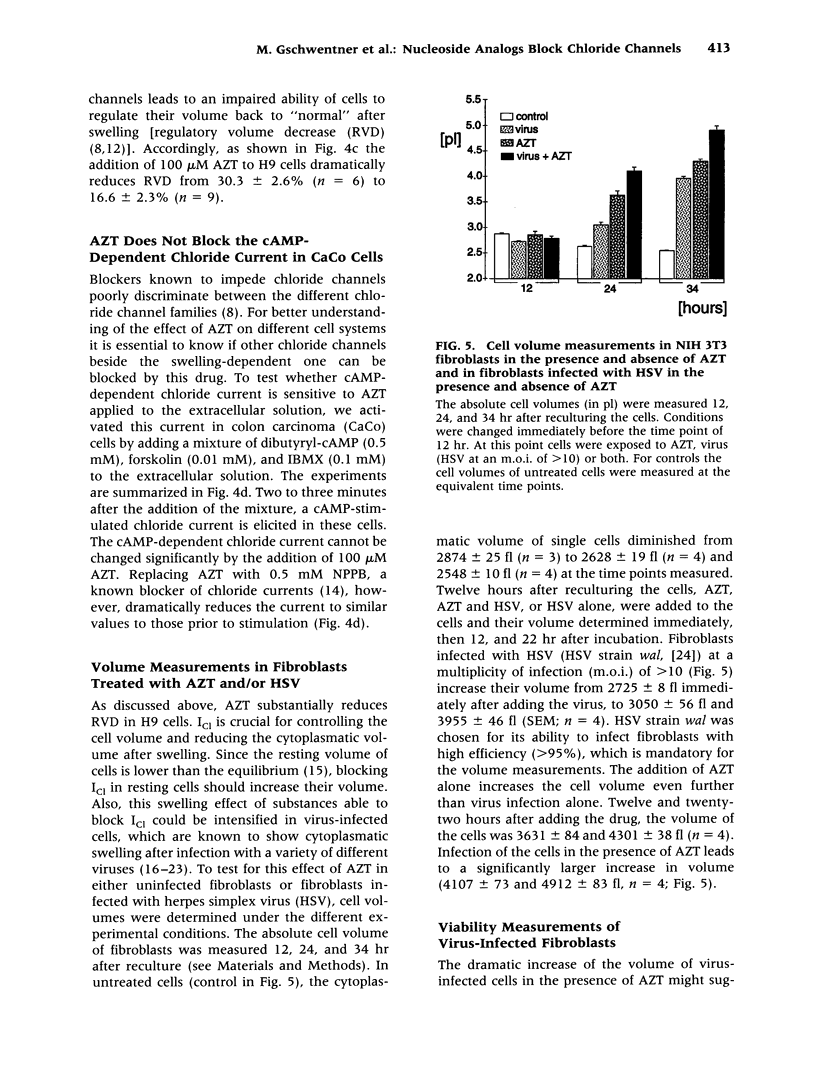
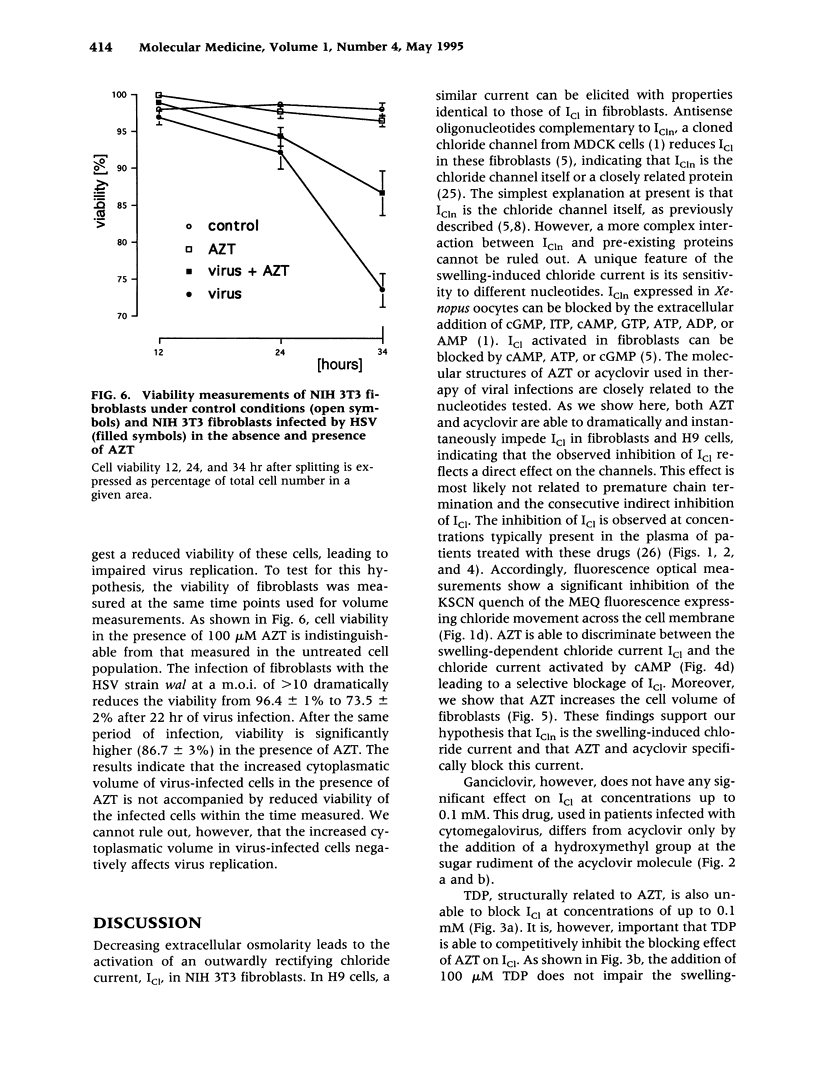
BACKGROUND: The antiviral drugs AZT and acyclovir are generally used in the treatment of infections with human immunodeficiency virus (HIV) and herpes simplex virus (HSV). These substances are known to impede virus replication by premature nucleic acid chain termination. It is not yet clear, however, if this is the sole mechanism responsible for the antiviral and/or the numerous side effects observed in patients treated with these agents. We investigated the swelling-induced chloride current in fibroblasts, which we demonstrated is closely related or identical to a cloned epithelial chloride channel, ICln: This chloride channel can be blocked by nucleotides. MATERIALS AND METHODS: Electrophysiological, fluorescence optical, and volume measurements were made to determine the effect of nucleoside analogs on the swelling-dependent chloride current (ICl) in NIH 3T3 fibroblasts and in human T cell lymphoma (H9) cells and the cAMP-dependent chloride current in CaCo cells. RESULTS: AZT and acyclovir block the swelling-dependent chloride current and the chloride flux in fibroblasts, and the regulatory volume decrease (RVD) and ICl in H9 cells. This immediate effect can be substantially reduced by the simultaneous incubation of the cells with thymidine-5'-diphosphate (TDP) or uridine, both of which are by themselves unable to affect ICl. CONCLUSIONS: We show here a novel molecular mechanism by which antiviral drugs of the nucleoside analog family could lead to impairments of the kidney, bone marrow, gastrointestinal, and neuronal functions, and how these side effects could possibly be restricted by the presence of TDP or uridine.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdul-Aziz T. A., Arp L. H. Progression of tracheal lesions in turkeys exposed by aerosol to LaSota strain of Newcastle disease virus. Avian Dis. 1983 Oct-Dec;27(4):1131–1141. [PubMed] [Google Scholar]
- Alton E. W., Manning S. D., Schlatter P. J., Geddes D. M., Williams A. J. Characterization of a Ca(2+)-dependent anion channel from sheep tracheal epithelium incorporated into planar bilayers. J Physiol. 1991 Nov;443:137–159. doi: 10.1113/jphysiol.1991.sp018827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bardadin K. A., Scheuer P. J. Endothelial cell changes in acute hepatitis. A light and electron microscopic study. J Pathol. 1984 Nov;144(3):213–220. doi: 10.1002/path.1711440308. [DOI] [PubMed] [Google Scholar]
- Bashford C. L., Micklem K. J., Pasternak C. A. Sequential onset of permeability changes in mouse ascites cells induced by Sendai virus. Biochim Biophys Acta. 1985 Apr 11;814(2):247–255. doi: 10.1016/0005-2736(85)90442-0. [DOI] [PubMed] [Google Scholar]
- Biwersi J., Verkman A. S. Cell-permeable fluorescent indicator for cytosolic chloride. Biochemistry. 1991 Aug 13;30(32):7879–7883. doi: 10.1021/bi00246a001. [DOI] [PubMed] [Google Scholar]
- Bubien J. K., Kirk K. L., Rado T. A., Frizzell R. A. Cell cycle dependence of chloride permeability in normal and cystic fibrosis lymphocytes. Science. 1990 Jun 15;248(4961):1416–1419. doi: 10.1126/science.2162561. [DOI] [PubMed] [Google Scholar]
- Jacobson M. A. Valaciclovir (BW256U87): the L-valyl ester of acyclovir. J Med Virol. 1993;Suppl 1:150–153. doi: 10.1002/jmv.1890410529. [DOI] [PubMed] [Google Scholar]
- Karayiannis P., Petrovic L. M., Fry M., Moore D., Enticott M., McGarvey M. J., Scheuer P. J., Thomas H. C. Studies of GB hepatitis agent in tamarins. Hepatology. 1989 Feb;9(2):186–192. doi: 10.1002/hep.1840090204. [DOI] [PubMed] [Google Scholar]
- Keilbaugh S. A., Hobbs G. A., Simpson M. V. Anti-human immunodeficiency virus type 1 therapy and peripheral neuropathy: prevention of 2',3'-dideoxycytidine toxicity in PC12 cells, a neuronal model, by uridine and pyruvate. Mol Pharmacol. 1993 Oct;44(4):702–706. [PubMed] [Google Scholar]
- Krapivinsky G. B., Ackerman M. J., Gordon E. A., Krapivinsky L. D., Clapham D. E. Molecular characterization of a swelling-induced chloride conductance regulatory protein, pICln. Cell. 1994 Feb 11;76(3):439–448. doi: 10.1016/0092-8674(94)90109-0. [DOI] [PubMed] [Google Scholar]
- Kroesen B. J., Mesander G., ter Haar J. G., The T. H., de Leij L. Direct visualisation and quantification of cellular cytotoxicity using two colour flourescence. J Immunol Methods. 1992 Nov 25;156(1):47–54. doi: 10.1016/0022-1759(92)90009-i. [DOI] [PubMed] [Google Scholar]
- Lang F., Ritter M., Völkl H., Häussinger D. The biological significance of cell volume. Ren Physiol Biochem. 1993 Jan-Apr;16(1-2):48–65. doi: 10.1159/000173751. [DOI] [PubMed] [Google Scholar]
- McNutt N. S., Kindel S., Lugo J. Cutaneous manifestations of measles in AIDS. J Cutan Pathol. 1992 Aug;19(4):315–324. doi: 10.1111/j.1600-0560.1992.tb01368.x. [DOI] [PubMed] [Google Scholar]
- Nagra R. M., Burrola P. G., Wiley C. A. Development of spongiform encephalopathy in retroviral infected mice. Lab Invest. 1992 Mar;66(3):292–302. [PubMed] [Google Scholar]
- Neser J. A., Phillips T., Thomson G. R., Gainaru M. D., Coetzee T. African swine fever. I. Morphological changes and virus replication in blood platelets of pigs infected with virulent haemadsorbing and non-haemadsorbing isolates. Onderstepoort J Vet Res. 1986 Sep;53(3):133–141. [PubMed] [Google Scholar]
- Paulmichl M., Friedrich F., Maly K., Lang F. The effect of hypoosmolarity on the electrical properties of Madin Darby canine kidney cells. Pflugers Arch. 1989 Mar;413(5):456–462. doi: 10.1007/BF00594173. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Lang F. Enhancement of intracellular calcium concentration by extracellular ATP and UTP in Madin Darby Canine Kidney cells. Biochem Biophys Res Commun. 1988 Nov 15;156(3):1139–1143. doi: 10.1016/s0006-291x(88)80751-4. [DOI] [PubMed] [Google Scholar]
- Paulmichl M., Li Y., Wickman K., Ackerman M., Peralta E., Clapham D. New mammalian chloride channel identified by expression cloning. Nature. 1992 Mar 19;356(6366):238–241. doi: 10.1038/356238a0. [DOI] [PubMed] [Google Scholar]
- Pierce S. K., Politis A. D. Ca2(+)-activated cell volume recovery mechanisms. Annu Rev Physiol. 1990;52:27–42. doi: 10.1146/annurev.ph.52.030190.000331. [DOI] [PubMed] [Google Scholar]
- Pohlenz J. F., Cheville N. F., Woode G. N., Mokresh A. H. Cellular lesions in intestinal mucosa of gnotobiotic calves experimentally infected with a new unclassified bovine virus (Breda virus). Vet Pathol. 1984 Jul;21(4):407–417. doi: 10.1177/030098588402100407. [DOI] [PubMed] [Google Scholar]
- Ritter M., Steidl M., Lang F. Inhibition of ion conductances by osmotic shrinkage of Madin-Darby canine kidney cells. Am J Physiol. 1991 Oct;261(4 Pt 1):C602–C607. doi: 10.1152/ajpcell.1991.261.4.C602. [DOI] [PubMed] [Google Scholar]
- Schröeder C. H., Engler H., Kirchner H. Protection of mice by an apathogenic strain HSV-1 against lethal infection by a pathogenic strain of HSV-1. J Gen Virol. 1981 Jan;52(Pt 1):159–161. doi: 10.1099/0022-1317-52-1-159. [DOI] [PubMed] [Google Scholar]
- Sommadossi J. P., Carlisle R., Schinazi R. F., Zhou Z. Uridine reverses the toxicity of 3'-azido-3'-deoxythymidine in normal human granulocyte-macrophage progenitor cells in vitro without impairment of antiretroviral activity. Antimicrob Agents Chemother. 1988 Jul;32(7):997–1001. doi: 10.1128/aac.32.7.997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Venglarik C. J., Singh A. K., Wang R., Bridges R. J. Trinitrophenyl-ATP blocks colonic Cl- channels in planar phospholipid bilayers. Evidence for two nucleotide binding sites. J Gen Physiol. 1993 Apr;101(4):545–569. doi: 10.1085/jgp.101.4.545. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wangemann P., Wittner M., Di Stefano A., Englert H. C., Lang H. J., Schlatter E., Greger R. Cl(-)-channel blockers in the thick ascending limb of the loop of Henle. Structure activity relationship. Pflugers Arch. 1986;407 (Suppl 2):S128–S141. doi: 10.1007/BF00584942. [DOI] [PubMed] [Google Scholar]