Abstract
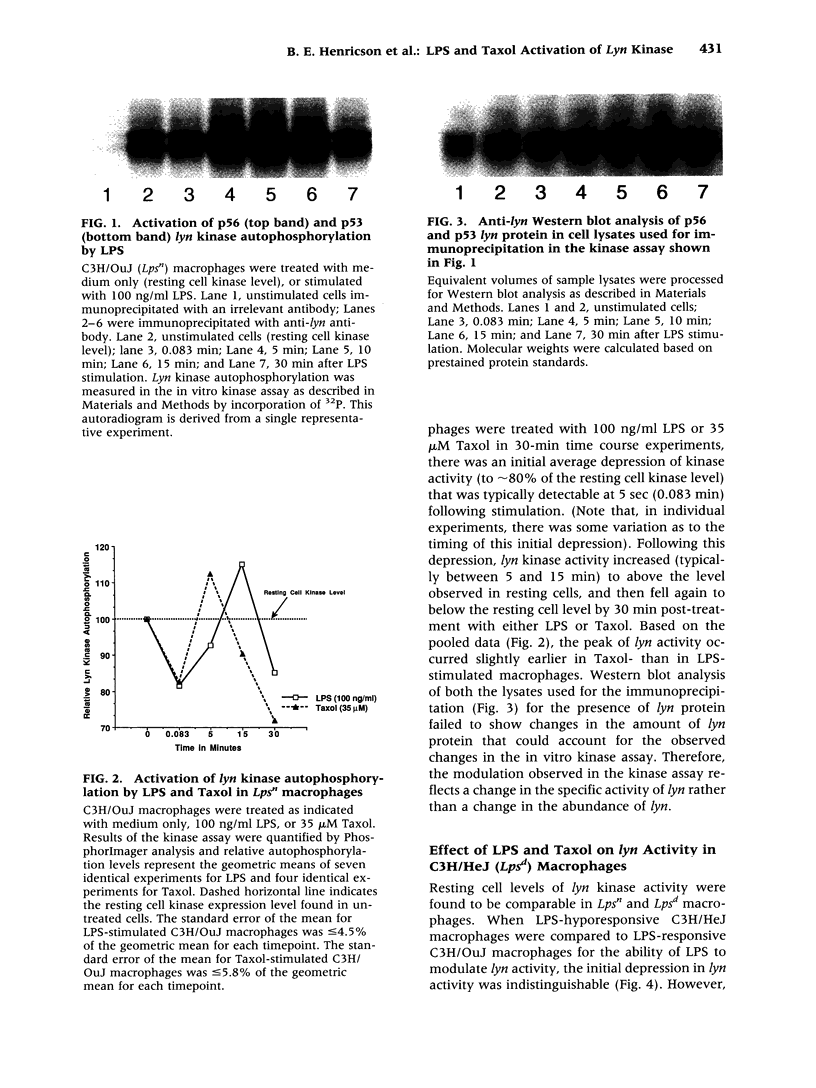
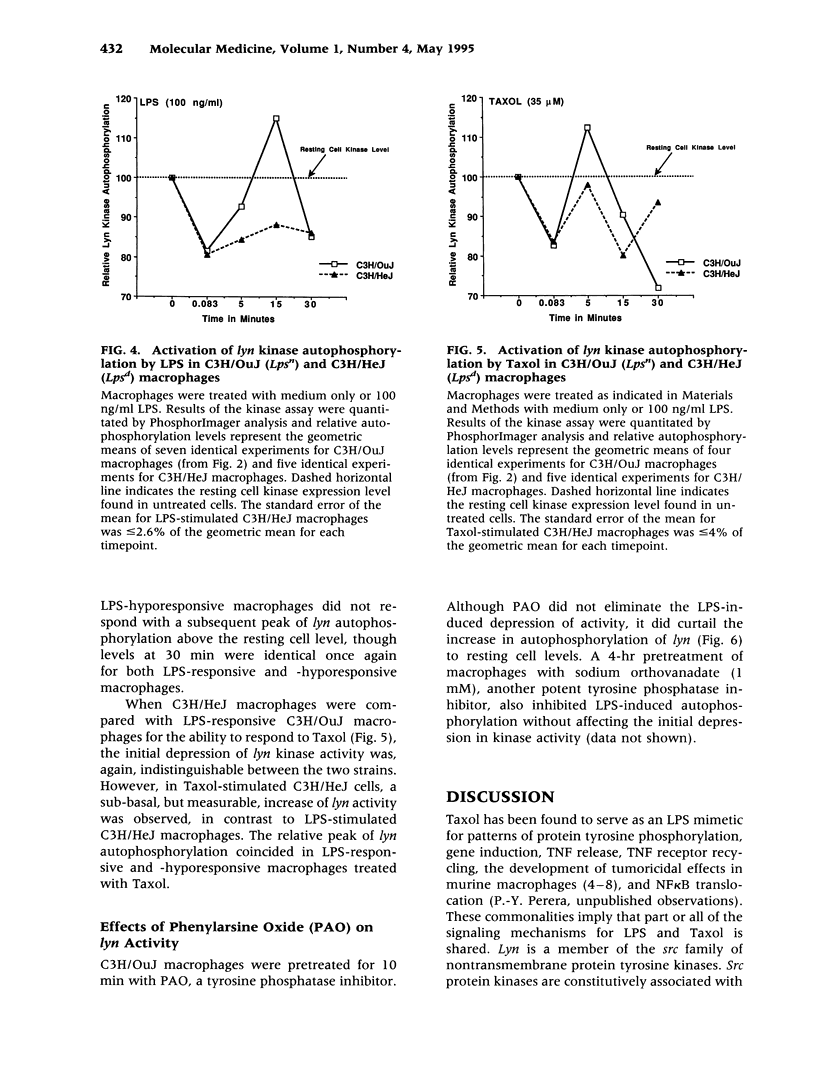
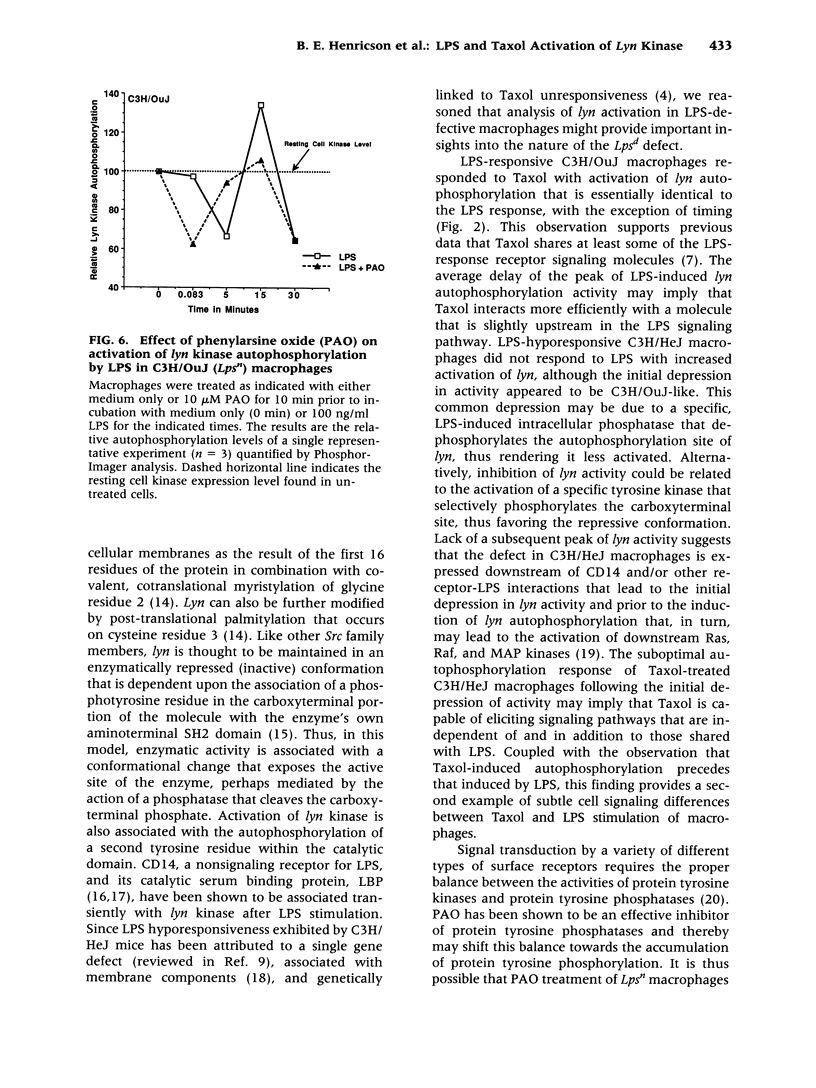
BACKGROUND: The anti-tumor agent, Taxol, has been shown in murine macrophages to stimulate tumor necrosis factor (TNF), modulate TNF receptors, induce a large panel of immediate-early genes, and induce protein tyrosine phosphorylation indistinguishably from LPS. These data, coupled with the finding that lipid A antagonists block Taxol-induced stimulation, support the hypothesis that these two structurally unrelated compounds activate a common, receptor-associated signaling apparatus. A very early event in LPS signaling of human monocytes is activation of lyn kinase activity. We therefore sought to evaluate the activation of lyn kinase by LPS and Taxol in LPS-responsive (Lps(n)) and LPS-hyporesponsive (Lps(d)) macrophages. MATERIALS AND METHODS: C3H/OuJ (Lps(n)) and C3H/HeJ (Lps(d)) macrophages were stimulated by LPS or Taxol. Cell lysates were subjected to immunoprecipitation with anti-lyn antibody, gel electrophoresis, and in vitro kinase assays. Autoradiography and Phosphor-Imager analysis were carried out to detect incorporation of 32P into lyn protein. RESULTS: Within seconds of stimulation, LPS and Taxol induce in Lps(n) macrophages a depression of autophosphorylation, followed within minutes by autophosphorylation of both p53 and p56 lyn species. Lps(d) macrophages respond to LPS and Taxol with the initial decrease in activity, but fail to respond to LPS with autophosphorylation, and respond only to a limited extent upon Taxol stimulation. Tyrosine phosphatase inhibitors exerted inhibitory effects on LPS stimulation of lyn autophosphorylation. CONCLUSIONS: Decreased lyn kinase activity within seconds and autophosphorylation within minutes of LPS or Taxol stimulation in Lps(n) macrophages strongly supports the hypothesis that LPS and Taxol share a common signaling pathway. The finding that C3H/HeJ macrophages respond to LPS and Taxol with a normal depression of lyn activity, but fail to autophosphorylate lyn normally in response to LPS or Taxol, suggests that the Lps(d) defect is distal to LPS-receptor interaction. Finally, the inhibitory effect of tyrosine phosphatase inhibitors on LPS-induced lyn autophosphorylation suggests that tyrosine phosphatase(s) may participate in the regulation of lyn kinase activity.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Carboni J. M., Singh C., Tepper M. A. Taxol and lipopolysaccharide activation of a murine macrophage cell line and induction of similar tyrosine phosphoproteins. J Natl Cancer Inst Monogr. 1993;(15):95–101. [PubMed] [Google Scholar]
- Cooper J. A., Howell B. The when and how of Src regulation. Cell. 1993 Jun 18;73(6):1051–1054. doi: 10.1016/0092-8674(93)90634-3. [DOI] [PubMed] [Google Scholar]
- Ding A. H., Porteu F., Sanchez E., Nathan C. F. Shared actions of endotoxin and taxol on TNF receptors and TNF release. Science. 1990 Apr 20;248(4953):370–372. doi: 10.1126/science.1970196. [DOI] [PubMed] [Google Scholar]
- Geppert T. D., Whitehurst C. E., Thompson P., Beutler B. Lipopolysaccharide signals activation of tumor necrosis factor biosynthesis through the ras/raf-1/MEK/MAPK pathway. Mol Med. 1994 Nov;1(1):93–103. [PMC free article] [PubMed] [Google Scholar]
- Hailman E., Lichenstein H. S., Wurfel M. M., Miller D. S., Johnson D. A., Kelley M., Busse L. A., Zukowski M. M., Wright S. D. Lipopolysaccharide (LPS)-binding protein accelerates the binding of LPS to CD14. J Exp Med. 1994 Jan 1;179(1):269–277. doi: 10.1084/jem.179.1.269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henricson B. E., Manthey C. L., Perera P. Y., Hamilton T. A., Vogel S. N. Dissociation of lipopolysaccharide (LPS)-inducible gene expression in murine macrophages pretreated with smooth LPS versus monophosphoryl lipid A. Infect Immun. 1993 Jun;61(6):2325–2333. doi: 10.1128/iai.61.6.2325-2333.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakobovits A., Sharon N., Zan-Bar I. Acquisition of mitogenic responsiveness by nonresponding lymphocytes upon insertion of appropriate membrane components. J Exp Med. 1982 Oct 1;156(4):1274–1279. doi: 10.1084/jem.156.4.1274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manthey C. L., Brandes M. E., Perera P. Y., Vogel S. N. Taxol increases steady-state levels of lipopolysaccharide-inducible genes and protein-tyrosine phosphorylation in murine macrophages. J Immunol. 1992 Oct 1;149(7):2459–2465. [PubMed] [Google Scholar]
- Manthey C. L., Perera P. Y., Salkowski C. A., Vogel S. N. Taxol provides a second signal for murine macrophage tumoricidal activity. J Immunol. 1994 Jan 15;152(2):825–831. [PubMed] [Google Scholar]
- Manthey C. L., Qureshi N., Stütz P. L., Vogel S. N. Lipopolysaccharide antagonists block taxol-induced signaling in murine macrophages. J Exp Med. 1993 Aug 1;178(2):695–702. doi: 10.1084/jem.178.2.695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntire F. C., Sievert H. W., Barlow G. H., Finley R. A., Lee A. Y. Chemical, physical, biological properties of a lipopolysaccharide from Escherichia coli K-235. Biochemistry. 1967 Aug;6(8):2363–2372. doi: 10.1021/bi00860a011. [DOI] [PubMed] [Google Scholar]
- Resh M. D. Myristylation and palmitylation of Src family members: the fats of the matter. Cell. 1994 Feb 11;76(3):411–413. doi: 10.1016/0092-8674(94)90104-x. [DOI] [PubMed] [Google Scholar]
- Rowinsky E. K., Donehower R. C., Jones R. J., Tucker R. W. Microtubule changes and cytotoxicity in leukemic cell lines treated with taxol. Cancer Res. 1988 Jul 15;48(14):4093–4100. [PubMed] [Google Scholar]
- Stefanová I., Corcoran M. L., Horak E. M., Wahl L. M., Bolen J. B., Horak I. D. Lipopolysaccharide induces activation of CD14-associated protein tyrosine kinase p53/56lyn. J Biol Chem. 1993 Oct 5;268(28):20725–20728. [PubMed] [Google Scholar]





