Abstract
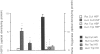
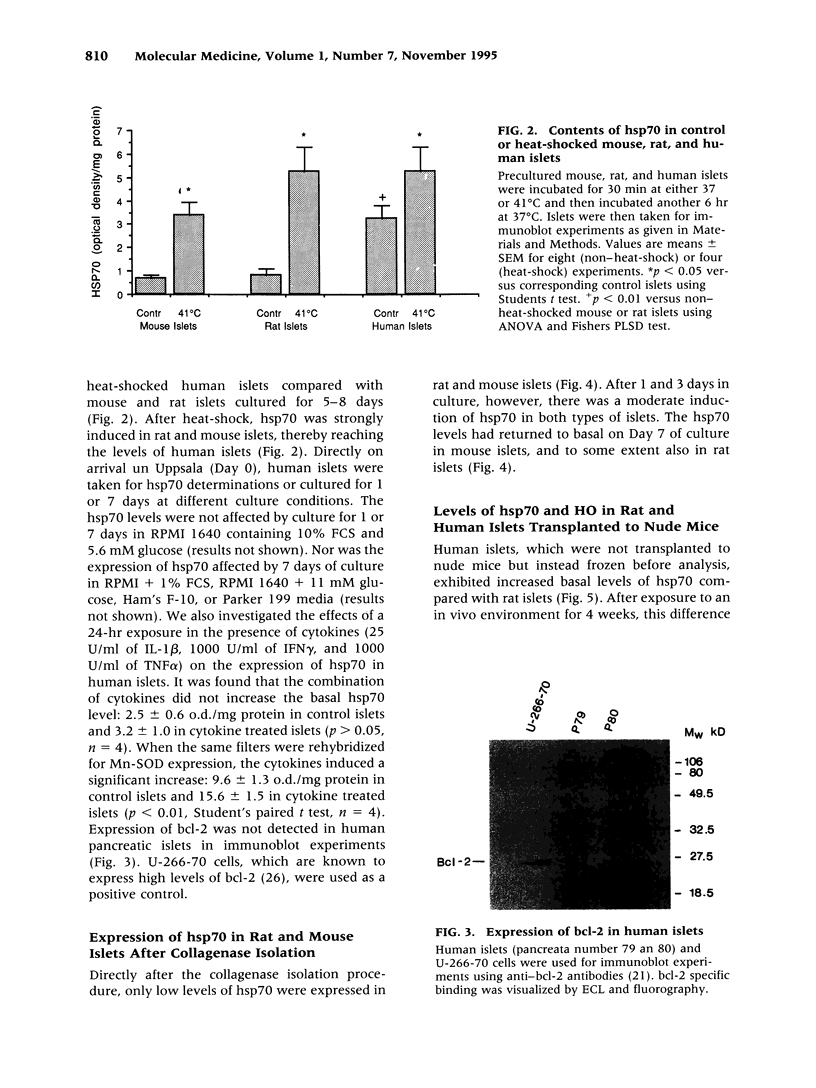
BACKGROUND: It has previously been observed that the insulin-producing cells of human pancreatic islets are more resistant to alloxan-, streptozotocin-, nitroprusside-, or cytokine-induced injury than those of mouse and rat islets. MATERIALS AND METHODS: Human pancreatic islets were obtained from heart-beating organ donors. The expression of the stress proteins heat shock protein 70 (hsp70) and heme oxygenase and the anti-apoptosis gene bcl-2 was determined in isolated rat, mouse, and human islets, either cultured in vitro or transplanted under the kidney capsule of nude mice, using immunoblot analysis. Rat and human islet sensitive hydrogen peroxide was assess by glucose oxidation measurements. Isolated islets were also analyzed for their catalase and superoxide dismutase activities, and the islet cell levels of reduced glutathione were determined in response to hydrogen peroxide and nitroprusside. Programmed cell death in human and rat islets in response to streptozotocin was evaluated using TUNEL staining. RESULTS: Cultured human islets expressed higher contents of hsp70 than mouse and rat islets at basal conditions. Also after 4 weeks under the kidney capsule of normoglycemic mice, the hsp70 levels were higher in human islets than in rat islets. The expression of another stress protein, heme oxygenase (HO), was strongly increased in cultured rat islets, but was not affected in human islets. Expression of the bcl-2 gene could not be detected in human islets. In spite of this, 0.5 mM streptozotocin induced apotosis in rat but not in human islet cells. Hydrogen peroxide (0.1 and 0.4 mM) decreased glucose oxidation rates in rat but not in human islets. The levels of reduced glutathione were moderately decreased in human and rat islet cells and sharply decreased in mouse islet cells in response to hydrogen peroxide. Moreover, the activities of catalase and superoxide dismutase (SOD) were markedly lower in mouse islets than in human islets. The activity of catalase was lower in rat islets than in human islets. CONCLUSION: Human islets differ clearly from mouse and rat islets in their increased expression of hsp70, catalase, and SOD, which may explain the increased resistance of human islets to beta cell toxins.
Full text
PDF



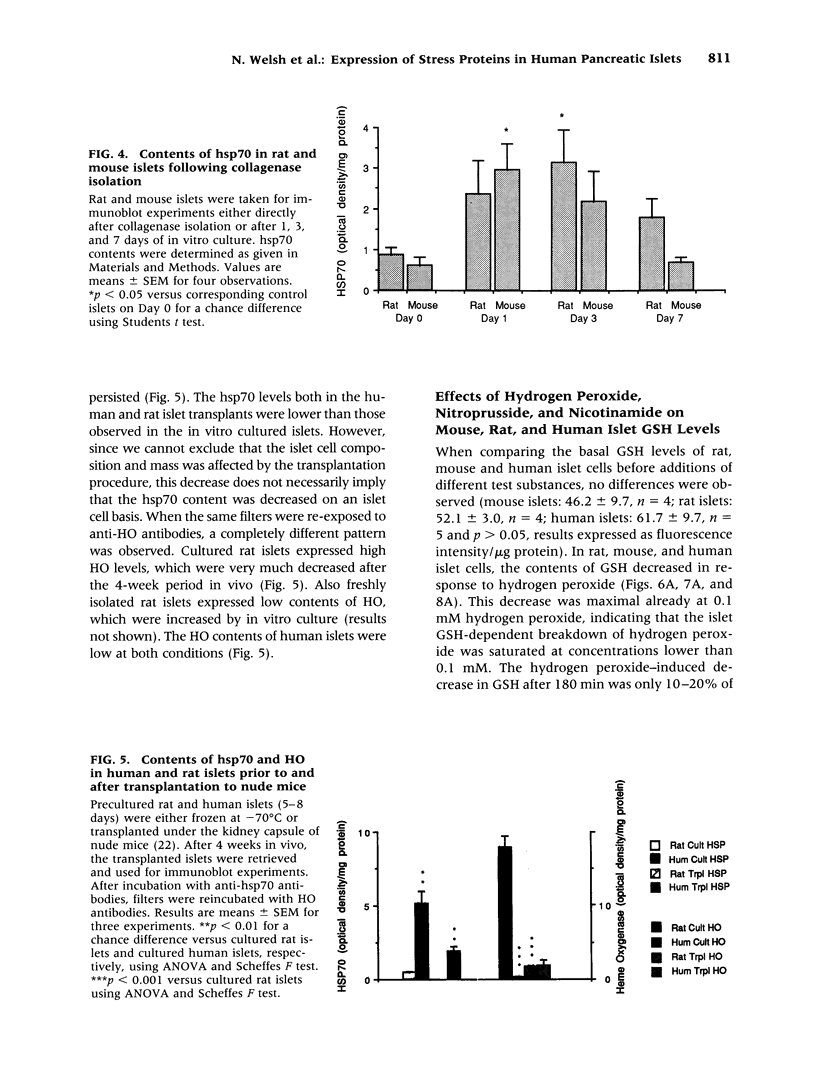
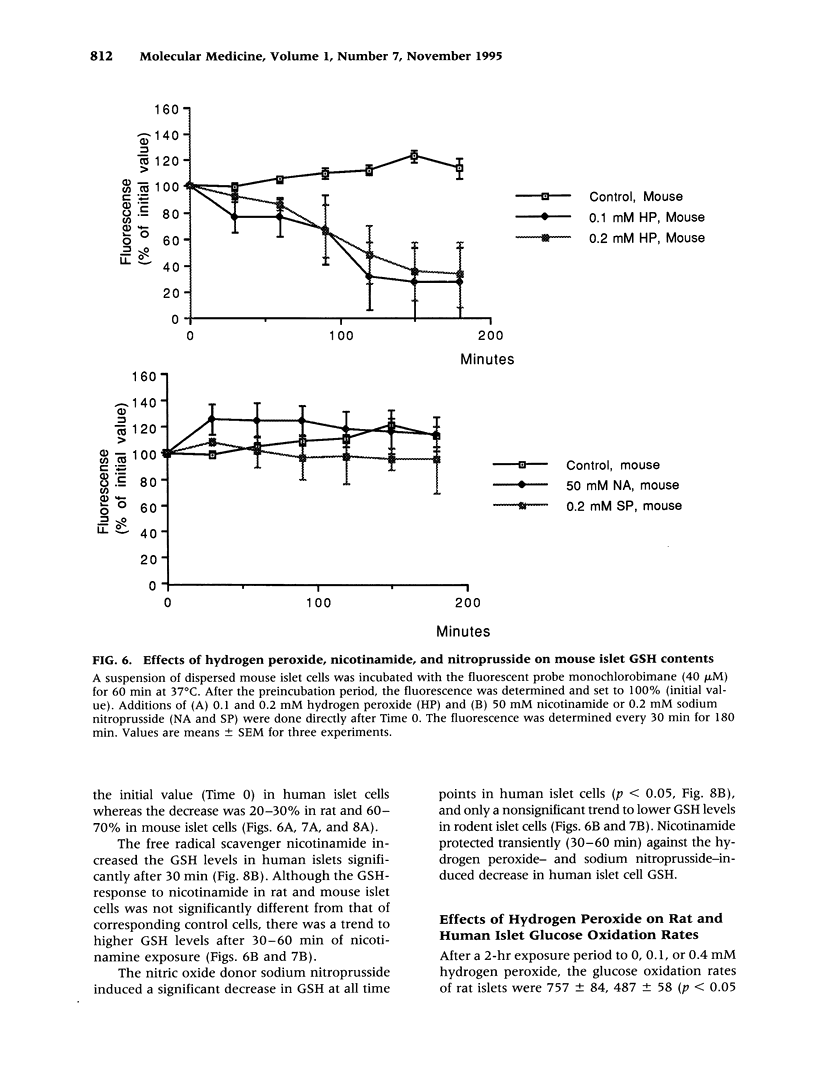
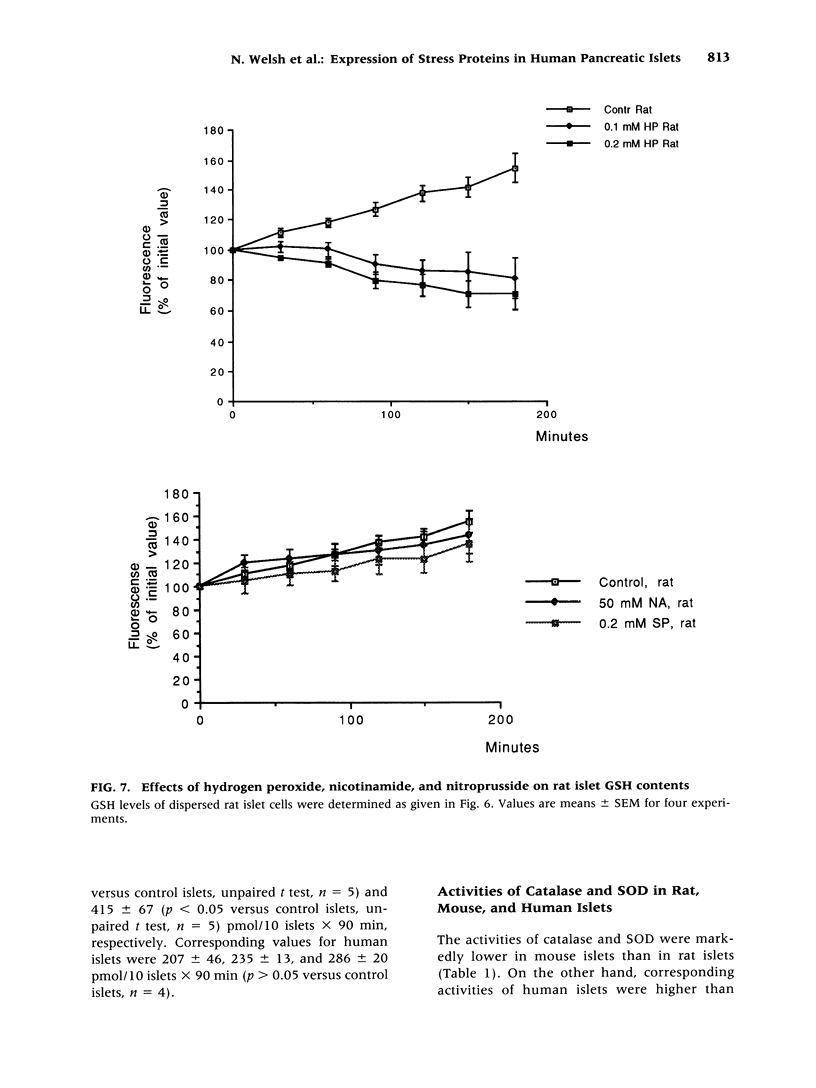
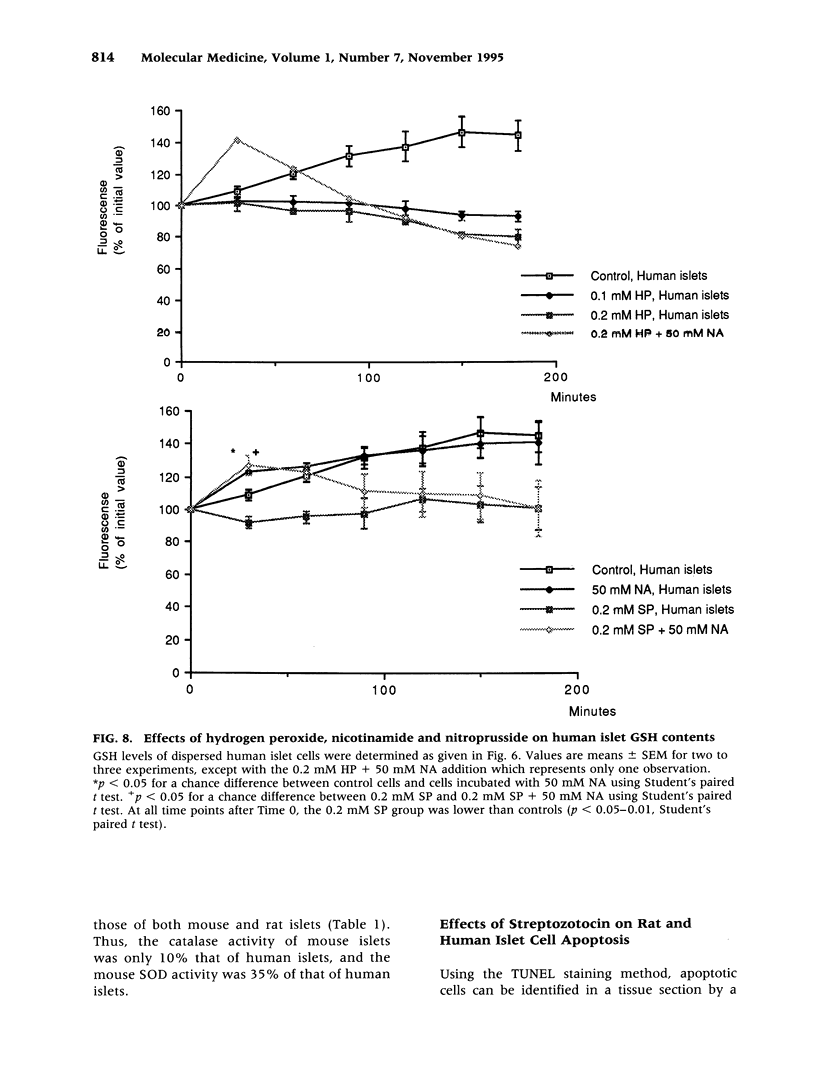

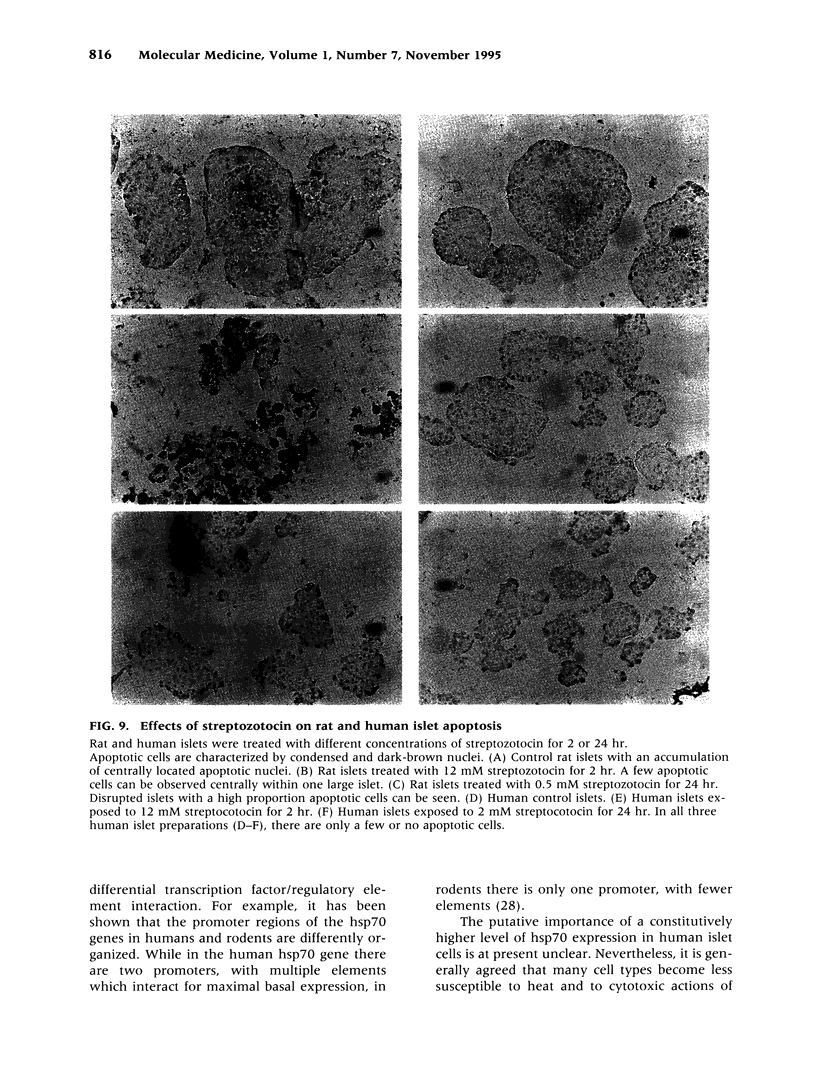




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Andersen H. U., Jørgensen K. H., Egeberg J., Mandrup-Poulsen T., Nerup J. Nicotinamide prevents interleukin-1 effects on accumulated insulin release and nitric oxide production in rat islets of Langerhans. Diabetes. 1994 Jun;43(6):770–777. doi: 10.2337/diab.43.6.770. [DOI] [PubMed] [Google Scholar]
- Andersson A. Isolated mouse pancreatic islets in culture: effects of serum and different culture media on the insulin production of the islets. Diabetologia. 1978 Jun;14(6):397–404. doi: 10.1007/BF01228134. [DOI] [PubMed] [Google Scholar]
- Bensaude O., Morange M. Spontaneous high expression of heat-shock proteins in mouse embryonal carcinoma cells and ectoderm from day 8 mouse embryo. EMBO J. 1983;2(2):173–177. doi: 10.1002/j.1460-2075.1983.tb01401.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg L. A., Cagliero E., Sandler S., Welsh N., Eizirik D. L. Interleukin-1 beta increases the activity of superoxide dismutase in rat pancreatic islets. Endocrinology. 1992 May;130(5):2851–2857. doi: 10.1210/endo.130.5.1533363. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cornelius J. G., Luttge B. G., Peck A. B. Antioxidant enzyme activities in IDD-prone and IDD-resistant mice: a comparative study. Free Radic Biol Med. 1993 Apr;14(4):409–420. doi: 10.1016/0891-5849(93)90090-h. [DOI] [PubMed] [Google Scholar]
- Craig E. A. The heat shock response. CRC Crit Rev Biochem. 1985;18(3):239–280. doi: 10.3109/10409238509085135. [DOI] [PubMed] [Google Scholar]
- Deneke S. M., Fanburg B. L. Regulation of cellular glutathione. Am J Physiol. 1989 Oct;257(4 Pt 1):L163–L173. doi: 10.1152/ajplung.1989.257.4.L163. [DOI] [PubMed] [Google Scholar]
- Eisenbarth G. S. Type I diabetes mellitus. A chronic autoimmune disease. N Engl J Med. 1986 May 22;314(21):1360–1368. doi: 10.1056/NEJM198605223142106. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Korbutt G. S., Hellerström C. Prolonged exposure of human pancreatic islets to high glucose concentrations in vitro impairs the beta-cell function. J Clin Invest. 1992 Oct;90(4):1263–1268. doi: 10.1172/JCI115989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik D. L., Pipeleers D. G., Ling Z., Welsh N., Hellerström C., Andersson A. Major species differences between humans and rodents in the susceptibility to pancreatic beta-cell injury. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9253–9256. doi: 10.1073/pnas.91.20.9253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eizirik D. L., Sandler S., Palmer J. P. Repair of pancreatic beta-cells. A relevant phenomenon in early IDDM? Diabetes. 1993 Oct;42(10):1383–1391. doi: 10.2337/diab.42.10.1383. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Sandler S., Welsh N., Bendtzen K., Hellerström C. Nicotinamide decreases nitric oxide production and partially protects human pancreatic islets against the suppressive effects of combinations of cytokines. Autoimmunity. 1994;19(3):193–198. doi: 10.3109/08916939408995694. [DOI] [PubMed] [Google Scholar]
- Eizirik D. L., Sandler S., Welsh N., Cetkovic-Cvrlje M., Nieman A., Geller D. A., Pipeleers D. G., Bendtzen K., Hellerström C. Cytokines suppress human islet function irrespective of their effects on nitric oxide generation. J Clin Invest. 1994 May;93(5):1968–1974. doi: 10.1172/JCI117188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson U. J., Borg L. A. Protection by free oxygen radical scavenging enzymes against glucose-induced embryonic malformations in vitro. Diabetologia. 1991 May;34(5):325–331. doi: 10.1007/BF00405004. [DOI] [PubMed] [Google Scholar]
- Grankvist K., Marklund S. L., Täljedal I. B. CuZn-superoxide dismutase, Mn-superoxide dismutase, catalase and glutathione peroxidase in pancreatic islets and other tissues in the mouse. Biochem J. 1981 Nov 1;199(2):393–398. doi: 10.1042/bj1990393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greene J. M., Larin Z., Taylor I. C., Prentice H., Gwinn K. A., Kingston R. E. Multiple basal elements of a human hsp70 promoter function differently in human and rodent cell lines. Mol Cell Biol. 1987 Oct;7(10):3646–3655. doi: 10.1128/mcb.7.10.3646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helqvist S., Polla B. S., Johannesen J., Nerup J. Heat shock protein induction in rat pancreatic islets by recombinant human interleukin 1 beta. Diabetologia. 1991 Mar;34(3):150–156. doi: 10.1007/BF00418268. [DOI] [PubMed] [Google Scholar]
- Horio F., Fukuda M., Katoh H., Petruzzelli M., Yano N., Rittershaus C., Bonner-Weir S., Hattori M. Reactive oxygen intermediates in autoimmune islet cell destruction of the NOD mouse induced by peritoneal exudate cells (rich in macrophages) but not T cells. Diabetologia. 1994 Jan;37(1):22–31. doi: 10.1007/BF00428773. [DOI] [PubMed] [Google Scholar]
- Johansson L. H., Borg L. A. A spectrophotometric method for determination of catalase activity in small tissue samples. Anal Biochem. 1988 Oct;174(1):331–336. doi: 10.1016/0003-2697(88)90554-4. [DOI] [PubMed] [Google Scholar]
- Juntti-Berggren L., Larsson O., Rorsman P., Ammälä C., Bokvist K., Wåhlander K., Nicotera P., Dypbukt J., Orrenius S., Hallberg A. Increased activity of L-type Ca2+ channels exposed to serum from patients with type I diabetes. Science. 1993 Jul 2;261(5117):86–90. doi: 10.1126/science.7686306. [DOI] [PubMed] [Google Scholar]
- Llesuy S. F., Tomaro M. L. Heme oxygenase and oxidative stress. Evidence of involvement of bilirubin as physiological protector against oxidative damage. Biochim Biophys Acta. 1994 Aug 11;1223(1):9–14. doi: 10.1016/0167-4889(94)90067-1. [DOI] [PubMed] [Google Scholar]
- Lorenzo A., Razzaboni B., Weir G. C., Yankner B. A. Pancreatic islet cell toxicity of amylin associated with type-2 diabetes mellitus. Nature. 1994 Apr 21;368(6473):756–760. doi: 10.1038/368756a0. [DOI] [PubMed] [Google Scholar]
- Malaisse W. J., Malaisse-Lagae F., Sener A., Pipeleers D. G. Determinants of the selective toxicity of alloxan to the pancreatic B cell. Proc Natl Acad Sci U S A. 1982 Feb;79(3):927–930. doi: 10.1073/pnas.79.3.927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margulis B. A., Nacharov P. V., Tsvetkova O. I., Welsh M., Kinev A. V. The characterization and use of different antibodies against the hsp70 major heat shock protein family for the development of an immunoassay. Electrophoresis. 1991 Sep;12(9):670–673. doi: 10.1002/elps.1150120913. [DOI] [PubMed] [Google Scholar]
- Margulis B. A., Sandler S., Eizirik D. L., Welsh N., Welsh M. Liposomal delivery of purified heat shock protein hsp70 into rat pancreatic islets as protection against interleukin 1 beta-induced impaired beta-cell function. Diabetes. 1991 Nov;40(11):1418–1422. doi: 10.2337/diab.40.11.1418. [DOI] [PubMed] [Google Scholar]
- Mellgren A., Schnell Landström A. H., Petersson B., Andersson A. The renal subcapsular site offers better growth conditions for transplanted mouse pancreatic islet cells than the liver or spleen. Diabetologia. 1986 Sep;29(9):670–672. doi: 10.1007/BF00869269. [DOI] [PubMed] [Google Scholar]
- Moreira J. E., Hand A. R., Håkan Borg L. A., Sandler S., Welsh M., Welsh N., Eizirik D. L. Decrease in insulin-containing secretory granules and mitochondrial gene expression in mouse pancreatic islets maintained in culture following streptozotocin exposure. Virchows Arch B Cell Pathol Incl Mol Pathol. 1991;60(5):337–344. doi: 10.1007/BF02899565. [DOI] [PubMed] [Google Scholar]
- Morgan N. G., Cable H. C., Newcombe N. R., Williams G. T. Treatment of cultured pancreatic B-cells with streptozotocin induces cell death by apoptosis. Biosci Rep. 1994 Oct;14(5):243–250. doi: 10.1007/BF01209729. [DOI] [PubMed] [Google Scholar]
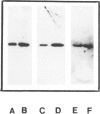
- Pettersson M., Jernberg-Wiklund H., Larsson L. G., Sundström C., Givol I., Tsujimoto Y., Nilsson K. Expression of the bcl-2 gene in human multiple myeloma cell lines and normal plasma cells. Blood. 1992 Jan 15;79(2):495–502. [PubMed] [Google Scholar]
- Pezzella F., Tse A. G., Cordell J. L., Pulford K. A., Gatter K. C., Mason D. Y. Expression of the bcl-2 oncogene protein is not specific for the 14;18 chromosomal translocation. Am J Pathol. 1990 Aug;137(2):225–232. [PMC free article] [PubMed] [Google Scholar]
- Pipeleers D. G., in't Veld P. A., Van de Winkel M., Maes E., Schuit F. C., Gepts W. A new in vitro model for the study of pancreatic A and B cells. Endocrinology. 1985 Sep;117(3):806–816. doi: 10.1210/endo-117-3-806. [DOI] [PubMed] [Google Scholar]
- Pociot F., Rønningen K. S., Nerup J. Polymorphic analysis of the human MHC-linked heat shock protein 70 (HSP70-2) and HSP70-Hom genes in insulin-dependent diabetes mellitus (IDDM). Scand J Immunol. 1993 Nov;38(5):491–495. doi: 10.1111/j.1365-3083.1993.tb02593.x. [DOI] [PubMed] [Google Scholar]
- Raju V. S., Maines M. D. Coordinated expression and mechanism of induction of HSP32 (heme oxygenase-1) mRNA by hyperthermia in rat organs. Biochim Biophys Acta. 1994 Apr 6;1217(3):273–280. doi: 10.1016/0167-4781(94)90286-0. [DOI] [PubMed] [Google Scholar]
- Shrieve D. C., Bump E. A., Rice G. C. Heterogeneity of cellular glutathione among cells derived from a murine fibrosarcoma or a human renal cell carcinoma detected by flow cytometric analysis. J Biol Chem. 1988 Oct 5;263(28):14107–14114. [PubMed] [Google Scholar]
- Strasser A., Harris A. W., Cory S. bcl-2 transgene inhibits T cell death and perturbs thymic self-censorship. Cell. 1991 Nov 29;67(5):889–899. doi: 10.1016/0092-8674(91)90362-3. [DOI] [PubMed] [Google Scholar]
- Tsujimoto Y. Stress-resistance conferred by high level of bcl-2 alpha protein in human B lymphoblastoid cell. Oncogene. 1989 Nov;4(11):1331–1336. [PubMed] [Google Scholar]
- Vaux D. L., Cory S., Adams J. M. Bcl-2 gene promotes haemopoietic cell survival and cooperates with c-myc to immortalize pre-B cells. Nature. 1988 Sep 29;335(6189):440–442. doi: 10.1038/335440a0. [DOI] [PubMed] [Google Scholar]
- Vile G. F., Basu-Modak S., Waltner C., Tyrrell R. M. Heme oxygenase 1 mediates an adaptive response to oxidative stress in human skin fibroblasts. Proc Natl Acad Sci U S A. 1994 Mar 29;91(7):2607–2610. doi: 10.1073/pnas.91.7.2607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welch W. J., Feramisco J. R. Rapid purification of mammalian 70,000-dalton stress proteins: affinity of the proteins for nucleotides. Mol Cell Biol. 1985 Jun;5(6):1229–1237. doi: 10.1128/mcb.5.6.1229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Welsh N., Hellerström C. In vitro restoration of insulin production in islets from adult rats treated neonatally with streptozotocin. Endocrinology. 1990 Apr;126(4):1842–1848. doi: 10.1210/endo-126-4-1842. [DOI] [PubMed] [Google Scholar]
- Welsh N., Sandler S. Protective action by hemin against interleukin-1 beta induced inhibition of rat pancreatic islet function. Mol Cell Endocrinol. 1994 Jul;103(1-2):109–114. doi: 10.1016/0303-7207(94)90076-0. [DOI] [PubMed] [Google Scholar]
- Welsh N., Welsh M., Lindquist S., Eizirik D. L., Bendtzen K., Sandler S. Interleukin-1 beta increases the biosynthesis of the heat shock protein hsp70 and selectively decreases the biosynthesis of five proteins in rat pancreatic islets. Autoimmunity. 1991;9(1):33–40. doi: 10.3109/08916939108997121. [DOI] [PubMed] [Google Scholar]
- Wilson G. L., Patton N. J., McCord J. M., Mullins D. W., Mossman B. T. Mechanisms of streptozotocin- and alloxan-induced damage in rat B cells. Diabetologia. 1984 Dec;27(6):587–591. doi: 10.1007/BF00276973. [DOI] [PubMed] [Google Scholar]