Abstract
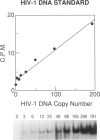
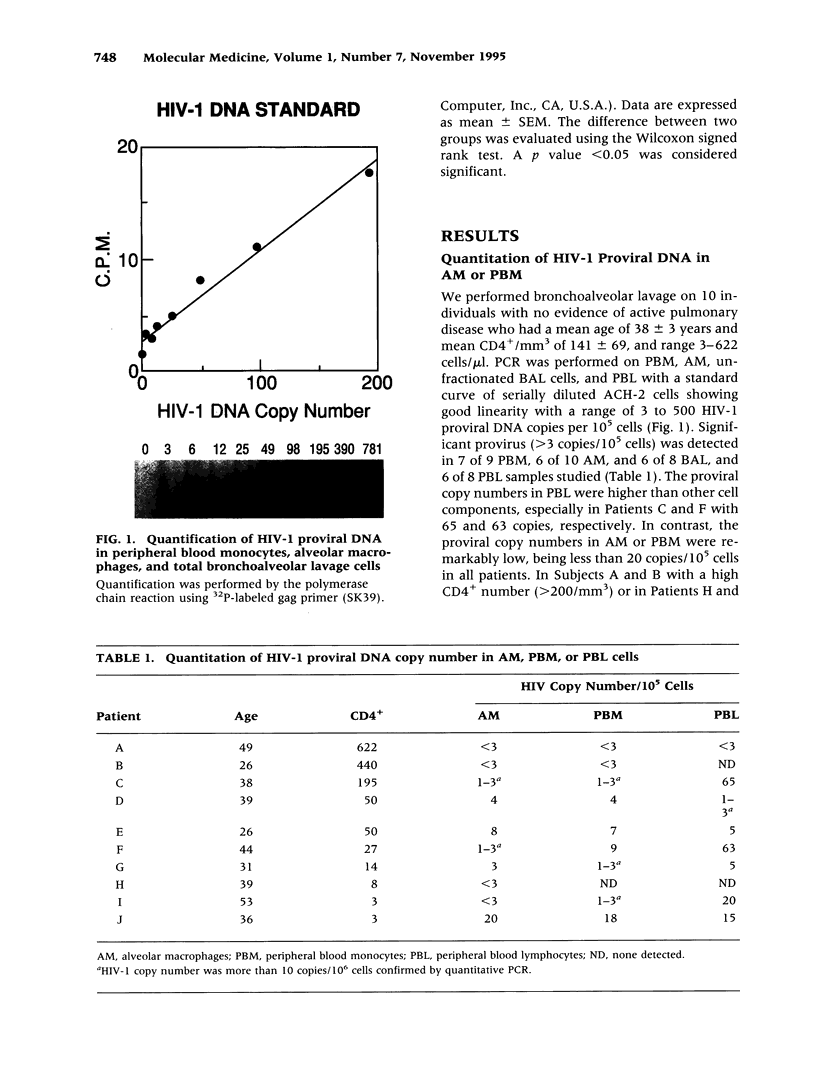
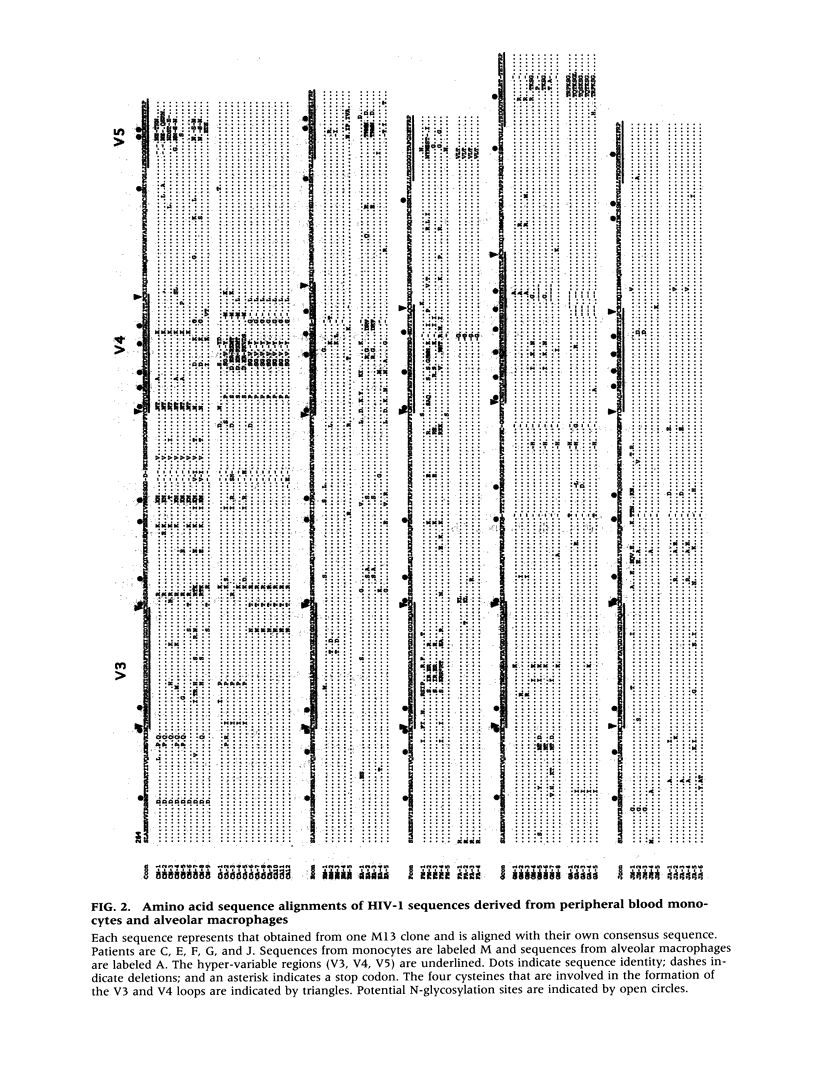
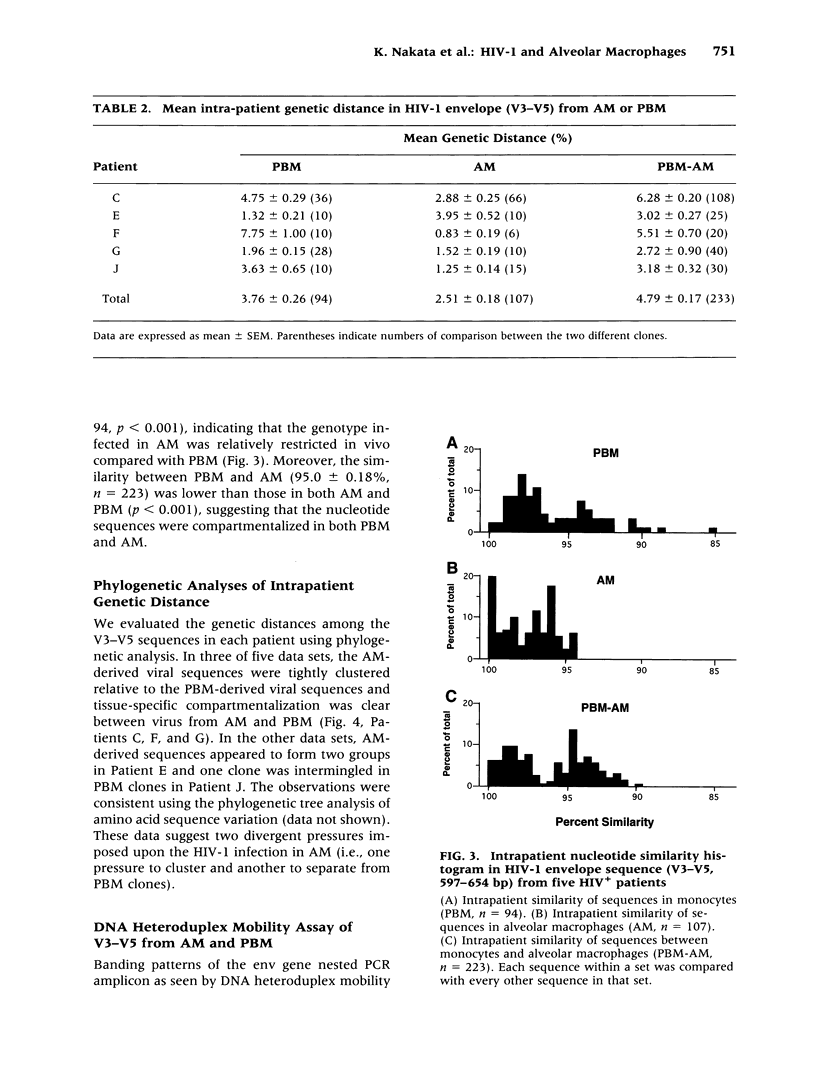
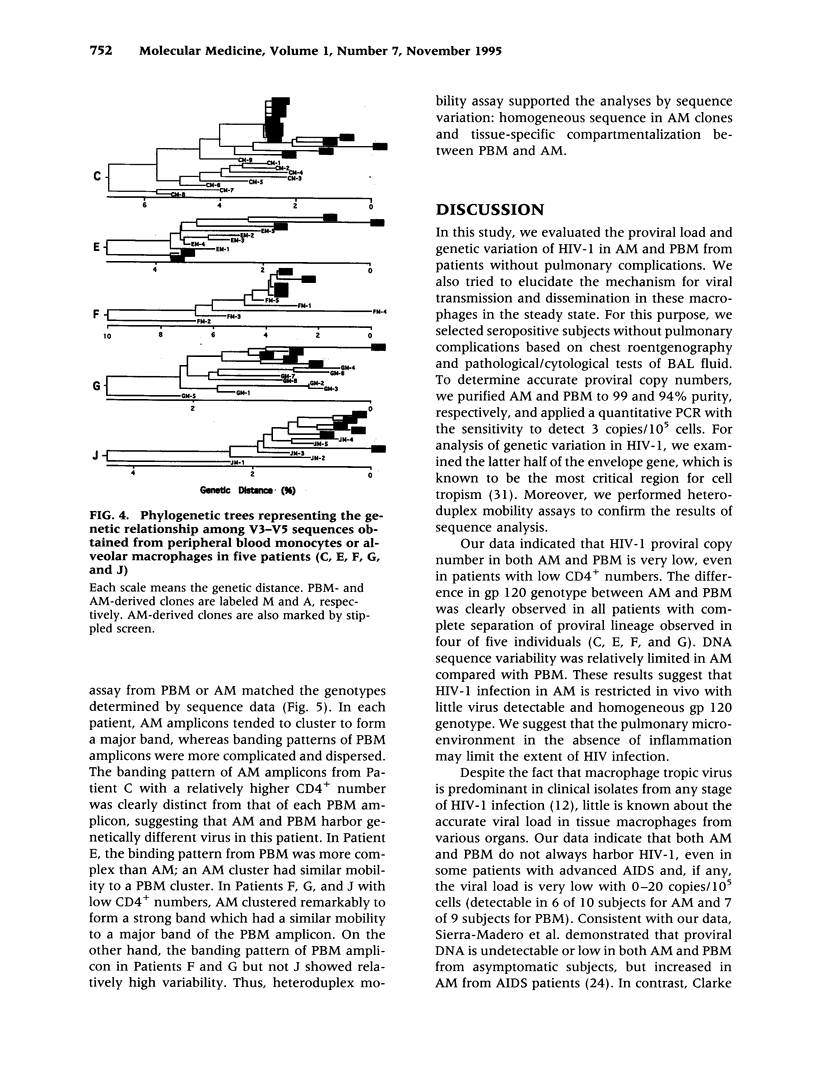
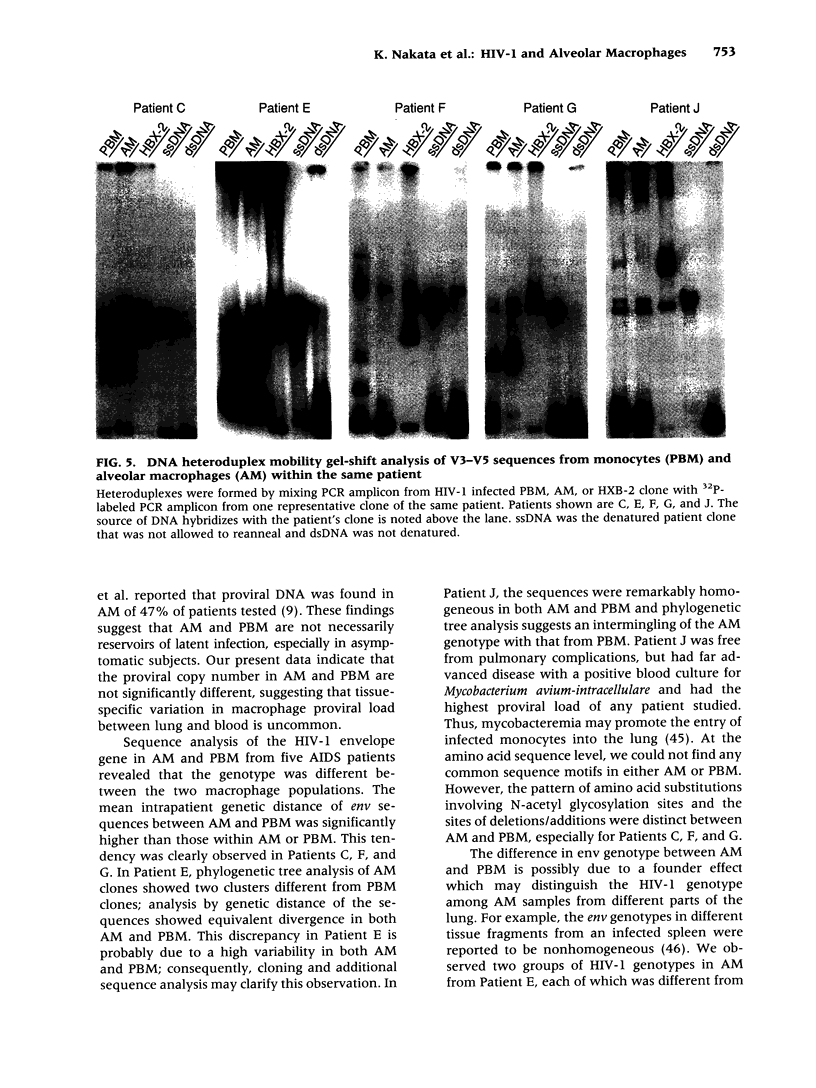
BACKGROUND: We investigated the human immunodeficiency virus (HIV) proviral DNA sequence and copy number in alveolar macrophages (AM) and peripheral blood monocytes (PBM) from 10 HIV-positive patients without any active concurrent pulmonary disease to understand the nature of HIV-1 infection in vivo in the lung microenvironment. MATERIALS AND METHODS: The 10 seropositive patients without active pulmonary disease were selected based on chest roentegenography and pathological/cytological test of bronchoalveolar (BAL) fluid. In order to determine accurate proviral copy numbers, AM and PBM were isolated to 99 and 94% purity, respectively, and quantitative polymerase chain reaction (PCR), with a sensitivity to detect three copies of HIV proviral DNA per 10(5) cells, was applied. For analysis of genetic variation in HIV-1, PCR-amplified HIV-1 DNA from AM and PBM of five patients were subcloned and 2-12 clones from each sample underwent DNA sequence analysis of HIV-1 gp120 V3-V5. Heteroduplex mobility assays were performed to confirm the results of the sequence analysis. RESULTS: The proviral copy number in AM or PBM were less than 20 copies/10(5) cells in all patients, and five patients had less than the detection limit. There was no significant difference in HIV copy number between AM and PBM. No correlation was found between PBM/AM HIV copy number and CD4+ lymphocyte count in the peripheral blood. Sequence analysis revealed that the mean intrapatient genetic similarity in AM was 97.5 +/- 0.18% (n = 107), which was significantly higher than that in PBM (96.2 +/- 0.26% (n = 94), p < 0.001), suggesting that variability of HIV-1 DNA in AM was relatively limited. Divergence occurred when AM derived HIV-1 sequence was compared with PBM derived sequence from the same patient (95.8 +/- 0.17% (n = 223) p < 0.001). Phylogenetic analysis of DNA sequence demonstrated complete separation of HIV lineages from lung and blood in four of five patients. CONCLUSIONS: The results suggest the HIV-1 infection in AM is restricted in vivo with low viral burden and homogenous genotype. We propose that the pulmonary microenvironment may limit the extent of HIV-1 infection.
Full text
PDF








Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Agostini C., Trentin L., Zambello R., Bulian P., Caenazzo C., Cipriani A., Cadrobbi P., Garbisa S., Semenzato G. Release of granulocyte-macrophage colony-stimulating factor by alveolar macrophages in the lung of HIV-1-infected patients. A mechanism accounting for macrophage and neutrophil accumulation. J Immunol. 1992 Nov 15;149(10):3379–3385. [PubMed] [Google Scholar]
- Albert J., Abrahamsson B., Nagy K., Aurelius E., Gaines H., Nyström G., Fenyö E. M. Rapid development of isolate-specific neutralizing antibodies after primary HIV-1 infection and consequent emergence of virus variants which resist neutralization by autologous sera. AIDS. 1990 Feb;4(2):107–112. doi: 10.1097/00002030-199002000-00002. [DOI] [PubMed] [Google Scholar]
- Archibald D. W., Cole G. A. In vitro inhibition of HIV-1 infectivity by human salivas. AIDS Res Hum Retroviruses. 1990 Dec;6(12):1425–1432. doi: 10.1089/aid.1990.6.1425. [DOI] [PubMed] [Google Scholar]
- Autran B., Mayaud C. M., Raphael M., Plata F., Denis M., Bourguin A., Guillon J. M., Debre P., Akoun G. Evidence for a cytotoxic T-lymphocyte alveolitis in human immunodeficiency virus-infected patients. AIDS. 1988 Jun;2(3):179–183. [PubMed] [Google Scholar]
- Baroni C. D., Pezzella F., Pezzella M., Macchi B., Vitolo D., Uccini S., Ruco L. P. Expression of HIV in lymph node cells of LAS patients. Immunohistology, in situ hybridization, and identification of target cells. Am J Pathol. 1988 Dec;133(3):498–506. [PMC free article] [PubMed] [Google Scholar]
- Civin C. I., Strauss L. C., Brovall C., Fackler M. J., Schwartz J. F., Shaper J. H. Antigenic analysis of hematopoiesis. III. A hematopoietic progenitor cell surface antigen defined by a monoclonal antibody raised against KG-1a cells. J Immunol. 1984 Jul;133(1):157–165. [PubMed] [Google Scholar]
- Clarke J. R., Krishnan V., Bennett J., Mitchell D., Jeffries D. J. Detection of HIV-1 in human lung macrophages using the polymerase chain reaction. AIDS. 1990 Nov;4(11):1133–1136. doi: 10.1097/00002030-199011000-00012. [DOI] [PubMed] [Google Scholar]
- Cloyd M. W., Moore B. E. Spectrum of biological properties of human immunodeficiency virus (HIV-1) isolates. Virology. 1990 Jan;174(1):103–116. doi: 10.1016/0042-6822(90)90059-z. [DOI] [PubMed] [Google Scholar]
- Delassus S., Cheynier R., Wain-Hobson S. Nonhomogeneous distribution of human immunodeficiency virus type 1 proviruses in the spleen. J Virol. 1992 Sep;66(9):5642–5645. doi: 10.1128/jvi.66.9.5642-5645.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delwart E. L., Shpaer E. G., Louwagie J., McCutchan F. E., Grez M., Rübsamen-Waigmann H., Mullins J. I. Genetic relationships determined by a DNA heteroduplex mobility assay: analysis of HIV-1 env genes. Science. 1993 Nov 19;262(5137):1257–1261. doi: 10.1126/science.8235655. [DOI] [PubMed] [Google Scholar]
- Embretson J., Zupancic M., Ribas J. L., Burke A., Racz P., Tenner-Racz K., Haase A. T. Massive covert infection of helper T lymphocytes and macrophages by HIV during the incubation period of AIDS. Nature. 1993 Mar 25;362(6418):359–362. doi: 10.1038/362359a0. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Gendelman H. E. Human immunodeficiency virus type 1 infection of the nervous system: pathogenetic mechanisms. Ann Neurol. 1993 May;33(5):429–436. doi: 10.1002/ana.410330502. [DOI] [PubMed] [Google Scholar]
- Epstein L. G., Kuiken C., Blumberg B. M., Hartman S., Sharer L. R., Clement M., Goudsmit J. HIV-1 V3 domain variation in brain and spleen of children with AIDS: tissue-specific evolution within host-determined quasispecies. Virology. 1991 Feb;180(2):583–590. doi: 10.1016/0042-6822(91)90072-j. [DOI] [PubMed] [Google Scholar]
- Farizo K. M., Buehler J. W., Chamberland M. E., Whyte B. M., Froelicher E. S., Hopkins S. G., Reed C. M., Mokotoff E. D., Cohn D. L., Troxler S. Spectrum of disease in persons with human immunodeficiency virus infection in the United States. JAMA. 1992 Apr 1;267(13):1798–1805. [PubMed] [Google Scholar]
- Fenyö E. M., Morfeldt-Månson L., Chiodi F., Lind B., von Gegerfelt A., Albert J., Olausson E., Asjö B. Distinct replicative and cytopathic characteristics of human immunodeficiency virus isolates. J Virol. 1988 Nov;62(11):4414–4419. doi: 10.1128/jvi.62.11.4414-4419.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher A. G., Ensoli B., Looney D., Rose A., Gallo R. C., Saag M. S., Shaw G. M., Hahn B. H., Wong-Staal F. Biologically diverse molecular variants within a single HIV-1 isolate. Nature. 1988 Aug 4;334(6181):444–447. doi: 10.1038/334444a0. [DOI] [PubMed] [Google Scholar]
- Ho D. D., Rota T. R., Hirsch M. S. Infection of monocyte/macrophages by human T lymphotropic virus type III. J Clin Invest. 1986 May;77(5):1712–1715. doi: 10.1172/JCI112491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Israël-Biet D., Cadranel J., Beldjord K., Andrieu J. M., Jeffrey A., Even P. Tumor necrosis factor production in HIV-seropositive subjects. Relationship with lung opportunistic infections and HIV expression in alveolar macrophages. J Immunol. 1991 Jul 15;147(2):490–494. [PubMed] [Google Scholar]
- Itescu S., Simonelli P. F., Winchester R. J., Ginsberg H. S. Human immunodeficiency virus type 1 strains in the lungs of infected individuals evolve independently from those in peripheral blood and are highly conserved in the C-terminal region of the envelope V3 loop. Proc Natl Acad Sci U S A. 1994 Nov 22;91(24):11378–11382. doi: 10.1073/pnas.91.24.11378. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Korber B. T., Kunstman K. J., Patterson B. K., Furtado M., McEvilly M. M., Levy R., Wolinsky S. M. Genetic differences between blood- and brain-derived viral sequences from human immunodeficiency virus type 1-infected patients: evidence of conserved elements in the V3 region of the envelope protein of brain-derived sequences. J Virol. 1994 Nov;68(11):7467–7481. doi: 10.1128/jvi.68.11.7467-7481.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lebargy F., Branellec A., Deforges L., Bignon J., Bernaudin J. F. HIV-1 in human alveolar macrophages from infected patients is latent in vivo but replicates after in vitro stimulation. Am J Respir Cell Mol Biol. 1994 Jan;10(1):72–78. doi: 10.1165/ajrcmb.10.1.8292383. [DOI] [PubMed] [Google Scholar]
- McElrath M. J., Steinman R. M., Cohn Z. A. Latent HIV-1 infection in enriched populations of blood monocytes and T cells from seropositive patients. J Clin Invest. 1991 Jan;87(1):27–30. doi: 10.1172/JCI114981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyerhans A., Cheynier R., Albert J., Seth M., Kwok S., Sninsky J., Morfeldt-Månson L., Asjö B., Wain-Hobson S. Temporal fluctuations in HIV quasispecies in vivo are not reflected by sequential HIV isolations. Cell. 1989 Sep 8;58(5):901–910. doi: 10.1016/0092-8674(89)90942-2. [DOI] [PubMed] [Google Scholar]
- Michaels J., Sharer L. R., Epstein L. G. Human immunodeficiency virus type 1 (HIV-1) infection of the nervous system: a review. Immunodefic Rev. 1988;1(1):71–104. [PubMed] [Google Scholar]
- Mosier D. E., Gulizia R. J., MacIsaac P. D., Torbett B. E., Levy J. A. Rapid loss of CD4+ T cells in human-PBL-SCID mice by noncytopathic HIV isolates. Science. 1993 Apr 30;260(5108):689–692. doi: 10.1126/science.8097595. [DOI] [PubMed] [Google Scholar]
- Nara P. L., Smit L., Dunlop N., Hatch W., Merges M., Waters D., Kelliher J., Gallo R. C., Fischinger P. J., Goudsmit J. Emergence of viruses resistant to neutralization by V3-specific antibodies in experimental human immunodeficiency virus type 1 IIIB infection of chimpanzees. J Virol. 1990 Aug;64(8):3779–3791. doi: 10.1128/jvi.64.8.3779-3791.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pang S., Vinters H. V., Akashi T., O'Brien W. A., Chen I. S. HIV-1 env sequence variation in brain tissue of patients with AIDS-related neurologic disease. J Acquir Immune Defic Syndr. 1991;4(11):1082–1092. [PubMed] [Google Scholar]
- Piatak M., Jr, Saag M. S., Yang L. C., Clark S. J., Kappes J. C., Luk K. C., Hahn B. H., Shaw G. M., Lifson J. D. High levels of HIV-1 in plasma during all stages of infection determined by competitive PCR. Science. 1993 Mar 19;259(5102):1749–1754. doi: 10.1126/science.8096089. [DOI] [PubMed] [Google Scholar]
- Plata F., Garcia-Pons F., Ryter A., Lebargy F., Goodenow M. M., Dat M. H., Autran B., Mayaud C. HIV-1 infection of lung alveolar fibroblasts and macrophages in humans. AIDS Res Hum Retroviruses. 1990 Aug;6(8):979–986. doi: 10.1089/aid.1990.6.979. [DOI] [PubMed] [Google Scholar]
- Pumarola-Sune T., Navia B. A., Cordon-Cardo C., Cho E. S., Price R. W. HIV antigen in the brains of patients with the AIDS dementia complex. Ann Neurol. 1987 May;21(5):490–496. doi: 10.1002/ana.410210513. [DOI] [PubMed] [Google Scholar]
- Rich E. A., Chen I. S., Zack J. A., Leonard M. L., O'Brien W. A. Increased susceptibility of differentiated mononuclear phagocytes to productive infection with human immunodeficiency virus-1 (HIV-1). J Clin Invest. 1992 Jan;89(1):176–183. doi: 10.1172/JCI115559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sasseville V. G., Newman W., Brodie S. J., Hesterberg P., Pauley D., Ringler D. J. Monocyte adhesion to endothelium in simian immunodeficiency virus-induced AIDS encephalitis is mediated by vascular cell adhesion molecule-1/alpha 4 beta 1 integrin interactions. Am J Pathol. 1994 Jan;144(1):27–40. [PMC free article] [PubMed] [Google Scholar]
- Schuurman H. J., Krone W. J., Broekhuizen R., Goudsmit J. Expression of RNA and antigens of human immunodeficiency virus type-1 (HIV-1) in lymph nodes from HIV-1 infected individuals. Am J Pathol. 1988 Dec;133(3):516–524. [PMC free article] [PubMed] [Google Scholar]
- Sharer L. R. Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol. 1992 Jan;51(1):3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Shioda T., Levy J. A., Cheng-Mayer C. Macrophage and T cell-line tropisms of HIV-1 are determined by specific regions of the envelope gp120 gene. Nature. 1991 Jan 10;349(6305):167–169. doi: 10.1038/349167a0. [DOI] [PubMed] [Google Scholar]
- Sierra-Madero J. G., Toossi Z., Hom D. L., Finegan C. K., Hoenig E., Rich E. A. Relationship between load of virus in alveolar macrophages from human immunodeficiency virus type 1-infected persons, production of cytokines, and clinical status. J Infect Dis. 1994 Jan;169(1):18–27. doi: 10.1093/infdis/169.1.18. [DOI] [PubMed] [Google Scholar]
- Tersmette M., Gruters R. A., de Wolf F., de Goede R. E., Lange J. M., Schellekens P. T., Goudsmit J., Huisman H. G., Miedema F. Evidence for a role of virulent human immunodeficiency virus (HIV) variants in the pathogenesis of acquired immunodeficiency syndrome: studies on sequential HIV isolates. J Virol. 1989 May;63(5):2118–2125. doi: 10.1128/jvi.63.5.2118-2125.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentin L., Garbisa S., Zambello R., Agostini C., Caenazzo C., Di Francesco C., Cipriani A., Francavilla E., Semenzato G. Spontaneous production of interleukin-6 by alveolar macrophages from human immunodeficiency virus type 1-infected patients. J Infect Dis. 1992 Oct;166(4):731–737. doi: 10.1093/infdis/166.4.731. [DOI] [PubMed] [Google Scholar]
- Twigg H. L., 3rd, Iwamoto G. K., Soliman D. M. Role of cytokines in alveolar macrophage accessory cell function in HIV-infected individuals. J Immunol. 1992 Aug 15;149(4):1462–1469. [PubMed] [Google Scholar]
- Twigg H. L., 3rd, Lipscomb M. F., Yoffe B., Barbaro D. J., Weissler J. C. Enhanced accessory cell function by alveolar macrophages from patients infected with the human immunodeficiency virus: potential role for depletion of CD4+ cells in the lung. Am J Respir Cell Mol Biol. 1989 Nov;1(5):391–400. doi: 10.1165/ajrcmb/1.5.391. [DOI] [PubMed] [Google Scholar]
- Velo G. P., Spector W. G. The origin and turnover of alveolar macrophages in experimental pneumonia. J Pathol. 1973 Jan;109(1):7–19. doi: 10.1002/path.1711090103. [DOI] [PubMed] [Google Scholar]
- Westervelt P., Trowbridge D. B., Epstein L. G., Blumberg B. M., Li Y., Hahn B. H., Shaw G. M., Price R. W., Ratner L. Macrophage tropism determinants of human immunodeficiency virus type 1 in vivo. J Virol. 1992 Apr;66(4):2577–2582. doi: 10.1128/jvi.66.4.2577-2582.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wiley C. A., Schrier R. D., Nelson J. A., Lampert P. W., Oldstone M. B. Cellular localization of human immunodeficiency virus infection within the brains of acquired immune deficiency syndrome patients. Proc Natl Acad Sci U S A. 1986 Sep;83(18):7089–7093. doi: 10.1073/pnas.83.18.7089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang Y., Nakata K., Weiden M., Rom W. N. Mycobacterium tuberculosis enhances human immunodeficiency virus-1 replication by transcriptional activation at the long terminal repeat. J Clin Invest. 1995 May;95(5):2324–2331. doi: 10.1172/JCI117924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu T., Mo H., Wang N., Nam D. S., Cao Y., Koup R. A., Ho D. D. Genotypic and phenotypic characterization of HIV-1 patients with primary infection. Science. 1993 Aug 27;261(5125):1179–1181. doi: 10.1126/science.8356453. [DOI] [PubMed] [Google Scholar]
- van Furth R. Origin and turnover of monocytes and macrophages. Curr Top Pathol. 1989;79:125–150. [PubMed] [Google Scholar]