Abstract
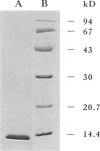
BACKGROUND: Recent studies of melanin biosynthesis have uncovered an unusual enzymatic activity which converts the non-naturally occurring D-isomer of 2-carboxy-2,3-dihydroindole-5,6-quinone (dopachrome) into 5,6-dihydroxyindole-2-carboxylic acid (DHICA). The aim of the present investigation was to isolate and characterize the enzyme catalyzing this tautomerization reaction. MATERIALS AND METHODS: After we performed a tissue survey of D-dopachrome tautomerase activity, 10 bovine lenses were homogenized and used as a source of enzyme. A soluble fraction was obtained by high-speed centrifugation and subjected to successive FPLC chromatography on Phenyl-sepharose, Mono S cation-exchange, and Superdex gel-filtration. The isolated enzyme was electrophoresed, blotted onto PVDF membrane, and the N terminus analyzed by gas phase micro-sequencing. RESULTS: The protein catalyzing the conversion of D-dopachrome to DHICA was purified to homogeneity in 14% yield and showed a molecular weight of 12 kD when analyzed by SDS-PAGE. The first 27 amino acid residues of this protein were sequenced and found to be identical with those of bovine macrophage migration inhibitory factor (MIF). The catalytic activity of native MIF was confirmed by studies of purified recombinant human MIF, which showed the same tautomerase activity. While L-dopachrome was not a substrate for this reaction, the methyl esters of the L- and D-isomers were found to be better substrates for MIF than D-dopachrome. CONCLUSIONS: MIF has been described recently to be an anterior pituitary hormone and to be released from immune cells stimulated by low concentrations of glucocorticoids. Once secreted, MIF acts to control, or counter-regulate, the immunosuppressive effects of glucocorticoids on the immune system. Although the tested substrate, D-dopachrome, does not occur naturally, the observation that MIF has tautomerase activity suggests that MIF may mediate its biological effects by an enzymatic reaction. These data also offer a potential approach for the design of small molecule pharmacological inhibitors of MIF that may modulate its potent immunoregulatory effects in vivo.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aroca P., Solano F., Garcia-Borrón J. C., Lozano J. A. Specificity of dopachrome tautomerase and inhibition by carboxylated indoles. Considerations on the enzyme active site. Biochem J. 1991 Jul 15;277(Pt 2):393–397. doi: 10.1042/bj2770393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernhagen J., Calandra T., Mitchell R. A., Martin S. B., Tracey K. J., Voelter W., Manogue K. R., Cerami A., Bucala R. MIF is a pituitary-derived cytokine that potentiates lethal endotoxaemia. Nature. 1993 Oct 21;365(6448):756–759. doi: 10.1038/365756a0. [DOI] [PubMed] [Google Scholar]
- Bernhagen J., Mitchell R. A., Calandra T., Voelter W., Cerami A., Bucala R. Purification, bioactivity, and secondary structure analysis of mouse and human macrophage migration inhibitory factor (MIF). Biochemistry. 1994 Nov 29;33(47):14144–14155. doi: 10.1021/bi00251a025. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Calandra T., Bernhagen J., Mitchell R. A., Bucala R. The macrophage is an important and previously unrecognized source of macrophage migration inhibitory factor. J Exp Med. 1994 Jun 1;179(6):1895–1902. doi: 10.1084/jem.179.6.1895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galat A., Rivière S., Bouet F. Purification of macrophage migration inhibitory factor (MIF) from bovine brain cytosol. FEBS Lett. 1993 Mar 22;319(3):233–236. doi: 10.1016/0014-5793(93)80553-7. [DOI] [PubMed] [Google Scholar]
- Jackson I. J. A cDNA encoding tyrosinase-related protein maps to the brown locus in mouse. Proc Natl Acad Sci U S A. 1988 Jun;85(12):4392–4396. doi: 10.1073/pnas.85.12.4392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson I. J., Chambers D. M., Tsukamoto K., Copeland N. G., Gilbert D. J., Jenkins N. A., Hearing V. A second tyrosinase-related protein, TRP-2, maps to and is mutated at the mouse slaty locus. EMBO J. 1992 Feb;11(2):527–535. doi: 10.1002/j.1460-2075.1992.tb05083.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Matsudaira P. Sequence from picomole quantities of proteins electroblotted onto polyvinylidene difluoride membranes. J Biol Chem. 1987 Jul 25;262(21):10035–10038. [PubMed] [Google Scholar]
- Odh G., Hindemith A., Rosengren A. M., Rosengren E., Rorsman H. Isolation of a new tautomerase monitored by the conversion of D-dopachrome to 5,6-dihydroxyindole. Biochem Biophys Res Commun. 1993 Dec 15;197(2):619–624. doi: 10.1006/bbrc.1993.2524. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M. After dopachrome? Pigment Cell Res. 1991 Mar;4(2):53–62. doi: 10.1111/j.1600-0749.1991.tb00315.x. [DOI] [PubMed] [Google Scholar]
- Pawelek J. M. Dopachrome conversion factor functions as an isomerase. Biochem Biophys Res Commun. 1990 Feb 14;166(3):1328–1333. doi: 10.1016/0006-291x(90)91011-g. [DOI] [PubMed] [Google Scholar]
- Sakai M., Nishihira J., Hibiya Y., Koyama Y., Nishi S. Glutathione binding rat liver 13k protein is the homologue of the macrophage migration inhibitory factor. Biochem Mol Biol Int. 1994 Jun;33(3):439–446. [PubMed] [Google Scholar]
- Schreiber S. L., Crabtree G. R. The mechanism of action of cyclosporin A and FK506. Immunol Today. 1992 Apr;13(4):136–142. doi: 10.1016/0167-5699(92)90111-J. [DOI] [PubMed] [Google Scholar]
- Sherry B., Yarlett N., Strupp A., Cerami A. Identification of cyclophilin as a proinflammatory secretory product of lipopolysaccharide-activated macrophages. Proc Natl Acad Sci U S A. 1992 Apr 15;89(8):3511–3515. doi: 10.1073/pnas.89.8.3511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shibahara S., Tomita Y., Sakakura T., Nager C., Chaudhuri B., Müller R. Cloning and expression of cDNA encoding mouse tyrosinase. Nucleic Acids Res. 1986 Mar 25;14(6):2413–2427. doi: 10.1093/nar/14.6.2413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugumaran M., Semensi V. Quinone methide as a new intermediate in eumelanin biosynthesis. J Biol Chem. 1991 Apr 5;266(10):6073–6078. [PubMed] [Google Scholar]
- Tsukamoto K., Jackson I. J., Urabe K., Montague P. M., Hearing V. J. A second tyrosinase-related protein, TRP-2, is a melanogenic enzyme termed DOPAchrome tautomerase. EMBO J. 1992 Feb;11(2):519–526. doi: 10.1002/j.1460-2075.1992.tb05082.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winder A. J., Wittbjer A., Rosengren E., Rorsman H. The mouse brown (b) locus protein has dopachrome tautomerase activity and is located in lysosomes in transfected fibroblasts. J Cell Sci. 1993 Sep;106(Pt 1):153–166. doi: 10.1242/jcs.106.1.153. [DOI] [PubMed] [Google Scholar]
- Wistow G. J., Shaughnessy M. P., Lee D. C., Hodin J., Zelenka P. S. A macrophage migration inhibitory factor is expressed in the differentiating cells of the eye lens. Proc Natl Acad Sci U S A. 1993 Feb 15;90(4):1272–1275. doi: 10.1073/pnas.90.4.1272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang M., Aman P., Grubb A., Panagopoulos I., Hindemith A., Rosengren E., Rorsman H. Cloning and sequencing of a cDNA encoding rat D-dopachrome tautomerase. FEBS Lett. 1995 Oct 16;373(3):203–206. doi: 10.1016/0014-5793(95)01041-c. [DOI] [PubMed] [Google Scholar]