Abstract
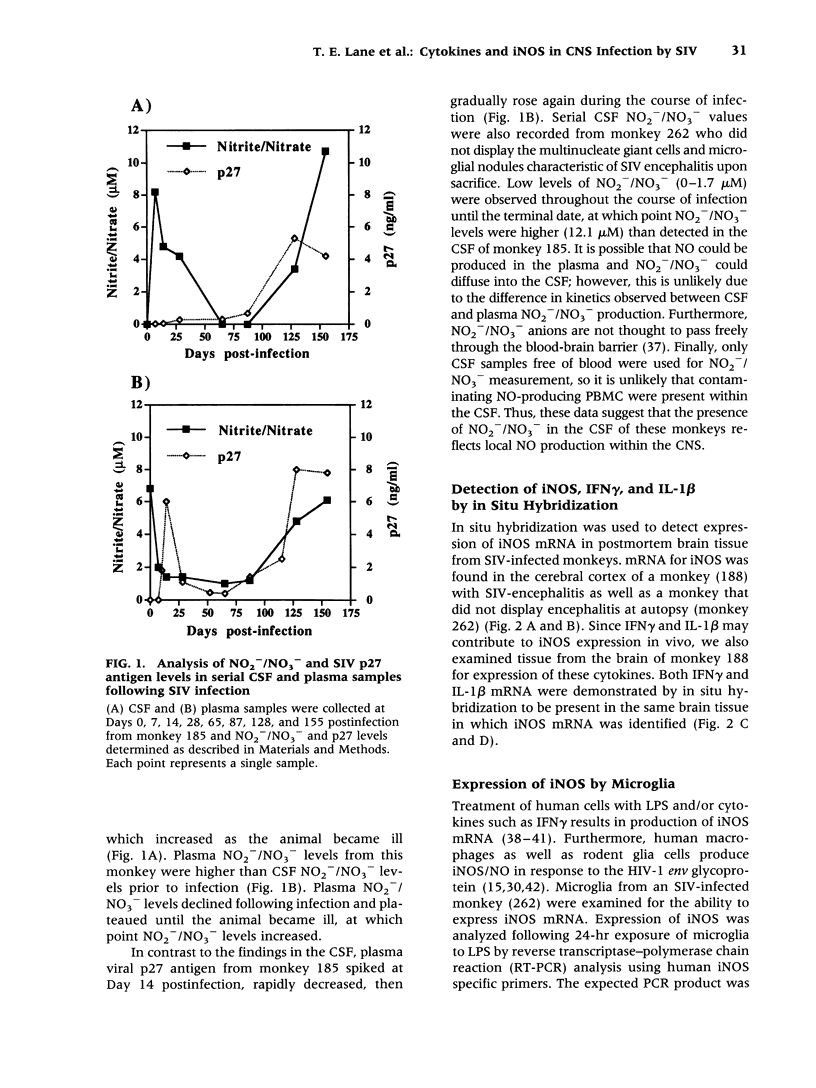
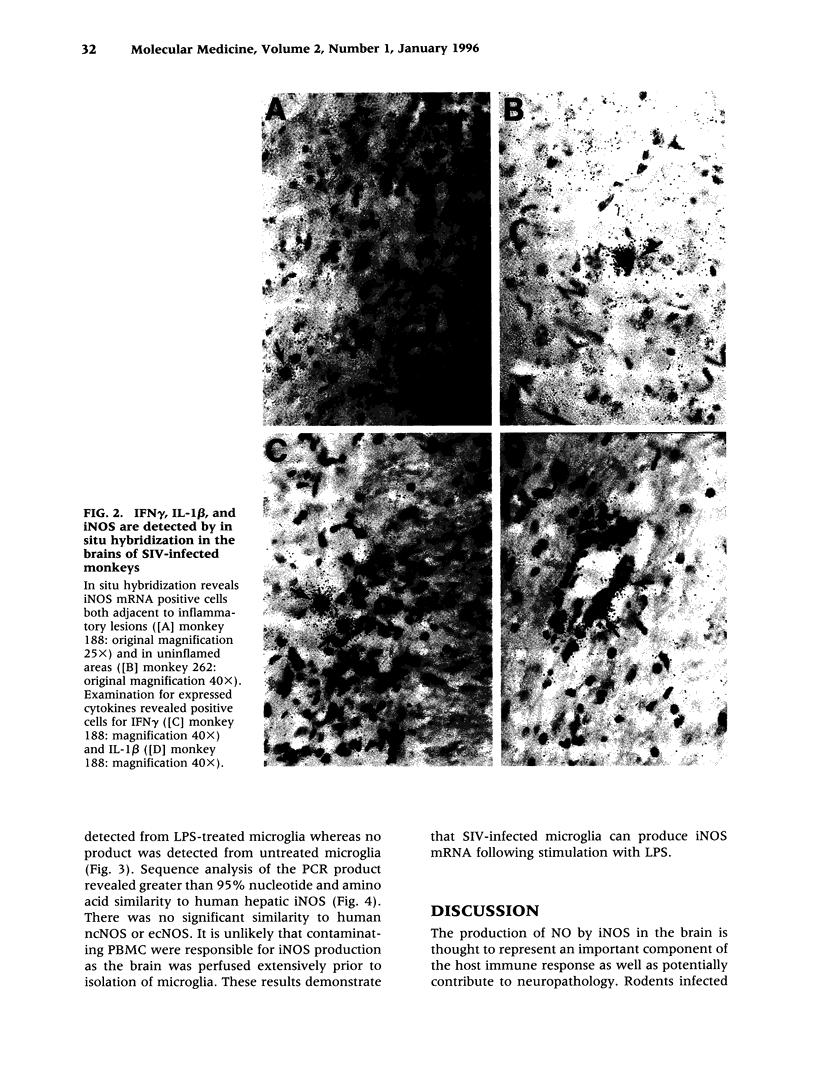
BACKGROUND: Human immunodeficiency virus type 1 (HIV-1) infection of the central nervous system (CNS) can lead to severe impairments in cognition, behavior, and motor skills. The mechanism(s) by which HIV-1 induces CNS disease are not well understood. Recent evidence suggests that expression of inducible nitric oxide synthase (iNOS) and nitric oxide (NO) may contribute to HIV-1-induced neurologic disease. We sought to determine if these factors were present in the CNS of rhesus monkeys with simian immunodeficiency virus (SIV)-induced CNS disease. MATERIALS AND METHODS: Total NO production in cerebral spinal fluid (CSF) from infected monkeys was determined by measuring nitrite (NO2-) and nitrate (NO3-) (stable NO degradation products) utilizing Greiss reagents. In situ hybridization revealed iNOS, interferon-gamma (IFNgamma), and interleukin 1 beta (IL-1 beta) mRNA in the brains of SIV-infected monkeys. Microglia were isolated from animals infected with SIV. Following stimulation with LPS, induction of iNOS mRNA in isolated microglia was analyzed by reverse transcriptase-polymerase chain reaction. RESULTS: Serial CSF samples from an SIV-infected monkey reveal increased levels of NO2-/NO3-. In situ hybridization demonstrated iNOS, IFN gamma, and IL-1 beta mRNAs in post-mortem brain tissue of SIV-infected monkeys. Furthermore, stimulated microglia from an SIV-infected monkey could produce iNOS mRNA. CONCLUSIONS: The presence of iNOS in the brain and NO2-/NO3- in the CSF indicates that NO is produced in the CNS of SIV-infected monkeys. The data suggest that iNOS and NO may be contributing to SIV-induced CNS disease.
Full text
PDF



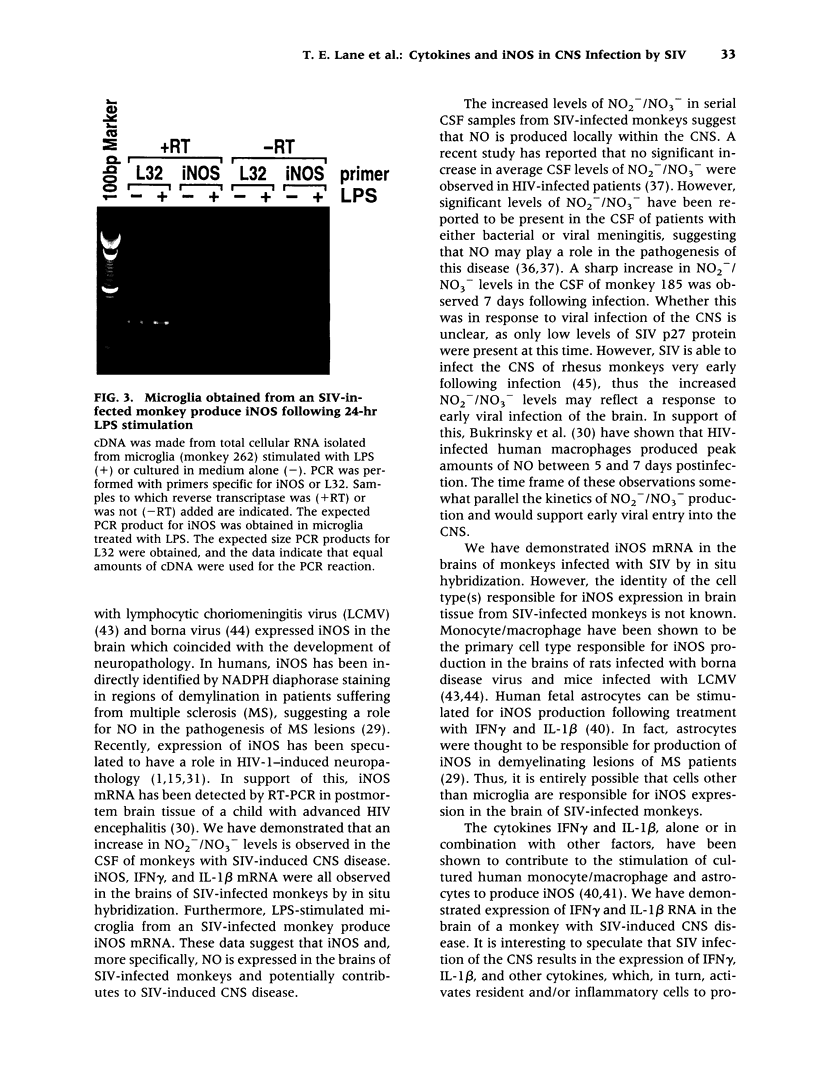
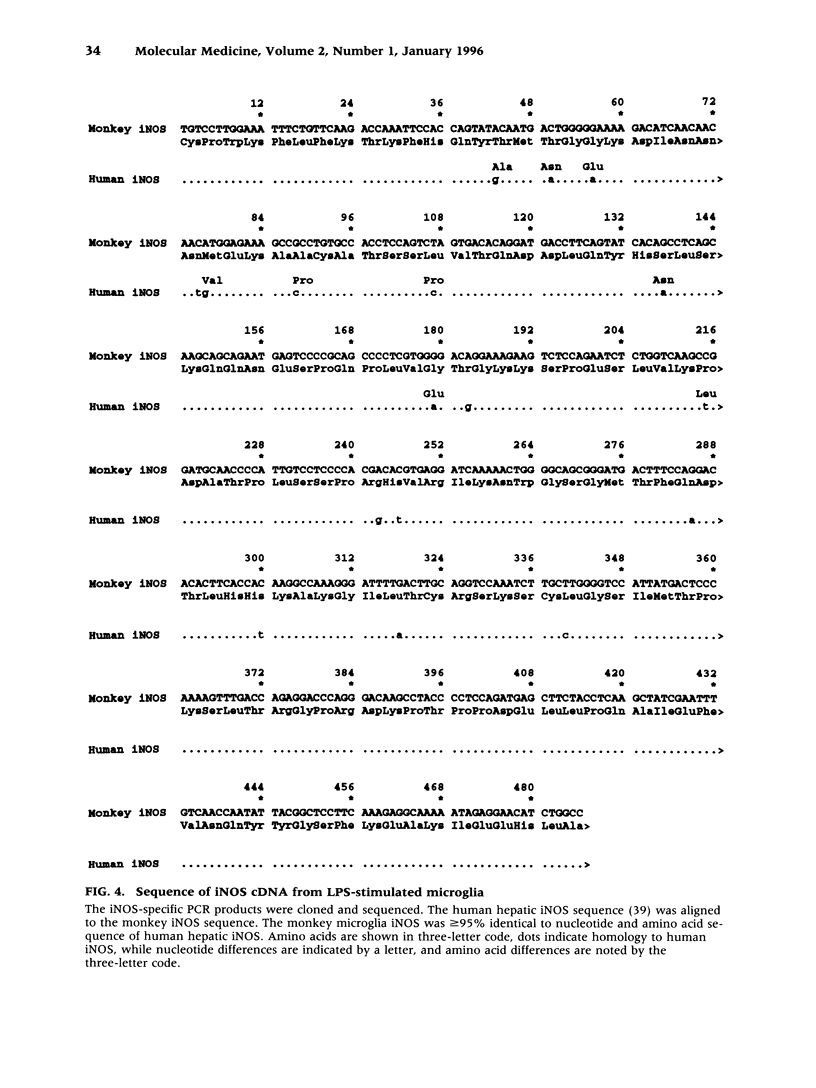



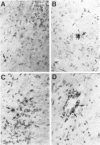
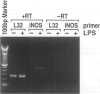
Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Asano K., Chee C. B., Gaston B., Lilly C. M., Gerard C., Drazen J. M., Stamler J. S. Constitutive and inducible nitric oxide synthase gene expression, regulation, and activity in human lung epithelial cells. Proc Natl Acad Sci U S A. 1994 Oct 11;91(21):10089–10093. doi: 10.1073/pnas.91.21.10089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beckman J. S. The double-edged role of nitric oxide in brain function and superoxide-mediated injury. J Dev Physiol. 1991 Jan;15(1):53–59. [PubMed] [Google Scholar]
- Beckmann J. S., Ye Y. Z., Anderson P. G., Chen J., Accavitti M. A., Tarpey M. M., White C. R. Extensive nitration of protein tyrosines in human atherosclerosis detected by immunohistochemistry. Biol Chem Hoppe Seyler. 1994 Feb;375(2):81–88. doi: 10.1515/bchm3.1994.375.2.81. [DOI] [PubMed] [Google Scholar]
- Benos D. J., Hahn B. H., Bubien J. K., Ghosh S. K., Mashburn N. A., Chaikin M. A., Shaw G. M., Benveniste E. N. Envelope glycoprotein gp120 of human immunodeficiency virus type 1 alters ion transport in astrocytes: implications for AIDS dementia complex. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):494–498. doi: 10.1073/pnas.91.2.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brinkmann R., Schwinn A., Müller J., Stahl-Hennig C., Coulibaly C., Hunsmann G., Czub S., Rethwilm A., Dörries R., ter Meulen V. In vitro and in vivo infection of rhesus monkey microglial cells by simian immunodeficiency virus. Virology. 1993 Aug;195(2):561–568. doi: 10.1006/viro.1993.1407. [DOI] [PubMed] [Google Scholar]
- Brinkmann R., Schwinn A., Narayan O., Zink C., Kreth H. W., Roggendorf W., Dörries R., Schwender S., Imrich H., ter Meulen V. Human immunodeficiency virus infection in microglia: correlation between cells infected in the brain and cells cultured from infectious brain tissue. Ann Neurol. 1992 Apr;31(4):361–365. doi: 10.1002/ana.410310403. [DOI] [PubMed] [Google Scholar]
- Bukrinsky M. I., Nottet H. S., Schmidtmayerova H., Dubrovsky L., Flanagan C. R., Mullins M. E., Lipton S. A., Gendelman H. E. Regulation of nitric oxide synthase activity in human immunodeficiency virus type 1 (HIV-1)-infected monocytes: implications for HIV-associated neurological disease. J Exp Med. 1995 Feb 1;181(2):735–745. doi: 10.1084/jem.181.2.735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bö L., Dawson T. M., Wesselingh S., Mörk S., Choi S., Kong P. A., Hanley D., Trapp B. D. Induction of nitric oxide synthase in demyelinating regions of multiple sclerosis brains. Ann Neurol. 1994 Nov;36(5):778–786. doi: 10.1002/ana.410360515. [DOI] [PubMed] [Google Scholar]
- Campbell I. L., Samimi A., Chiang C. S. Expression of the inducible nitric oxide synthase. Correlation with neuropathology and clinical features in mice with lymphocytic choriomeningitis. J Immunol. 1994 Oct 15;153(8):3622–3629. [PubMed] [Google Scholar]
- Chakrabarti L., Hurtrel M., Maire M. A., Vazeux R., Dormont D., Montagnier L., Hurtrel B. Early viral replication in the brain of SIV-infected rhesus monkeys. Am J Pathol. 1991 Dec;139(6):1273–1280. [PMC free article] [PubMed] [Google Scholar]
- Dawson V. L., Dawson T. M., Uhl G. R., Snyder S. H. Human immunodeficiency virus type 1 coat protein neurotoxicity mediated by nitric oxide in primary cortical cultures. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3256–3259. doi: 10.1073/pnas.90.8.3256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desrosiers R. C. The simian immunodeficiency viruses. Annu Rev Immunol. 1990;8:557–578. doi: 10.1146/annurev.iy.08.040190.003013. [DOI] [PubMed] [Google Scholar]
- Geller D. A., Lowenstein C. J., Shapiro R. A., Nussler A. K., Di Silvio M., Wang S. C., Nakayama D. K., Simmons R. L., Snyder S. H., Billiar T. R. Molecular cloning and expression of inducible nitric oxide synthase from human hepatocytes. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3491–3495. doi: 10.1073/pnas.90.8.3491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendelman H. E., Lipton S. A., Tardieu M., Bukrinsky M. I., Nottet H. S. The neuropathogenesis of HIV-1 infection. J Leukoc Biol. 1994 Sep;56(3):389–398. doi: 10.1002/jlb.56.3.389. [DOI] [PubMed] [Google Scholar]
- Genis P., Jett M., Bernton E. W., Boyle T., Gelbard H. A., Dzenko K., Keane R. W., Resnick L., Mizrachi Y., Volsky D. J. Cytokines and arachidonic metabolites produced during human immunodeficiency virus (HIV)-infected macrophage-astroglia interactions: implications for the neuropathogenesis of HIV disease. J Exp Med. 1992 Dec 1;176(6):1703–1718. doi: 10.1084/jem.176.6.1703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Griffin D. E., McArthur J. C., Cornblath D. R. Neopterin and interferon-gamma in serum and cerebrospinal fluid of patients with HIV-associated neurologic disease. Neurology. 1991 Jan;41(1):69–74. doi: 10.1212/wnl.41.1.69. [DOI] [PubMed] [Google Scholar]
- Grimaldi L. M., Martino G. V., Franciotta D. M., Brustia R., Castagna A., Pristerà R., Lazzarin A. Elevated alpha-tumor necrosis factor levels in spinal fluid from HIV-1-infected patients with central nervous system involvement. Ann Neurol. 1991 Jan;29(1):21–25. doi: 10.1002/ana.410290106. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Rubinow D., Lane C., Markey S. P. Cerebrospinal fluid quinolinic acid concentrations are increased in acquired immune deficiency syndrome. Ann Neurol. 1989 Aug;26(2):275–277. doi: 10.1002/ana.410260215. [DOI] [PubMed] [Google Scholar]
- Heyes M. P., Saito K., Crowley J. S., Davis L. E., Demitrack M. A., Der M., Dilling L. A., Elia J., Kruesi M. J., Lackner A. Quinolinic acid and kynurenine pathway metabolism in inflammatory and non-inflammatory neurological disease. Brain. 1992 Oct;115(Pt 5):1249–1273. doi: 10.1093/brain/115.5.1249. [DOI] [PubMed] [Google Scholar]
- Hurtrel B., Chakrabarti L., Hurtrel M., Maire M. A., Dormont D., Montagnier L. Early SIV encephalopathy. J Med Primatol. 1991 Jun;20(4):159–166. [PubMed] [Google Scholar]
- Hurtrel B., Chakrabarti L., Hurtrel M., Montagnier L. Target cells during early SIV encephalopathy. Res Virol. 1993 Jan-Feb;144(1):41–46. doi: 10.1016/s0923-2516(06)80010-5. [DOI] [PubMed] [Google Scholar]
- Kestler H., Kodama T., Ringler D., Marthas M., Pedersen N., Lackner A., Regier D., Sehgal P., Daniel M., King N. Induction of AIDS in rhesus monkeys by molecularly cloned simian immunodeficiency virus. Science. 1990 Jun 1;248(4959):1109–1112. doi: 10.1126/science.2160735. [DOI] [PubMed] [Google Scholar]
- Kindt T. J., Hirsch V. M., Johnson P. R., Sawasdikosol S. Animal models for acquired immunodeficiency syndrome. Adv Immunol. 1992;52:425–474. doi: 10.1016/s0065-2776(08)60880-9. [DOI] [PubMed] [Google Scholar]
- Lackner A. A., Smith M. O., Munn R. J., Martfeld D. J., Gardner M. B., Marx P. A., Dandekar S. Localization of simian immunodeficiency virus in the central nervous system of rhesus monkeys. Am J Pathol. 1991 Sep;139(3):609–621. [PMC free article] [PubMed] [Google Scholar]
- Lairmore M. D., Post A. A., Goldsmith C. S., Folks T. M. Cytokine enhancement of simian immunodeficiency virus (SIV/mac) from a chronically infected cloned T-cell line (HuT-78). Arch Virol. 1991;121(1-4):43–53. doi: 10.1007/BF01316743. [DOI] [PubMed] [Google Scholar]
- Lancaster J. R., Jr, Hibbs J. B., Jr EPR demonstration of iron-nitrosyl complex formation by cytotoxic activated macrophages. Proc Natl Acad Sci U S A. 1990 Feb;87(3):1223–1227. doi: 10.1073/pnas.87.3.1223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee S. C., Dickson D. W., Liu W., Brosnan C. F. Induction of nitric oxide synthase activity in human astrocytes by interleukin-1 beta and interferon-gamma. J Neuroimmunol. 1993 Jul;46(1-2):19–24. doi: 10.1016/0165-5728(93)90229-r. [DOI] [PubMed] [Google Scholar]
- Marletta M. A., Yoon P. S., Iyengar R., Leaf C. D., Wishnok J. S. Macrophage oxidation of L-arginine to nitrite and nitrate: nitric oxide is an intermediate. Biochemistry. 1988 Nov 29;27(24):8706–8711. doi: 10.1021/bi00424a003. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Chen I. S. HIV-1, macrophages, glial cells, and cytokines in AIDS nervous system disease. FASEB J. 1991 Jul;5(10):2391–2397. doi: 10.1096/fasebj.5.10.2065887. [DOI] [PubMed] [Google Scholar]
- Merrill J. E., Ignarro L. J., Sherman M. P., Melinek J., Lane T. E. Microglial cell cytotoxicity of oligodendrocytes is mediated through nitric oxide. J Immunol. 1993 Aug 15;151(4):2132–2141. [PubMed] [Google Scholar]
- Milstien S., Sakai N., Brew B. J., Krieger C., Vickers J. H., Saito K., Heyes M. P. Cerebrospinal fluid nitrite/nitrate levels in neurologic diseases. J Neurochem. 1994 Sep;63(3):1178–1180. doi: 10.1046/j.1471-4159.1994.63031178.x. [DOI] [PubMed] [Google Scholar]
- Nathan C. F., Hibbs J. B., Jr Role of nitric oxide synthesis in macrophage antimicrobial activity. Curr Opin Immunol. 1991 Feb;3(1):65–70. doi: 10.1016/0952-7915(91)90079-g. [DOI] [PubMed] [Google Scholar]
- Nathan C., Xie Q. W. Nitric oxide synthases: roles, tolls, and controls. Cell. 1994 Sep 23;78(6):915–918. doi: 10.1016/0092-8674(94)90266-6. [DOI] [PubMed] [Google Scholar]
- Peterson P. K., Hu S., Anderson W. R., Chao C. C. Nitric oxide production and neurotoxicity mediated by activated microglia from human versus mouse brain. J Infect Dis. 1994 Aug;170(2):457–460. doi: 10.1093/infdis/170.2.457. [DOI] [PubMed] [Google Scholar]
- Pietraforte D., Tritarelli E., Testa U., Minetti M. gp120 HIV envelope glycoprotein increases the production of nitric oxide in human monocyte-derived macrophages. J Leukoc Biol. 1994 Feb;55(2):175–182. doi: 10.1002/jlb.55.2.175. [DOI] [PubMed] [Google Scholar]
- Reiling N., Ulmer A. J., Duchrow M., Ernst M., Flad H. D., Hauschildt S. Nitric oxide synthase: mRNA expression of different isoforms in human monocytes/macrophages. Eur J Immunol. 1994 Aug;24(8):1941–1944. doi: 10.1002/eji.1830240836. [DOI] [PubMed] [Google Scholar]
- Sarih M., Souvannavong V., Adam A. Nitric oxide synthase induces macrophage death by apoptosis. Biochem Biophys Res Commun. 1993 Mar 15;191(2):503–508. doi: 10.1006/bbrc.1993.1246. [DOI] [PubMed] [Google Scholar]
- Sharer L. R., Baskin G. B., Cho E. S., Murphey-Corb M., Blumberg B. M., Epstein L. G. Comparison of simian immunodeficiency virus and human immunodeficiency virus encephalitides in the immature host. Ann Neurol. 1988;23 (Suppl):S108–S112. doi: 10.1002/ana.410230727. [DOI] [PubMed] [Google Scholar]
- Sharer L. R. Pathology of HIV-1 infection of the central nervous system. A review. J Neuropathol Exp Neurol. 1992 Jan;51(1):3–11. doi: 10.1097/00005072-199201000-00002. [DOI] [PubMed] [Google Scholar]
- Tyor W. R., Glass J. D., Griffin J. W., Becker P. S., McArthur J. C., Bezman L., Griffin D. E. Cytokine expression in the brain during the acquired immunodeficiency syndrome. Ann Neurol. 1992 Apr;31(4):349–360. doi: 10.1002/ana.410310402. [DOI] [PubMed] [Google Scholar]
- Visser J. J., Scholten R. J., Hoekman K. Nitric oxide synthesis in meningococcal meningitis. Ann Intern Med. 1994 Feb 15;120(4):345–346. doi: 10.7326/0003-4819-120-4-199402150-00023. [DOI] [PubMed] [Google Scholar]
- Watry D., Lane T. E., Streb M., Fox H. S. Transfer of neuropathogenic simian immunodeficiency virus with naturally infected microglia. Am J Pathol. 1995 Apr;146(4):914–923. [PMC free article] [PubMed] [Google Scholar]
- Zhang J., Dawson V. L., Dawson T. M., Snyder S. H. Nitric oxide activation of poly(ADP-ribose) synthetase in neurotoxicity. Science. 1994 Feb 4;263(5147):687–689. doi: 10.1126/science.8080500. [DOI] [PubMed] [Google Scholar]
- Zheng Y. M., Schäfer M. K., Weihe E., Sheng H., Corisdeo S., Fu Z. F., Koprowski H., Dietzschold B. Severity of neurological signs and degree of inflammatory lesions in the brains of rats with Borna disease correlate with the induction of nitric oxide synthase. J Virol. 1993 Oct;67(10):5786–5791. doi: 10.1128/jvi.67.10.5786-5791.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Herrath M., Oldstone M. B., Fox H. S. Simian immunodeficiency virus (SIV)-specific CTL in cerebrospinal fluid and brains of SIV-infected rhesus macaques. J Immunol. 1995 May 15;154(10):5582–5589. [PubMed] [Google Scholar]