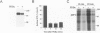Abstract
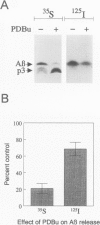
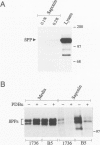
BACKGROUND: Amyloid beta-protein (A beta), the major constituent of amyloid deposits found in Alzheimer's disease, is derived from the beta-amyloid precursor protein (beta PP). Constitutive proteolysis by alpha-secretase and secretion of soluble beta PP (beta PPs) are stimulated by protein kinase C (PKC) activation, whereas A beta production and release are inhibited. The cellular mechanism that underlies the PKC-mediated down-regulation of A beta generation is unclear. Because endocytic processing of beta PP from the cell surface is a major pathway of A beta production, the effect of PKC activation by phorbol 12,13-dibutyrate (PDBu) on endocytic trafficking of beta PP was examined. MATERIALS AND METHODS: In this study, trafficking of beta PP was assayed in Chinese hamster ovary cells (CHO) cells stably transfected with full-length beta PP751. RESULTS: Treatment with PDBu resulted in a rapid and striking reduction of up to 80% in the amount of beta PP at the cell surface. This loss of cell-surface molecules could not be accounted for by changes in the trafficking of cell-surface beta PP molecules, as determined by a radiolabeled antibody assay. Rather, the decrease in beta PP was due primarily to a reduction in the sorting of beta PP to the cell surface. This alteration was correlated with accelerated intracellular alpha-secretase-mediated beta PP cleavage and accelerated beta PP trafficking in the exocytic pathway. CONCLUSIONS: The data suggest that the displacement of beta PP away from the cell surface after phorbol ester treatment reduces the substrate available for endocytic processing and in turn, results in the inhibition of A beta production.
Full text
PDF
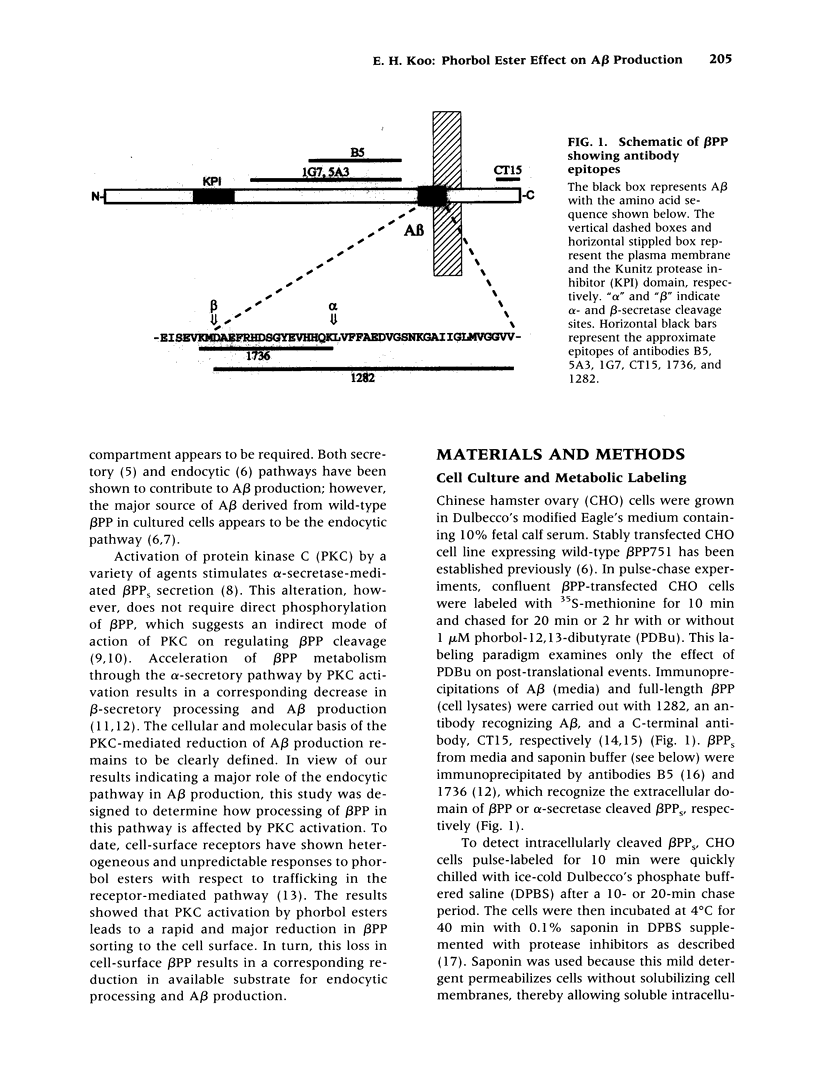

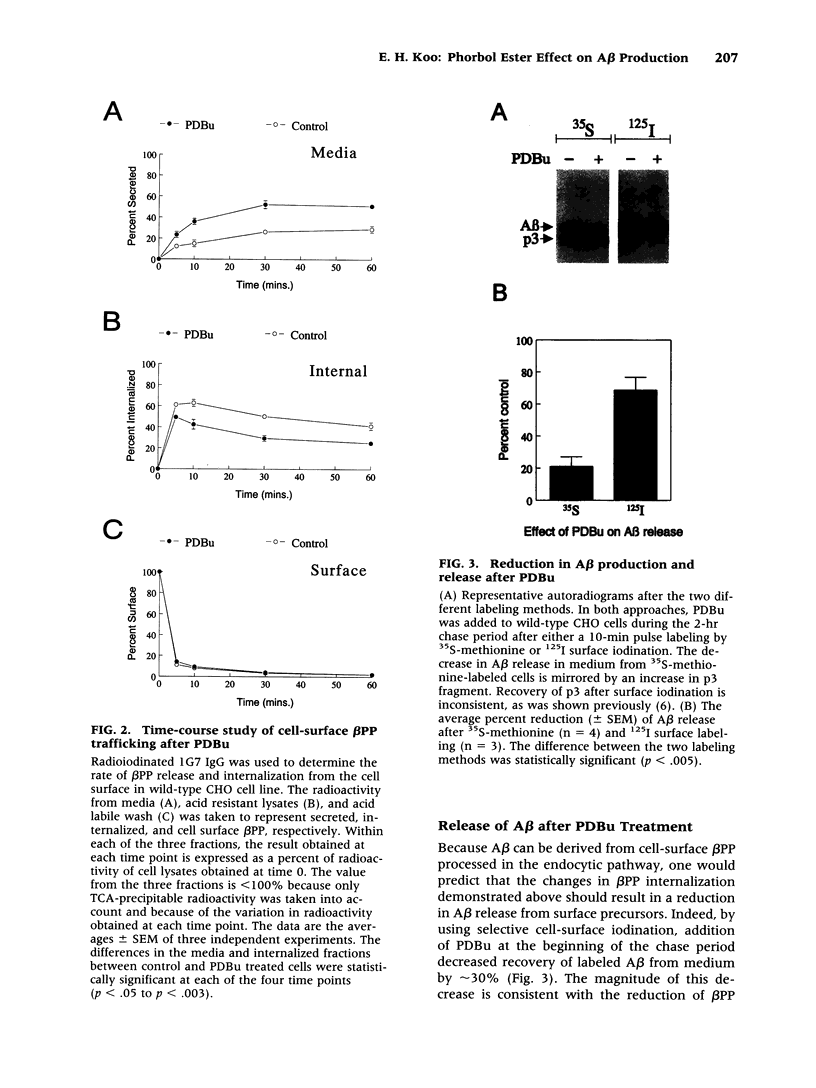
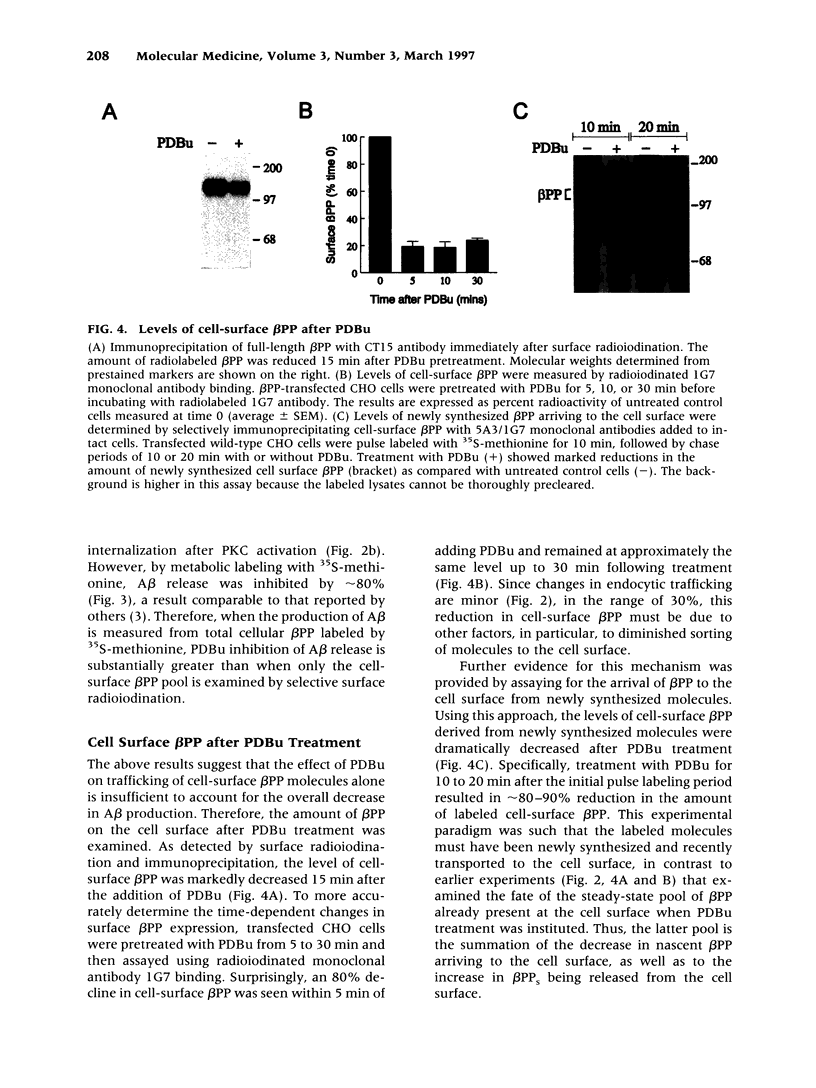
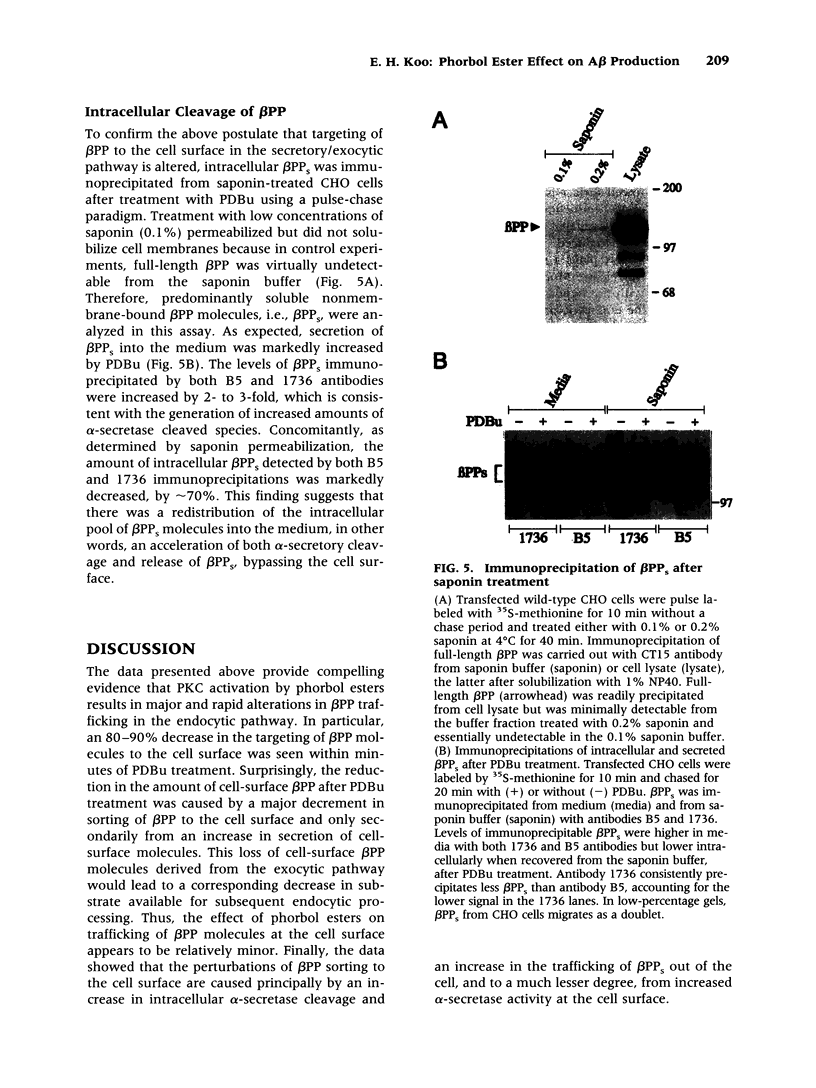


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arribas J., Massagué J. Transforming growth factor-alpha and beta-amyloid precursor protein share a secretory mechanism. J Cell Biol. 1995 Feb;128(3):433–441. doi: 10.1083/jcb.128.3.433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Backer J. M., King G. L. Regulation of receptor-mediated endocytosis by phorbol esters. Biochem Pharmacol. 1991 May 1;41(9):1267–1277. doi: 10.1016/0006-2952(91)90097-o. [DOI] [PubMed] [Google Scholar]
- Busciglio J., Gabuzda D. H., Matsudaira P., Yankner B. A. Generation of beta-amyloid in the secretory pathway in neuronal and nonneuronal cells. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):2092–2096. doi: 10.1073/pnas.90.5.2092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J. D., Koo E. H., Greengard P. Protein phosphorylation inhibits production of Alzheimer amyloid beta/A4 peptide. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9195–9198. doi: 10.1073/pnas.90.19.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Checler F. Processing of the beta-amyloid precursor protein and its regulation in Alzheimer's disease. J Neurochem. 1995 Oct;65(4):1431–1444. doi: 10.1046/j.1471-4159.1995.65041431.x. [DOI] [PubMed] [Google Scholar]
- Esch F. S., Keim P. S., Beattie E. C., Blacher R. W., Culwell A. R., Oltersdorf T., McClure D., Ward P. J. Cleavage of amyloid beta peptide during constitutive processing of its precursor. Science. 1990 Jun 1;248(4959):1122–1124. doi: 10.1126/science.2111583. [DOI] [PubMed] [Google Scholar]
- Felsenstein K. M., Ingalls K. M., Hunihan L. W., Roberts S. B. Reversal of the Swedish familial Alzheimer's disease mutant phenotype in cultured cells treated with phorbol 12,13-dibutyrate. Neurosci Lett. 1994 Jun 20;174(2):173–176. doi: 10.1016/0304-3940(94)90014-0. [DOI] [PubMed] [Google Scholar]
- Gandy S., Greengard P. Processing of Alzheimer A beta-amyloid precursor protein: cell biology, regulation, and role in Alzheimer disease. Int Rev Neurobiol. 1994;36:29–50. doi: 10.1016/s0074-7742(08)60302-5. [DOI] [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993 Dec 17;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Hung A. Y., Haass C., Nitsch R. M., Qiu W. Q., Citron M., Wurtman R. J., Growdon J. H., Selkoe D. J. Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J Biol Chem. 1993 Nov 5;268(31):22959–22962. [PubMed] [Google Scholar]
- Koo E. H., Squazzo S. L. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994 Jul 1;269(26):17386–17389. [PubMed] [Google Scholar]
- Koo E. H., Squazzo S. L., Selkoe D. J., Koo C. H. Trafficking of cell-surface amyloid beta-protein precursor. I. Secretion, endocytosis and recycling as detected by labeled monoclonal antibody. J Cell Sci. 1996 May;109(Pt 5):991–998. doi: 10.1242/jcs.109.5.991. [DOI] [PubMed] [Google Scholar]
- LeBlanc A. C., Gambetti P. Production of Alzheimer 4kDa beta-amyloid peptide requires the C-terminal cytosolic domain of the amyloid precursor protein. Biochem Biophys Res Commun. 1994 Nov 15;204(3):1371–1380. doi: 10.1006/bbrc.1994.2615. [DOI] [PubMed] [Google Scholar]
- Oltersdorf T., Ward P. J., Henriksson T., Beattie E. C., Neve R., Lieberburg I., Fritz L. C. The Alzheimer amyloid precursor protein. Identification of a stable intermediate in the biosynthetic/degradative pathway. J Biol Chem. 1990 Mar 15;265(8):4492–4497. [PubMed] [Google Scholar]
- Perez R. G., Squazzo S. L., Koo E. H. Enhanced release of amyloid beta-protein from codon 670/671 "Swedish" mutant beta-amyloid precursor protein occurs in both secretory and endocytic pathways. J Biol Chem. 1996 Apr 12;271(15):9100–9107. doi: 10.1074/jbc.271.15.9100. [DOI] [PubMed] [Google Scholar]
- Podlisny M. B., Ostaszewski B. L., Squazzo S. L., Koo E. H., Rydell R. E., Teplow D. B., Selkoe D. J. Aggregation of secreted amyloid beta-protein into sodium dodecyl sulfate-stable oligomers in cell culture. J Biol Chem. 1995 Apr 21;270(16):9564–9570. doi: 10.1074/jbc.270.16.9564. [DOI] [PubMed] [Google Scholar]
- Simmons C. F., Jr, Schwartz A. L. Cellular pathways of galactose-terminal ligand movement in a cloned human hepatoma cell line. Mol Pharmacol. 1984 Nov;26(3):509–519. [PubMed] [Google Scholar]
- Sisodia S. S., Koo E. H., Hoffman P. N., Perry G., Price D. L. Identification and transport of full-length amyloid precursor proteins in rat peripheral nervous system. J Neurosci. 1993 Jul;13(7):3136–3142. doi: 10.1523/JNEUROSCI.13-07-03136.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]
- Xu H., Greengard P., Gandy S. Regulated formation of Golgi secretory vesicles containing Alzheimer beta-amyloid precursor protein. J Biol Chem. 1995 Oct 6;270(40):23243–23245. doi: 10.1074/jbc.270.40.23243. [DOI] [PubMed] [Google Scholar]
- da Cruz e Silva O. A., Iverfeldt K., Oltersdorf T., Sinha S., Lieberburg I., Ramabhadran T. V., Suzuki T., Sisodia S. S., Gandy S., Greengard P. Regulated cleavage of Alzheimer beta-amyloid precursor protein in the absence of the cytoplasmic tail. Neuroscience. 1993 Dec;57(4):873–877. doi: 10.1016/0306-4522(93)90031-a. [DOI] [PubMed] [Google Scholar]