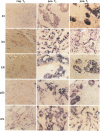
Abstract
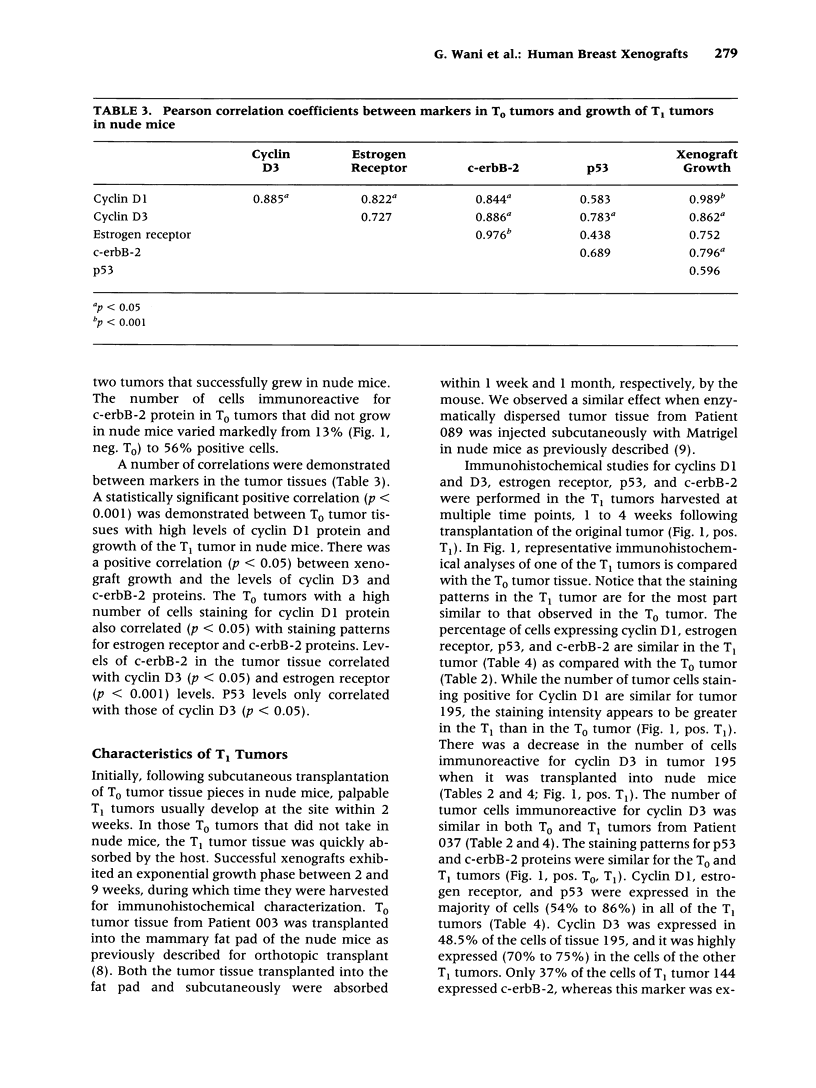
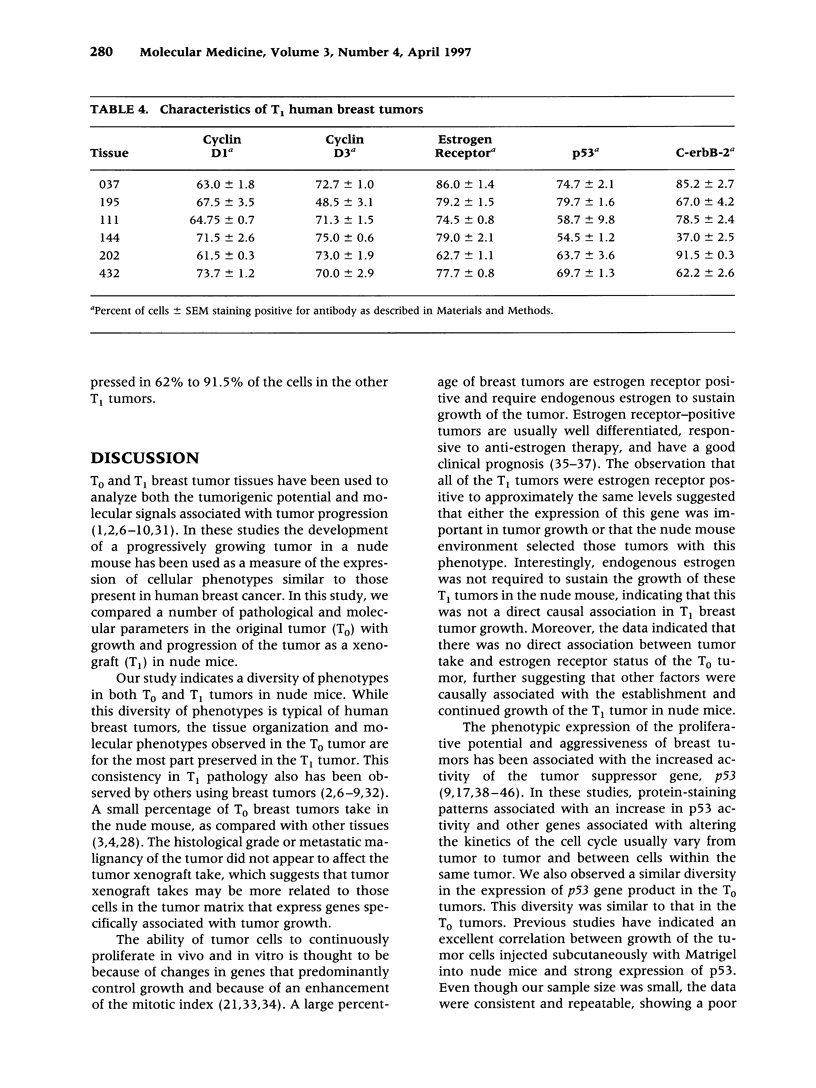
BACKGROUND: The overexpression or amplification of tumor suppressor and proto-oncogenes are important factors in the progression of breast cancer. Recent attention has focused on the cyclin genes, whose involvement in signal transduction pathways regulate cell cycle progression. The amplification of the cyclins D1 and D3 genes usually leads to loss of normal growth control and is thought to play an important growth regulatory role in tumor development and progression. In this report, we investigate the association of altered cyclin expression with other prognostic indicators (histological grade, lymph node status, estrogen receptor, p53, and c-erbB-2) in the progression of human breast cancer. MATERIALS AND METHODS: Surgical tumor specimens were obtained from 16 breast tubular ductal, and invasive ductal carcinomas and grafted onto gnotobiotic nude (nu/nu) mice. The expression diversity and distribution of the localization of the protein products of the c-erbB-2, cyclins D1 and D3, p53, and estrogen receptor were characterized immunohistochemically and the results in the original tumor (T0) were compared with those in the tumors that developed in nude mice (T1) xenografts. RESULTS: The T0 tumors exhibited a diversity of cellular morphology in the tumor matrix and diversity in expression of these proteins. These specific changes were also preserved in the T1 tumors. Whereas 67% of the T1 tumors exhibited high numbers of estrogen receptorpositive nuclei, only 50% of these tumors grew when grafted onto nude mice. The histological grade (14/15 were G2 to G3) and metastatic malignancy in the lymph nodes (10/15) did not appear to be related to tumor growth in the nude mouse. There was no relationship between those tumors which exhibited high percentages of c-erbB-2- and p53-positive cells and growth in nude mice. A strong association (p < 0.001) was observed between the overexpression of cyclin D1 transcripts in the T0 tumors and the continued growth of the T1 tumors in nude mice. In the T1 tumors, both cyclins D1 and D3, estrogen receptor, and p53 were observed in 49% to 86% of the cells of the T1 tumors examined; the number of cells expressing c-erbB-2 protein varied widely in these tumors. CONCLUSIONS: The results indicate that the tumor matrix exhibits a diversity in the level of phenotypic expression of genes involved in cellular growth of breast tumors in both the T0 or T1 host environment. Changes in cyclin activity appear to correlate with the vigorous level of breast tumor growth and progression.
Full text
PDF


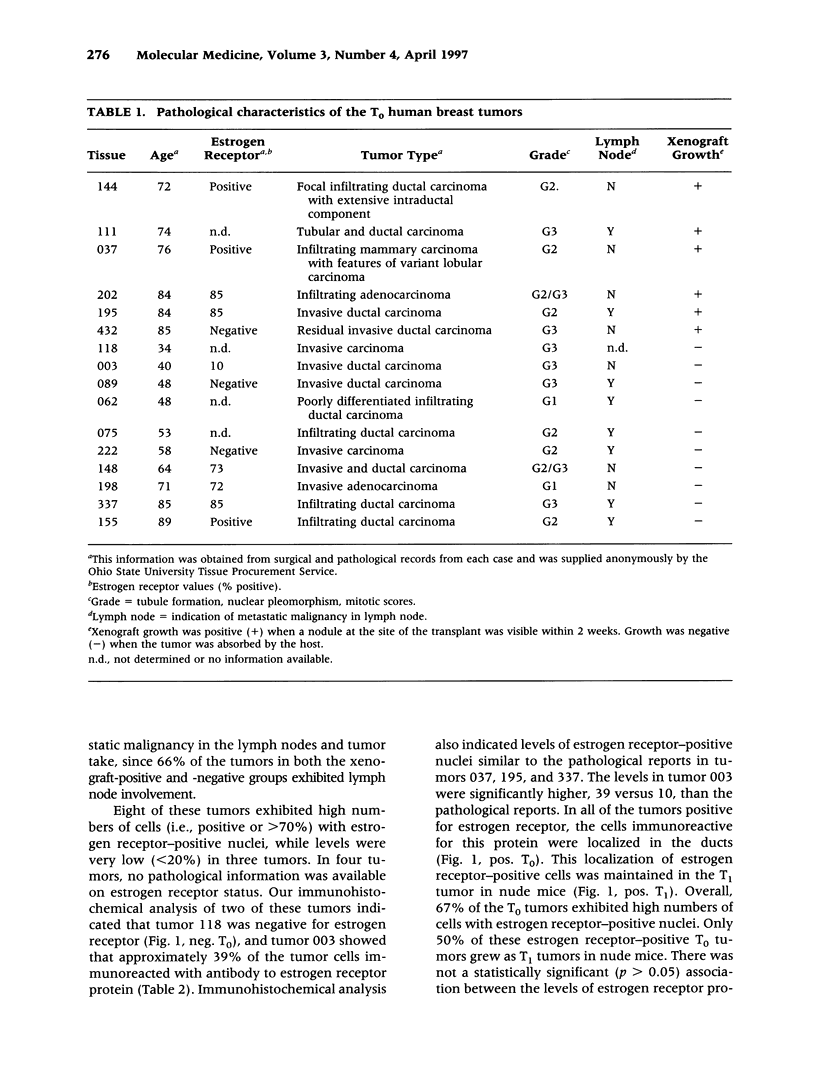



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allred D. C., Elledge R., Clark G. M., Fuqua S. A. The p53 tumor-suppressor gene in human breast cancer. Cancer Treat Res. 1994;71:63–77. doi: 10.1007/978-1-4615-2592-9_4. [DOI] [PubMed] [Google Scholar]
- Allred D. C., O'Connell P., Fuqua S. A. Biomarkers in early breast neoplasia. J Cell Biochem Suppl. 1993;17G:125–131. doi: 10.1002/jcb.240531125. [DOI] [PubMed] [Google Scholar]
- Allred D. C., O'Connell P., Fuqua S. A., Osborne C. K. Immunohistochemical studies of early breast cancer evolution. Breast Cancer Res Treat. 1994;32(1):13–18. doi: 10.1007/BF00666202. [DOI] [PubMed] [Google Scholar]
- Andrews B. J., Mason S. W. Gene expression and the cell cycle: a family affair. Science. 1993 Sep 17;261(5128):1543–1544. doi: 10.1126/science.8372349. [DOI] [PubMed] [Google Scholar]
- Barbareschi M., Caffo O., Doglioni C., Fina P., Marchetti A., Buttitta F., Leek R., Morelli L., Leonardi E., Bevilacqua G. p21WAF1 immunohistochemical expression in breast carcinoma: correlations with clinicopathological data, oestrogen receptor status, MIB1 expression, p53 gene and protein alterations and relapse-free survival. Br J Cancer. 1996 Jul;74(2):208–215. doi: 10.1038/bjc.1996.339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartkova J., Zemanova M., Bartek J. Abundance and subcellular localisation of cyclin D3 in human tumours. Int J Cancer. 1996 Jan 26;65(3):323–327. doi: 10.1002/(SICI)1097-0215(19960126)65:3<323::AID-IJC8>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Becker K., Dosch J., Gregel C. M., Martin B. A., Kaina B. Targeted expression of human O(6)-methylguanine-DNA methyltransferase (MGMT) in transgenic mice protects against tumor initiation in two-stage skin carcinogenesis. Cancer Res. 1996 Jul 15;56(14):3244–3249. [PubMed] [Google Scholar]
- Bosari S., Viale G. The clinical significance of p53 aberrations in human tumours. Virchows Arch. 1995;427(3):229–241. doi: 10.1007/BF00203389. [DOI] [PubMed] [Google Scholar]
- Boulikas T. Control of DNA replication by protein phosphorylation. Anticancer Res. 1994 Nov-Dec;14(6B):2465–2472. [PubMed] [Google Scholar]
- Brünner N., Boysen B., Rømer J., Spang-Thomsen M. The nude mouse as an in vivo model for human breast cancer invasion and metastasis. Breast Cancer Res Treat. 1993;24(3):257–264. doi: 10.1007/BF01833265. [DOI] [PubMed] [Google Scholar]
- Carson D. A., Lois A. Cancer progression and p53. Lancet. 1995 Oct 14;346(8981):1009–1011. doi: 10.1016/s0140-6736(95)91693-8. [DOI] [PubMed] [Google Scholar]
- Chen J. C., Shuler C. F., Zhang C. X., Schuller D. E., Milo G. E. Histopathologic comparison between human oral squamous cell carcinomas and their xenografts in nude mice. Oral Surg Oral Med Oral Pathol. 1991 Apr;71(4):457–463. doi: 10.1016/0030-4220(91)90430-k. [DOI] [PubMed] [Google Scholar]
- Chen J., Milo G. E., Shuler C. F., Schuller D. E. Xenograft growth and histodifferentiation of squamous cell carcinomas of the pharynx and larynx. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996 Feb;81(2):197–202. doi: 10.1016/s1079-2104(96)80415-x. [DOI] [PubMed] [Google Scholar]
- Clarke R. Human breast cancer cell line xenografts as models of breast cancer. The immunobiologies of recipient mice and the characteristics of several tumorigenic cell lines. Breast Cancer Res Treat. 1996;39(1):69–86. doi: 10.1007/BF01806079. [DOI] [PubMed] [Google Scholar]
- Clarke R., Skaar T., Baumann K., Leonessa F., James M., Lippman J., Thompson E. W., Freter C., Brunner N. Hormonal carcinogenesis in breast cancer: cellular and molecular studies of malignant progression. Breast Cancer Res Treat. 1994;31(2-3):237–248. doi: 10.1007/BF00666157. [DOI] [PubMed] [Google Scholar]
- Cox L. A., Chen G., Lee E. Y. Tumor suppressor genes and their roles in breast cancer. Breast Cancer Res Treat. 1994;32(1):19–38. doi: 10.1007/BF00666203. [DOI] [PubMed] [Google Scholar]
- Elledge R. M., Allred D. C. The p53 tumor suppressor gene in breast cancer. Breast Cancer Res Treat. 1994;32(1):39–47. doi: 10.1007/BF00666204. [DOI] [PubMed] [Google Scholar]
- Friedrichs K., Gluba S., Eidtmann H., Jonat W. Overexpression of p53 and prognosis in breast cancer. Cancer. 1993 Dec 15;72(12):3641–3647. doi: 10.1002/1097-0142(19931215)72:12<3641::aid-cncr2820721215>3.0.co;2-8. [DOI] [PubMed] [Google Scholar]
- Gillett C., Smith P., Gregory W., Richards M., Millis R., Peters G., Barnes D. Cyclin D1 and prognosis in human breast cancer. Int J Cancer. 1996 Apr 22;69(2):92–99. doi: 10.1002/(SICI)1097-0215(19960422)69:2<92::AID-IJC4>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- Giovanella B. C., Vardeman D. M., Williams L. J., Taylor D. J., de Ipolyi P. D., Greeff P. J., Stehlin J. S., Ullrich A., Cailleau R., Slamon D. J. Heterotransplantation of human breast carcinomas in nude mice. Correlation between successful heterotransplants, poor prognosis and amplification of the HER-2/neu oncogene. Int J Cancer. 1991 Jan 2;47(1):66–71. doi: 10.1002/ijc.2910470113. [DOI] [PubMed] [Google Scholar]
- Gottlieb T. M., Oren M. p53 in growth control and neoplasia. Biochim Biophys Acta. 1996 Jun 7;1287(2-3):77–102. doi: 10.1016/0304-419x(95)00019-c. [DOI] [PubMed] [Google Scholar]
- Han E. K., Sgambato A., Jiang W., Zhang Y. J., Santella R. M., Doki Y., Cacace A. M., Schieren I., Weinstein I. B. Stable overexpression of cyclin D1 in a human mammary epithelial cell line prolongs the S-phase and inhibits growth. Oncogene. 1995 Mar 2;10(5):953–961. [PubMed] [Google Scholar]
- Horne G. M., Anderson J. J., Tiniakos D. G., McIntosh G. G., Thomas M. D., Angus B., Henry J. A., Lennard T. W., Horne C. H. p53 protein as a prognostic indicator in breast carcinoma: a comparison of four antibodies for immunohistochemistry. Br J Cancer. 1996 Jan;73(1):29–35. doi: 10.1038/bjc.1996.6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hurst J., Maniar N., Tombarkiewicz J., Lucas F., Roberson C., Steplewski Z., James W., Perras J. A novel model of a metastatic human breast tumour xenograft line. Br J Cancer. 1993 Aug;68(2):274–276. doi: 10.1038/bjc.1993.327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katoh A., Breier S., Stemmler N., Specht S., Blanock K., D'Amico F. p53 protein expression in human breast carcinoma: lack of prognostic potential for recurrence of the disease. Anticancer Res. 1996 May-Jun;16(3A):1301–1304. [PubMed] [Google Scholar]
- Lewko W. M., Vaghmar R., Hubbard D., Moore M., He Y. J., Chang L., Husseini S., Wallwork K., Thurman G. B., Oldham R. K. Cultured cell lines from human breast cancer biopsies and xenografts. Breast Cancer Res Treat. 1990 Dec;17(2):121–129. doi: 10.1007/BF01806292. [DOI] [PubMed] [Google Scholar]
- Lipponen P., Ji H., Aaltomaa S., Syrjänen S., Syrjänen K. p53 protein expression in breast cancer as related to histopathological characteristics and prognosis. Int J Cancer. 1993 Aug 19;55(1):51–56. doi: 10.1002/ijc.2910550110. [DOI] [PubMed] [Google Scholar]
- Makris A., Powles T. J., Dowsett M., Allred C. p53 protein overexpression and chemosensitivity in breast cancer. Lancet. 1995 May 6;345(8958):1181–1182. doi: 10.1016/s0140-6736(95)91014-x. [DOI] [PubMed] [Google Scholar]
- Martinazzi M. Expression of p53 oncoprotein in different histological types of breast carcinoma. Am J Pathol. 1994 Jan;144(1):205–205. [PMC free article] [PubMed] [Google Scholar]
- McIntosh G. G., Anderson J. J., Milton I., Steward M., Parr A. H., Thomas M. D., Henry J. A., Angus B., Lennard T. W., Horne C. H. Determination of the prognostic value of cyclin D1 overexpression in breast cancer. Oncogene. 1995 Sep 7;11(5):885–891. [PubMed] [Google Scholar]
- Mehta R. R., Graves J. M., Warso M. A., Das Gupta T. K. Overexpression of mutant p53 and c-erbB-2 proteins and breast tumour take in mice. Br J Cancer. 1995 Nov;72(5):1160–1164. doi: 10.1038/bjc.1995.480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo G. R., Venesio T., Taverna D., Marte B. M., Callahan R., Hynes N. E. Growth suppression of normal mammary epithelial cells by wild-type p53. Oncogene. 1994 Feb;9(2):443–453. [PubMed] [Google Scholar]
- Molina R., Jo J., Filella X., Zanon G., Pahisa J., Muñoz M., Farrus B., Latre M. L., Gimenez N., Hage M. C-erbB-2 oncoprotein in the sera and tissue of patients with breast cancer. Utility in prognosis. Anticancer Res. 1996 Jul-Aug;16(4B):2295–2300. [PubMed] [Google Scholar]
- Nagai M. A., Marques L. A., Yamamoto L., Fujiyama C. T., Brentani M. M. Estrogen and progesterone receptor mRNA levels in primary breast cancer: association with patient survival and other clinical and tumor features. Int J Cancer. 1994 Nov 1;59(3):351–356. doi: 10.1002/ijc.2910590310. [DOI] [PubMed] [Google Scholar]
- Ozzello L., de Rosa C. M., Cantell K., Kauppinen H. L., Habif D. V., Sr Regression of human breast cancer xenografts in response to intralesional treatment with interferons alpha and gamma potentiated by tumor necrosis factor. J Interferon Cytokine Res. 1995 Oct;15(10):839–848. doi: 10.1089/jir.1995.15.839. [DOI] [PubMed] [Google Scholar]
- Price J. E., Polyzos A., Zhang R. D., Daniels L. M. Tumorigenicity and metastasis of human breast carcinoma cell lines in nude mice. Cancer Res. 1990 Feb 1;50(3):717–721. [PubMed] [Google Scholar]
- Price J. E., Zhang R. D. Studies of human breast cancer metastasis using nude mice. Cancer Metastasis Rev. 1990 Feb;8(4):285–297. doi: 10.1007/BF00052605. [DOI] [PubMed] [Google Scholar]
- Pujol P., Hilsenbeck S. G., Chamness G. C., Elledge R. M. Rising levels of estrogen receptor in breast cancer over 2 decades. Cancer. 1994 Sep 1;74(5):1601–1606. doi: 10.1002/1097-0142(19940901)74:5<1601::aid-cncr2820740517>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- Quénel N., Wafflart J., Bonichon F., de Mascarel I., Trojani M., Durand M., Avril A., Coindre J. M. The prognostic value of c-erbB2 in primary breast carcinomas: a study on 942 cases. Breast Cancer Res Treat. 1995 Sep;35(3):283–291. doi: 10.1007/BF00665980. [DOI] [PubMed] [Google Scholar]
- Schuuring E. The involvement of the chromosome 11q13 region in human malignancies: cyclin D1 and EMS1 are two new candidate oncogenes--a review. Gene. 1995 Jun 14;159(1):83–96. doi: 10.1016/0378-1119(94)00562-7. [DOI] [PubMed] [Google Scholar]
- Sgambato A., Han E. K., Zhang Y. J., Moon R. C., Santella R. M., Weinstein I. B. Deregulated expression of cyclin D1 and other cell cycle-related genes in carcinogen-induced rat mammary tumors. Carcinogenesis. 1995 Sep;16(9):2193–2198. doi: 10.1093/carcin/16.9.2193. [DOI] [PubMed] [Google Scholar]
- Shi S. R., Key M. E., Kalra K. L. Antigen retrieval in formalin-fixed, paraffin-embedded tissues: an enhancement method for immunohistochemical staining based on microwave oven heating of tissue sections. J Histochem Cytochem. 1991 Jun;39(6):741–748. doi: 10.1177/39.6.1709656. [DOI] [PubMed] [Google Scholar]
- Singleton T. P., Strickler J. G. Clinical and pathologic significance of the c-erbB-2 (HER-2/neu) oncogene. Pathol Annu. 1992;27(Pt 1):165–190. [PubMed] [Google Scholar]
- Smith H. S., Lu Y., Deng G., Martinez O., Krams S., Ljung B. M., Thor A., Lagios M. Molecular aspects of early stages of breast cancer progression. J Cell Biochem Suppl. 1993;17G:144–152. doi: 10.1002/jcb.240531128. [DOI] [PubMed] [Google Scholar]
- Stanton P. D., Cooke T. G., Forster G., Smith D., Going J. J. Cell kinetics in vivo of human breast cancer. Br J Surg. 1996 Jan;83(1):98–102. doi: 10.1002/bjs.1800830130. [DOI] [PubMed] [Google Scholar]
- Strobl J. S., Wonderlin W. F., Flynn D. C. Mitogenic signal transduction in human breast cancer cells. Gen Pharmacol. 1995 Dec;26(8):1643–1649. doi: 10.1016/0306-3623(95)00062-3. [DOI] [PubMed] [Google Scholar]
- Symmans W. F., Liu J., Knowles D. M., Inghirami G. Breast cancer heterogeneity: evaluation of clonality in primary and metastatic lesions. Hum Pathol. 1995 Feb;26(2):210–216. doi: 10.1016/0046-8177(95)90039-x. [DOI] [PubMed] [Google Scholar]
- Szöllösi J., Balázs M., Feuerstein B. G., Benz C. C., Waldman F. M. ERBB-2 (HER2/neu) gene copy number, p185HER-2 overexpression, and intratumor heterogeneity in human breast cancer. Cancer Res. 1995 Nov 15;55(22):5400–5407. [PubMed] [Google Scholar]
- Wani G., D'Ambrosio S. M. Differential expression of the O6-alkylguanine-DNA alkyltransferase gene in normal human breast and skin tissue: in situ mapping of cell type-specific expression. Mol Carcinog. 1995 Mar;12(3):177–184. doi: 10.1002/mc.2940120309. [DOI] [PubMed] [Google Scholar]
- Wuarin J., Nurse P. Regulating S phase: CDKs, licensing and proteolysis. Cell. 1996 Jun 14;85(6):785–787. doi: 10.1016/s0092-8674(00)81261-1. [DOI] [PubMed] [Google Scholar]
- Yohn J., Lehman T. A., Kurian P., Ribovich M., Milo G. E. Benzo[a]pyrene diol epoxide I modification of DNA in human skin xenografts. J Invest Dermatol. 1988 Oct;91(4):363–368. doi: 10.1111/1523-1747.ep12475997. [DOI] [PubMed] [Google Scholar]
- Zhang S. Y., Caamano J., Cooper F., Guo X., Klein-Szanto A. J. Immunohistochemistry of cyclin D1 in human breast cancer. Am J Clin Pathol. 1994 Nov;102(5):695–698. doi: 10.1093/ajcp/102.5.695. [DOI] [PubMed] [Google Scholar]