Abstract
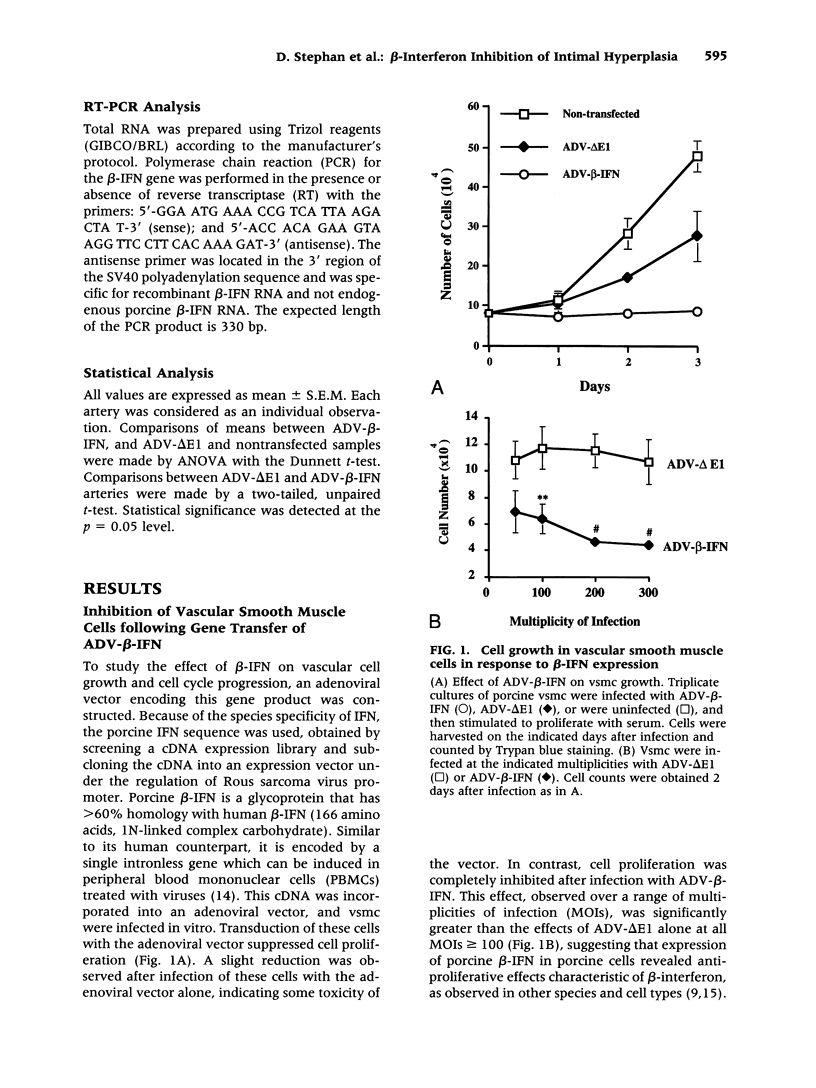
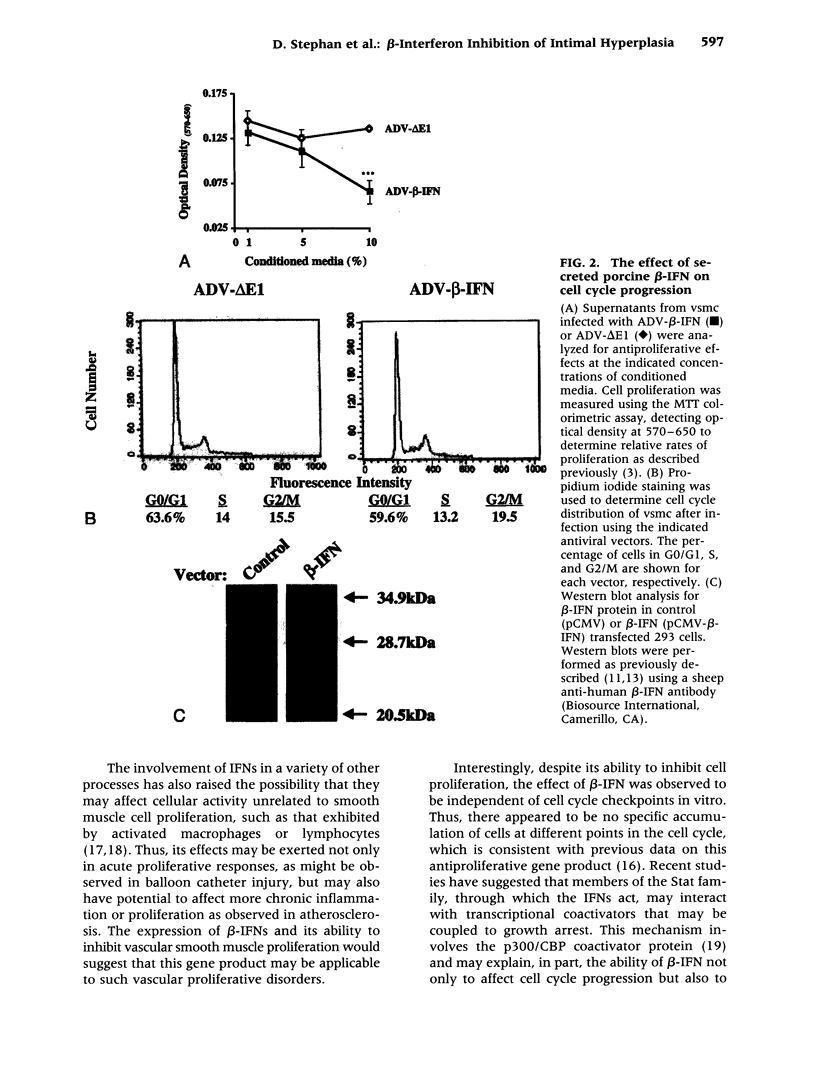
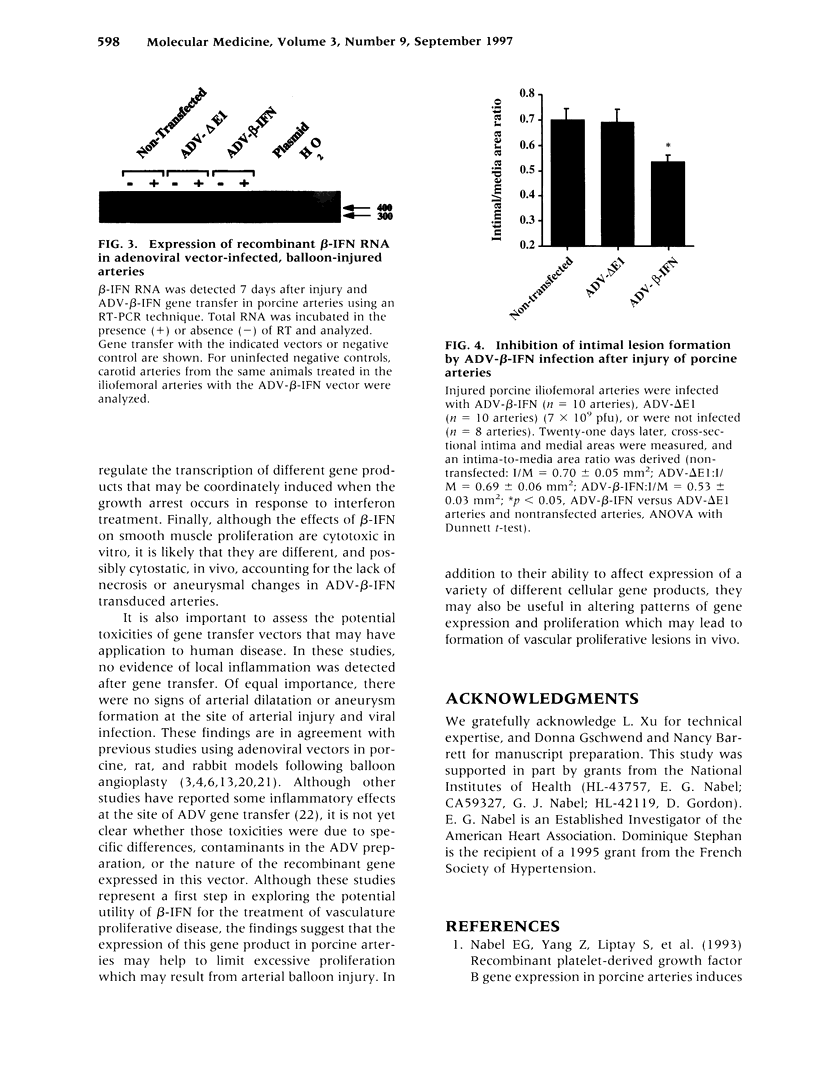
BACKGROUND: Balloon injury of the arterial wall induces increased vascular smooth cell proliferation, enhanced elastic recoil, and abnormalities in thrombosis, each of which contribute to regrowth of intima and the lesion of restenosis. Several gene transfer approaches have been used to inhibit such intimal smooth muscle cell growth. In this report, adenoviral gene transfer of beta-interferon (beta-IFN) was analyzed in a porcine model of balloon injury to determine whether a secreted growth inhibitory protein might affect the regrowth of vascular smooth muscle cells in vitro and in arteries. MATERIALS AND METHODS: An adenoviral vector encoding beta-interferon (ADV-beta-IFN) was prepared and used to infect porcine vascular smooth muscle cells in a porcine balloon injury model. Its antiproliferative effect was analyzed in vitro and in vivo. RESULTS: Expression of recombinant porcine beta-IFN in vascular smooth muscle cells reduced cell proliferation significantly in vitro, and supernatants derived from the beta-IFN vector inhibited vascular smooth muscle cell proliferation relative to controls. When introduced into porcine arteries after balloon injury, a reduction in cell proliferation was observed 7 days after gene transfer measured by BrdC incorporation (ADV-delta E1 arteries 14.5 +/- 1.2%, ADV-beta IFN 6.8 +/- 0.8%, p < 0.05, unpaired, two-tailed t-test). The intima-to-media area ratio was also reduced (nontransfected arteries, 0.70 +/- 0.05; ADV-delta E1 infected arteries, 0.69 +/- 0.06; ADV-beta-IFN infected arteries, 0.53 +/- 0.03; p < 0.05, ANOVA with Dunnett t-test). No evidence of organ toxicity was observed, and regrowth of the endothelial cell surface was observed 3-6 weeks after balloon injury. CONCLUSIONS: Gene transfer of an adenoviral vector encoding beta-IFN into balloon-injured arteries reduced vascular smooth muscle proliferation and intimal formation. Expression of this gene product may have potential application for the treatment of vascular proliferative diseases.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Artursson K., Gobl A., Lindersson M., Johansson M., Alm G. Molecular cloning of a gene encoding porcine interferon-beta. J Interferon Res. 1992 Jun;12(3):153–160. doi: 10.1089/jir.1992.12.153. [DOI] [PubMed] [Google Scholar]
- Barr E., Carroll J., Kalynych A. M., Tripathy S. K., Kozarsky K., Wilson J. M., Leiden J. M. Efficient catheter-mediated gene transfer into the heart using replication-defective adenovirus. Gene Ther. 1994 Jan;1(1):51–58. [PubMed] [Google Scholar]
- Bhattacharya S., Eckner R., Grossman S., Oldread E., Arany Z., D'Andrea A., Livingston D. M. Cooperation of Stat2 and p300/CBP in signalling induced by interferon-alpha. Nature. 1996 Sep 26;383(6598):344–347. doi: 10.1038/383344a0. [DOI] [PubMed] [Google Scholar]
- Chang M. W., Barr E., Seltzer J., Jiang Y. Q., Nabel G. J., Nabel E. G., Parmacek M. S., Leiden J. M. Cytostatic gene therapy for vascular proliferative disorders with a constitutively active form of the retinoblastoma gene product. Science. 1995 Jan 27;267(5197):518–522. doi: 10.1126/science.7824950. [DOI] [PubMed] [Google Scholar]
- David M. Transcription factors in interferon signaling. Pharmacol Ther. 1995 Feb;65(2):149–161. doi: 10.1016/0163-7258(94)00050-d. [DOI] [PubMed] [Google Scholar]
- French B. A., Mazur W., Ali N. M., Geske R. S., Finnigan J. P., Rodgers G. P., Roberts R., Raizner A. E. Percutaneous transluminal in vivo gene transfer by recombinant adenovirus in normal porcine coronary arteries, atherosclerotic arteries, and two models of coronary restenosis. Circulation. 1994 Nov;90(5):2402–2413. doi: 10.1161/01.cir.90.5.2402. [DOI] [PubMed] [Google Scholar]
- Fukumoto Y., Kawahara Y., Kariya K., Araki S., Fukuzaki H., Takai Y. Independent inhibition of DNA synthesis by protein kinase C, cyclic AMP and interferon alpha/beta in rabbit aortic smooth muscle cells. Biochem Biophys Res Commun. 1988 Nov 30;157(1):337–345. doi: 10.1016/s0006-291x(88)80052-4. [DOI] [PubMed] [Google Scholar]
- Heyns A. D., Eldor A., Vlodavsky I., Kaiser N., Fridman R., Panet A. The antiproliferative effect of interferon and the mitogenic activity of growth factors are independent cell cycle events. Studies with vascular smooth muscle cells and endothelial cells. Exp Cell Res. 1985 Dec;161(2):297–306. doi: 10.1016/0014-4827(85)90087-4. [DOI] [PubMed] [Google Scholar]
- ISAACS A., LINDENMANN J. Virus interference. I. The interferon. Proc R Soc Lond B Biol Sci. 1957 Sep 12;147(927):258–267. doi: 10.1098/rspb.1957.0048. [DOI] [PubMed] [Google Scholar]
- Indolfi C., Avvedimento E. V., Rapacciuolo A., Di Lorenzo E., Esposito G., Stabile E., Feliciello A., Mele E., Giuliano P., Condorelli G. Inhibition of cellular ras prevents smooth muscle cell proliferation after vascular injury in vivo. Nat Med. 1995 Jun;1(6):541–545. doi: 10.1038/nm0695-541. [DOI] [PubMed] [Google Scholar]
- Kuo P. T., Wilson A. C., Goldstein R. C., Schaub R. G. Suppression of experimental atherosclerosis in rabbits by interferon-inducing agents. J Am Coll Cardiol. 1984 Jan;3(1):129–134. doi: 10.1016/s0735-1097(84)80438-6. [DOI] [PubMed] [Google Scholar]
- Min W., Ghosh S., Lengyel P. The interferon-inducible p202 protein as a modulator of transcription: inhibition of NF-kappa B, c-Fos, and c-Jun activities. Mol Cell Biol. 1996 Jan;16(1):359–368. doi: 10.1128/mcb.16.1.359. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel E. G., Shum L., Pompili V. J., Yang Z. Y., San H., Shu H. B., Liptay S., Gold L., Gordon D., Derynck R. Direct transfer of transforming growth factor beta 1 gene into arteries stimulates fibrocellular hyperplasia. Proc Natl Acad Sci U S A. 1993 Nov 15;90(22):10759–10763. doi: 10.1073/pnas.90.22.10759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nabel E. G., Yang Z., Liptay S., San H., Gordon D., Haudenschild C. C., Nabel G. J. Recombinant platelet-derived growth factor B gene expression in porcine arteries induce intimal hyperplasia in vivo. J Clin Invest. 1993 Apr;91(4):1822–1829. doi: 10.1172/JCI116394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman K. D., Dunn P. F., Owens J. W., Schulick A. H., Virmani R., Sukhova G., Libby P., Dichek D. A. Adenovirus-mediated gene transfer into normal rabbit arteries results in prolonged vascular cell activation, inflammation, and neointimal hyperplasia. J Clin Invest. 1995 Dec;96(6):2955–2965. doi: 10.1172/JCI118367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ohno T., Gordon D., San H., Pompili V. J., Imperiale M. J., Nabel G. J., Nabel E. G. Gene therapy for vascular smooth muscle cell proliferation after arterial injury. Science. 1994 Aug 5;265(5173):781–784. doi: 10.1126/science.8047883. [DOI] [PubMed] [Google Scholar]
- Palmer H., Libby P. Interferon-beta. A potential autocrine regulator of human vascular smooth muscle cell growth. Lab Invest. 1992 Jun;66(6):715–721. [PubMed] [Google Scholar]
- Stephan D. J., Yang Z. Y., San H., Simari R. D., Wheeler C. J., Felgner P. L., Gordon D., Nabel G. J., Nabel E. G. A new cationic liposome DNA complex enhances the efficiency of arterial gene transfer in vivo. Hum Gene Ther. 1996 Oct 1;7(15):1803–1812. doi: 10.1089/hum.1996.7.15-1803. [DOI] [PubMed] [Google Scholar]
- Wilson A. C., Schaub R. G., Goldstein R. C., Kuo P. T. Suppression of aortic atherosclerosis in cholesterol-fed rabbits by purified rabbit interferon. Arteriosclerosis. 1990 Mar-Apr;10(2):208–214. doi: 10.1161/01.atv.10.2.208. [DOI] [PubMed] [Google Scholar]
- Yang Z. Y., Perkins N. D., Ohno T., Nabel E. G., Nabel G. J. The p21 cyclin-dependent kinase inhibitor suppresses tumorigenicity in vivo. Nat Med. 1995 Oct;1(10):1052–1056. doi: 10.1038/nm1095-1052. [DOI] [PubMed] [Google Scholar]
- Yang Z. Y., Simari R. D., Perkins N. D., San H., Gordon D., Nabel G. J., Nabel E. G. Role of the p21 cyclin-dependent kinase inhibitor in limiting intimal cell proliferation in response to arterial injury. Proc Natl Acad Sci U S A. 1996 Jul 23;93(15):7905–7910. doi: 10.1073/pnas.93.15.7905. [DOI] [PMC free article] [PubMed] [Google Scholar]




