Abstract
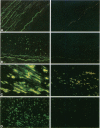
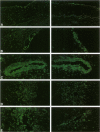
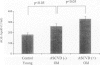
BACKGROUND: Advanced glycation endproducts (AGEs) are implicated in the pathogenesis of atherosclerotic vascular disease of diabetic and nondiabetic etiology. Recent research suggests that advanced glycation of ApoB contributes to the development of hyperlipidemia. AGE-specific receptors, expressed on vascular endothelium and mononuclear cells, may be involved in both the clearance of, and the inflammatory responses to AGEs. The aim of this study was to examine whether there is a relationship between serum AGE-ApoB and AGEs in arterial tissue of older normolipidemic nondiabetic patients with occlusive atherosclerotic disease, compared with age-matched and younger asymptomatic persons. MATERIALS AND METHODS: Serum AGE-ApoB was measured by ELISA in 21 cardiac bypass patients. Furthermore, an AGE-specific monoclonal antibody, and polyclonal antibodies against anti-AGE-receptor (anti-AGE-R) 1 and 2 were used to explore the localization and distribution of AGEs and AGE-R immunoreactivity (IR) in arterial segments excised from these patients. RESULTS: Serum AGE-ApoB levels were significantly elevated in the asymptomatic, older population, compared with those in young healthy persons (259 +/- 24 versus 180 +/- 21 AGE U/mg of ApoB, p < 0.01). Higher AGE-ApoB levels were observed in those patients with atherosclerosis (329 +/- 23 versus 259 +/- 24 AGE U/mg ApoB, p < 0.05). Comparisons of tissue AGE-collagen with serum AGE-ApoB levels showed a significant correlation (r = 0.707, p < 0.01). In early lesions, AGE-IR occurred mostly extracellularly. In fatty streaks and dense, cellular atheromatous lesions, AGE-IR was visible within lipid-containing smooth muscle cells and macrophages, while in late-stage, acellular plaques, AGE-IR occurred mostly extracellularly. AGE-R1 and -R2 were observed on vascular endothelial and smooth-muscle cells and on infiltrating mononuclear cells in the early-stage lesions, whereas in dense, late-stage plaques, they colocalized mostly with lipid-laden macrophages. On tissue sections, scoring of AGE-immunofluorescence correlated with tissue AGE and plasma AGE-ApoB. CONCLUSIONS: (1) The correlation between arterial tissue AGEs and circulating AGE-ApoB suggests a causal link between AGE modification of lipoproteins and atherosclerosis. AGE-specific receptors may contribute to this process. (2) Serum AGE-ApoB may serve to predict atherosclerosis in asymptomatic patients.
Full text
PDF


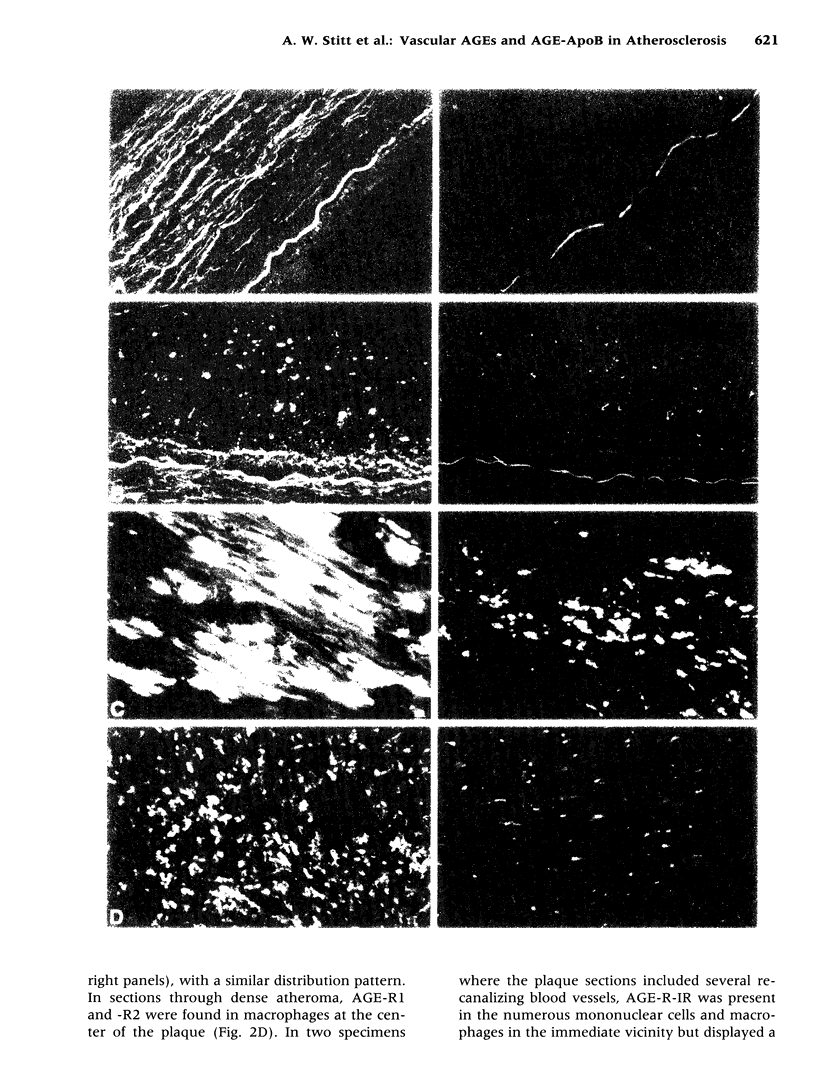

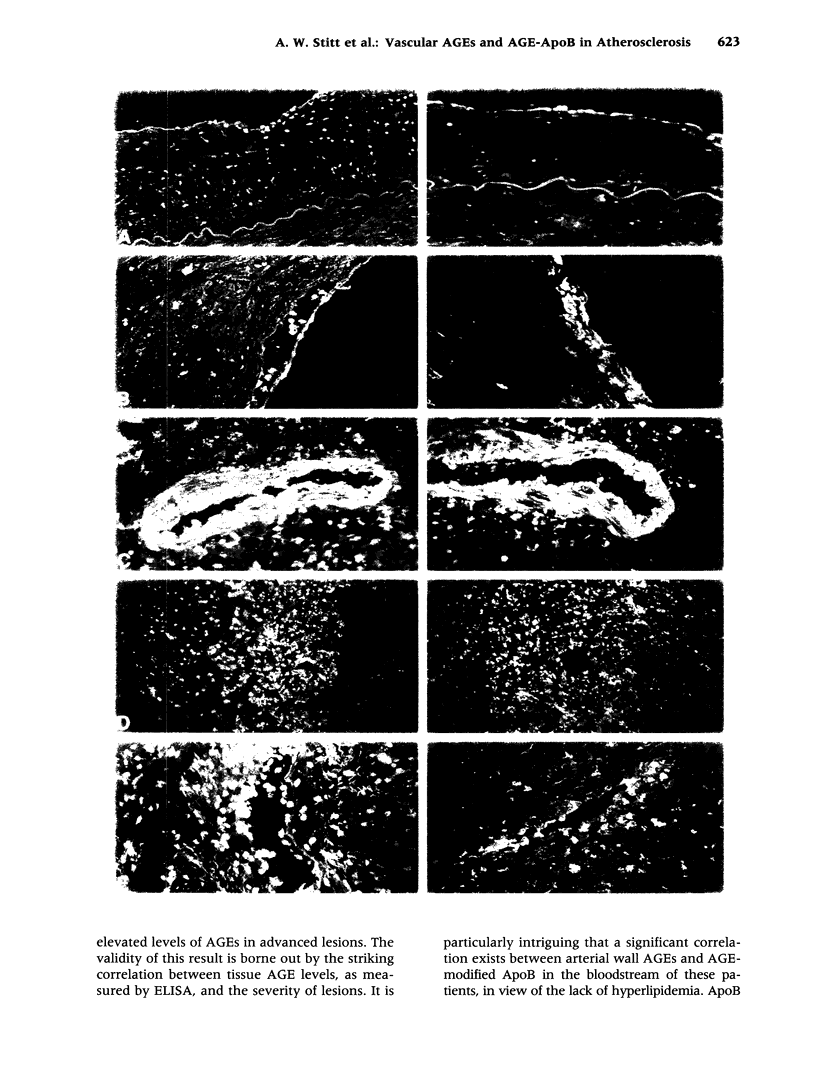
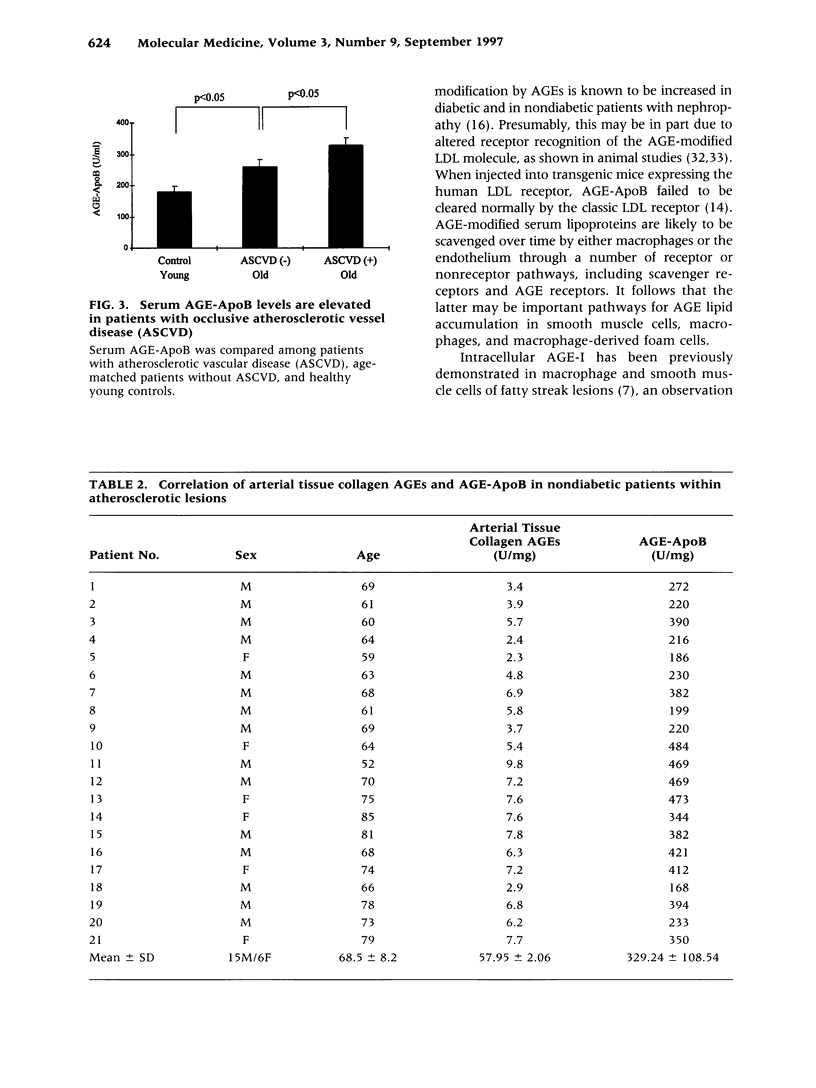
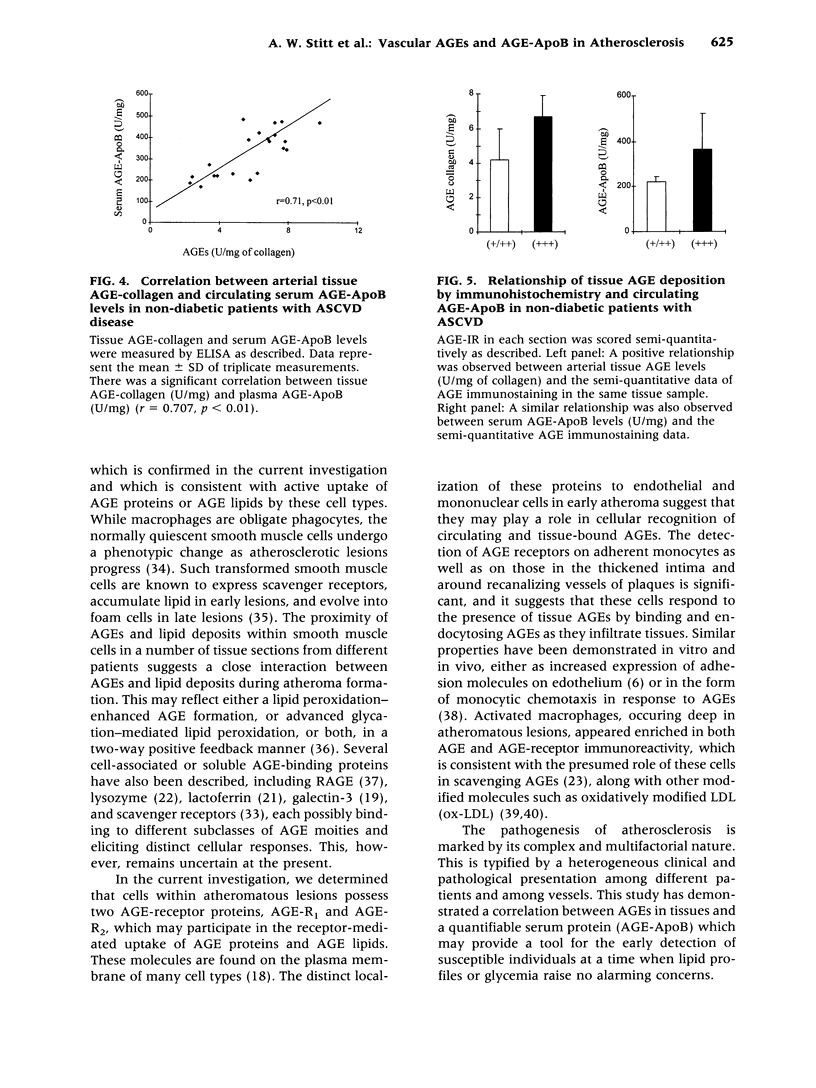


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Brownlee M., Pongor S., Cerami A. Covalent attachment of soluble proteins by nonenzymatically glycosylated collagen. Role in the in situ formation of immune complexes. J Exp Med. 1983 Nov 1;158(5):1739–1744. doi: 10.1084/jem.158.5.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R., Makita Z., Koschinsky T., Cerami A., Vlassara H. Lipid advanced glycosylation: pathway for lipid oxidation in vivo. Proc Natl Acad Sci U S A. 1993 Jul 15;90(14):6434–6438. doi: 10.1073/pnas.90.14.6434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R., Makita Z., Vega G., Grundy S., Koschinsky T., Cerami A., Vlassara H. Modification of low density lipoprotein by advanced glycation end products contributes to the dyslipidemia of diabetes and renal insufficiency. Proc Natl Acad Sci U S A. 1994 Sep 27;91(20):9441–9445. doi: 10.1073/pnas.91.20.9441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bucala R., Mitchell R., Arnold K., Innerarity T., Vlassara H., Cerami A. Identification of the major site of apolipoprotein B modification by advanced glycosylation end products blocking uptake by the low density lipoprotein receptor. J Biol Chem. 1995 May 5;270(18):10828–10832. doi: 10.1074/jbc.270.18.10828. [DOI] [PubMed] [Google Scholar]
- Bucala R., Tracey K. J., Cerami A. Advanced glycosylation products quench nitric oxide and mediate defective endothelium-dependent vasodilatation in experimental diabetes. J Clin Invest. 1991 Feb;87(2):432–438. doi: 10.1172/JCI115014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esposito C., Gerlach H., Brett J., Stern D., Vlassara H. Endothelial receptor-mediated binding of glucose-modified albumin is associated with increased monolayer permeability and modulation of cell surface coagulant properties. J Exp Med. 1989 Oct 1;170(4):1387–1407. doi: 10.1084/jem.170.4.1387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fogelman A. M., Haberland M. E., Seager J., Hokom M., Edwards P. A. Factors regulating the activities of the low density lipoprotein receptor and the scavenger receptor on human monocyte-macrophages. J Lipid Res. 1981 Sep;22(7):1131–1141. [PubMed] [Google Scholar]
- Goldstein J. L., Ho Y. K., Basu S. K., Brown M. S. Binding site on macrophages that mediates uptake and degradation of acetylated low density lipoprotein, producing massive cholesterol deposition. Proc Natl Acad Sci U S A. 1979 Jan;76(1):333–337. doi: 10.1073/pnas.76.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hunt J. V., Skamarauskas J. T., Mitchinson M. J. Protein glycation and fluorescent material in human atheroma. Atherosclerosis. 1994 Dec;111(2):255–265. doi: 10.1016/0021-9150(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Imani F., Horii Y., Suthanthiran M., Skolnik E. Y., Makita Z., Sharma V., Sehajpal P., Vlassara H. Advanced glycosylation endproduct-specific receptors on human and rat T-lymphocytes mediate synthesis of interferon gamma: role in tissue remodeling. J Exp Med. 1993 Dec 1;178(6):2165–2172. doi: 10.1084/jem.178.6.2165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ismail N. A., Alavi M. Z., Moore S. Lipoprotein-proteoglycan complexes from injured rabbit aortas accelerate lipoprotein uptake by arterial smooth muscle cells. Atherosclerosis. 1994 Jan;105(1):79–87. doi: 10.1016/0021-9150(94)90010-8. [DOI] [PubMed] [Google Scholar]
- Kirstein M., Brett J., Radoff S., Ogawa S., Stern D., Vlassara H. Advanced protein glycosylation induces transendothelial human monocyte chemotaxis and secretion of platelet-derived growth factor: role in vascular disease of diabetes and aging. Proc Natl Acad Sci U S A. 1990 Nov;87(22):9010–9014. doi: 10.1073/pnas.87.22.9010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kume S., Takeya M., Mori T., Araki N., Suzuki H., Horiuchi S., Kodama T., Miyauchi Y., Takahashi K. Immunohistochemical and ultrastructural detection of advanced glycation end products in atherosclerotic lesions of human aorta with a novel specific monoclonal antibody. Am J Pathol. 1995 Sep;147(3):654–667. [PMC free article] [PubMed] [Google Scholar]
- Lee W. K., Bell J., Kilpatrick E., Hayes M., Lindop G. B., Dominiczak M. H. Collagen-linked fluorescence in human atherosclerotic plaques. Atherosclerosis. 1993 Jan 25;98(2):219–227. doi: 10.1016/0021-9150(93)90131-d. [DOI] [PubMed] [Google Scholar]
- Li H., Freeman M. W., Libby P. Regulation of smooth muscle cell scavenger receptor expression in vivo by atherogenic diets and in vitro by cytokines. J Clin Invest. 1995 Jan;95(1):122–133. doi: 10.1172/JCI117628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. M., Mitsuhashi T., Wojciechowicz D., Shimizu N., Li J., Stitt A., He C., Banerjee D., Vlassara H. Molecular identity and cellular distribution of advanced glycation endproduct receptors: relationship of p60 to OST-48 and p90 to 80K-H membrane proteins. Proc Natl Acad Sci U S A. 1996 Oct 1;93(20):11047–11052. doi: 10.1073/pnas.93.20.11047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y. M., Tan A. X., Vlassara H. Antibacterial activity of lysozyme and lactoferrin is inhibited by binding of advanced glycation-modified proteins to a conserved motif. Nat Med. 1995 Oct;1(10):1057–1061. doi: 10.1038/nm1095-1057. [DOI] [PubMed] [Google Scholar]
- Luoma J., Hiltunen T., Särkioja T., Moestrup S. K., Gliemann J., Kodama T., Nikkari T., Ylä-Herttuala S. Expression of alpha 2-macroglobulin receptor/low density lipoprotein receptor-related protein and scavenger receptor in human atherosclerotic lesions. J Clin Invest. 1994 May;93(5):2014–2021. doi: 10.1172/JCI117195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Makita Z., Radoff S., Rayfield E. J., Yang Z., Skolnik E., Delaney V., Friedman E. A., Cerami A., Vlassara H. Advanced glycosylation end products in patients with diabetic nephropathy. N Engl J Med. 1991 Sep 19;325(12):836–842. doi: 10.1056/NEJM199109193251202. [DOI] [PubMed] [Google Scholar]
- Makita Z., Vlassara H., Cerami A., Bucala R. Immunochemical detection of advanced glycosylation end products in vivo. J Biol Chem. 1992 Mar 15;267(8):5133–5138. [PubMed] [Google Scholar]
- Monnier V. M., Kohn R. R., Cerami A. Accelerated age-related browning of human collagen in diabetes mellitus. Proc Natl Acad Sci U S A. 1984 Jan;81(2):583–587. doi: 10.1073/pnas.81.2.583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nakamura Y., Horii Y., Nishino T., Shiiki H., Sakaguchi Y., Kagoshima T., Dohi K., Makita Z., Vlassara H., Bucala R. Immunohistochemical localization of advanced glycosylation end products in coronary atheroma and cardiac tissue in diabetes mellitus. Am J Pathol. 1993 Dec;143(6):1649–1656. [PMC free article] [PubMed] [Google Scholar]
- Neeper M., Schmidt A. M., Brett J., Yan S. D., Wang F., Pan Y. C., Elliston K., Stern D., Shaw A. Cloning and expression of a cell surface receptor for advanced glycosylation end products of proteins. J Biol Chem. 1992 Jul 25;267(21):14998–15004. [PubMed] [Google Scholar]
- Palinski W., Koschinsky T., Butler S. W., Miller E., Vlassara H., Cerami A., Witztum J. L. Immunological evidence for the presence of advanced glycosylation end products in atherosclerotic lesions of euglycemic rabbits. Arterioscler Thromb Vasc Biol. 1995 May;15(5):571–582. doi: 10.1161/01.atv.15.5.571. [DOI] [PubMed] [Google Scholar]
- Reiser K. M. Nonenzymatic glycation of collagen in aging and diabetes. Proc Soc Exp Biol Med. 1991 Jan;196(1):17–29. doi: 10.3181/00379727-196-43158c. [DOI] [PubMed] [Google Scholar]
- Ross R. The pathogenesis of atherosclerosis: a perspective for the 1990s. Nature. 1993 Apr 29;362(6423):801–809. doi: 10.1038/362801a0. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Hori O., Brett J., Yan S. D., Wautier J. L., Stern D. Cellular receptors for advanced glycation end products. Implications for induction of oxidant stress and cellular dysfunction in the pathogenesis of vascular lesions. Arterioscler Thromb. 1994 Oct;14(10):1521–1528. doi: 10.1161/01.atv.14.10.1521. [DOI] [PubMed] [Google Scholar]
- Schmidt A. M., Vianna M., Gerlach M., Brett J., Ryan J., Kao J., Esposito C., Hegarty H., Hurley W., Clauss M. Isolation and characterization of two binding proteins for advanced glycosylation end products from bovine lung which are present on the endothelial cell surface. J Biol Chem. 1992 Jul 25;267(21):14987–14997. [PubMed] [Google Scholar]
- Schnider S. L., Kohn R. R. Effects of age and diabetes mellitus on the solubility of collagen from human skin, tracheal cartilage and dura mater. Exp Gerontol. 1982;17(3):185–194. doi: 10.1016/0531-5565(82)90024-9. [DOI] [PubMed] [Google Scholar]
- Sell D. R., Lapolla A., Odetti P., Fogarty J., Monnier V. M. Pentosidine formation in skin correlates with severity of complications in individuals with long-standing IDDM. Diabetes. 1992 Oct;41(10):1286–1292. doi: 10.2337/diab.41.10.1286. [DOI] [PubMed] [Google Scholar]
- Thyberg J., Hedin U., Sjölund M., Palmberg L., Bottger B. A. Regulation of differentiated properties and proliferation of arterial smooth muscle cells. Arteriosclerosis. 1990 Nov-Dec;10(6):966–990. doi: 10.1161/01.atv.10.6.966. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Brownlee M., Manogue K. R., Dinarello C. A., Pasagian A. Cachectin/TNF and IL-1 induced by glucose-modified proteins: role in normal tissue remodeling. Science. 1988 Jun 10;240(4858):1546–1548. doi: 10.1126/science.3259727. [DOI] [PubMed] [Google Scholar]
- Vlassara H., Bucala R., Striker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994 Feb;70(2):138–151. [PubMed] [Google Scholar]
- Vlassara H., Fuh H., Donnelly T., Cybulsky M. Advanced glycation endproducts promote adhesion molecule (VCAM-1, ICAM-1) expression and atheroma formation in normal rabbits. Mol Med. 1995 May;1(4):447–456. [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Fuh H., Makita Z., Krungkrai S., Cerami A., Bucala R. Exogenous advanced glycosylation end products induce complex vascular dysfunction in normal animals: a model for diabetic and aging complications. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):12043–12047. doi: 10.1073/pnas.89.24.12043. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vlassara H., Li Y. M., Imani F., Wojciechowicz D., Yang Z., Liu F. T., Cerami A. Identification of galectin-3 as a high-affinity binding protein for advanced glycation end products (AGE): a new member of the AGE-receptor complex. Mol Med. 1995 Sep;1(6):634–646. [PMC free article] [PubMed] [Google Scholar]
- Vlassara H. Receptor-mediated interactions of advanced glycosylation end products with cellular components within diabetic tissues. Diabetes. 1992 Oct;41 (Suppl 2):52–56. doi: 10.2337/diab.41.2.s52. [DOI] [PubMed] [Google Scholar]
- Yang Z., Makita Z., Horii Y., Brunelle S., Cerami A., Sehajpal P., Suthanthiran M., Vlassara H. Two novel rat liver membrane proteins that bind advanced glycosylation endproducts: relationship to macrophage receptor for glucose-modified proteins. J Exp Med. 1991 Sep 1;174(3):515–524. doi: 10.1084/jem.174.3.515. [DOI] [PMC free article] [PubMed] [Google Scholar]