Abstract
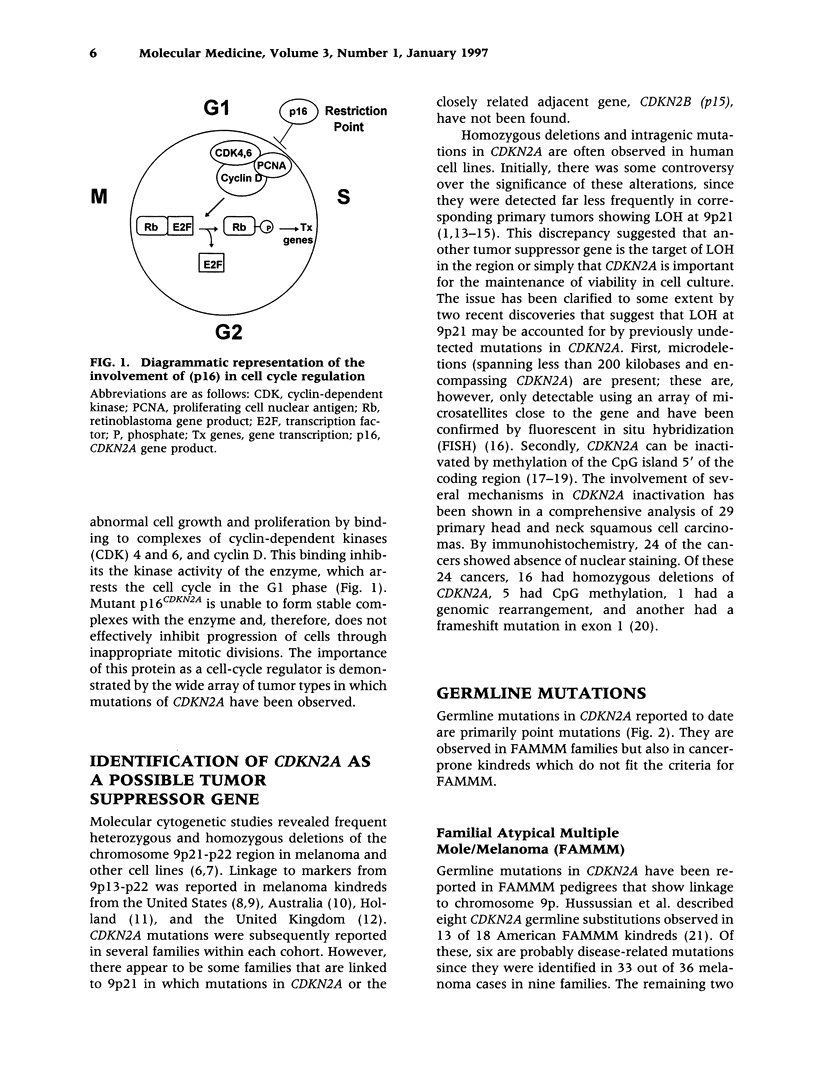
CDKN2A, the gene encoding the cell-cycle inhibitor p16CDKN2A, was first identified in 1994. Since then, somatic mutations have been observed in many cancers and germline alterations have been found in kindreds with familial atypical multiple mole/melanoma (FAMMM), also known as atypical mole syndrome. In this review we tabulate the known mutations in this gene and discuss specific aspects, particularly with respect to germline mutations and cancer predisposition.
Full text
PDF




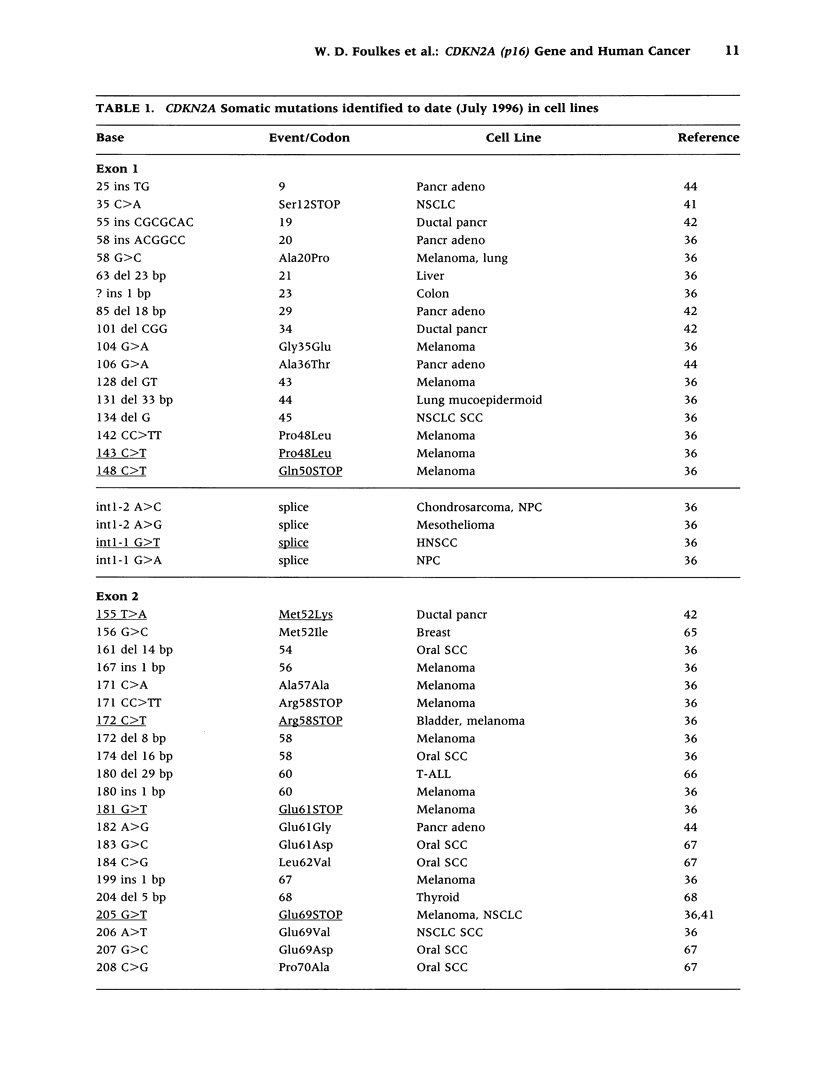
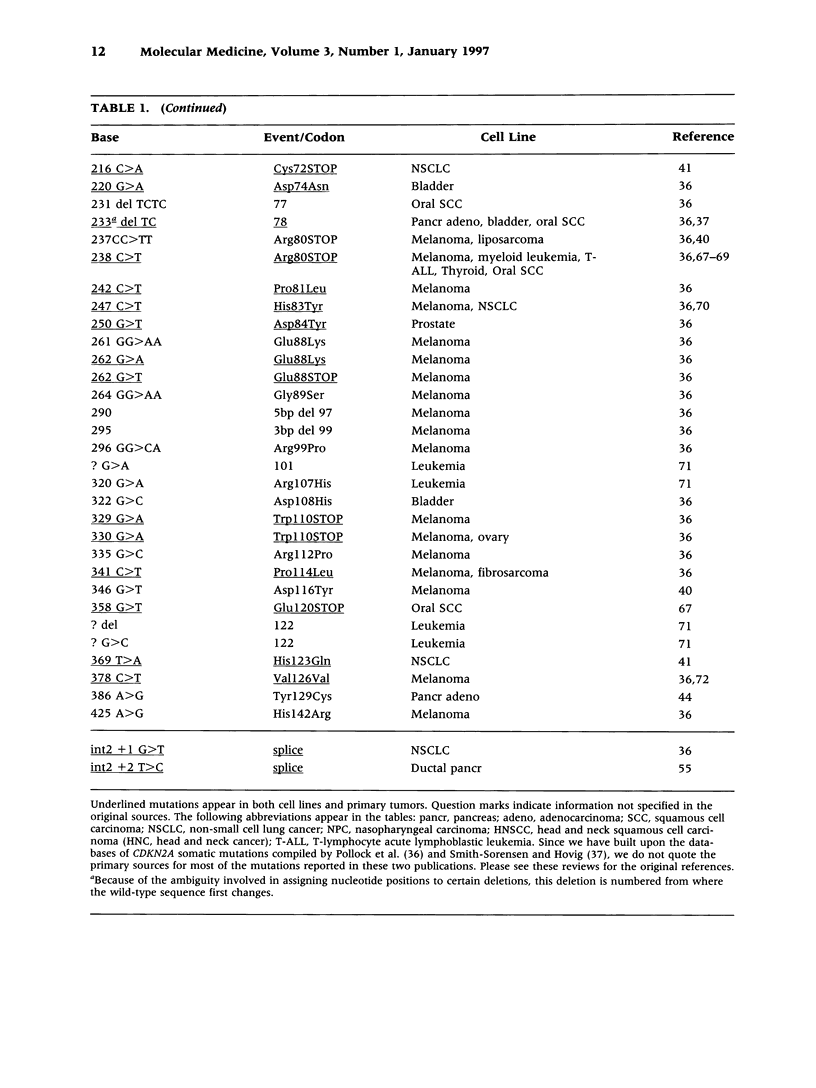
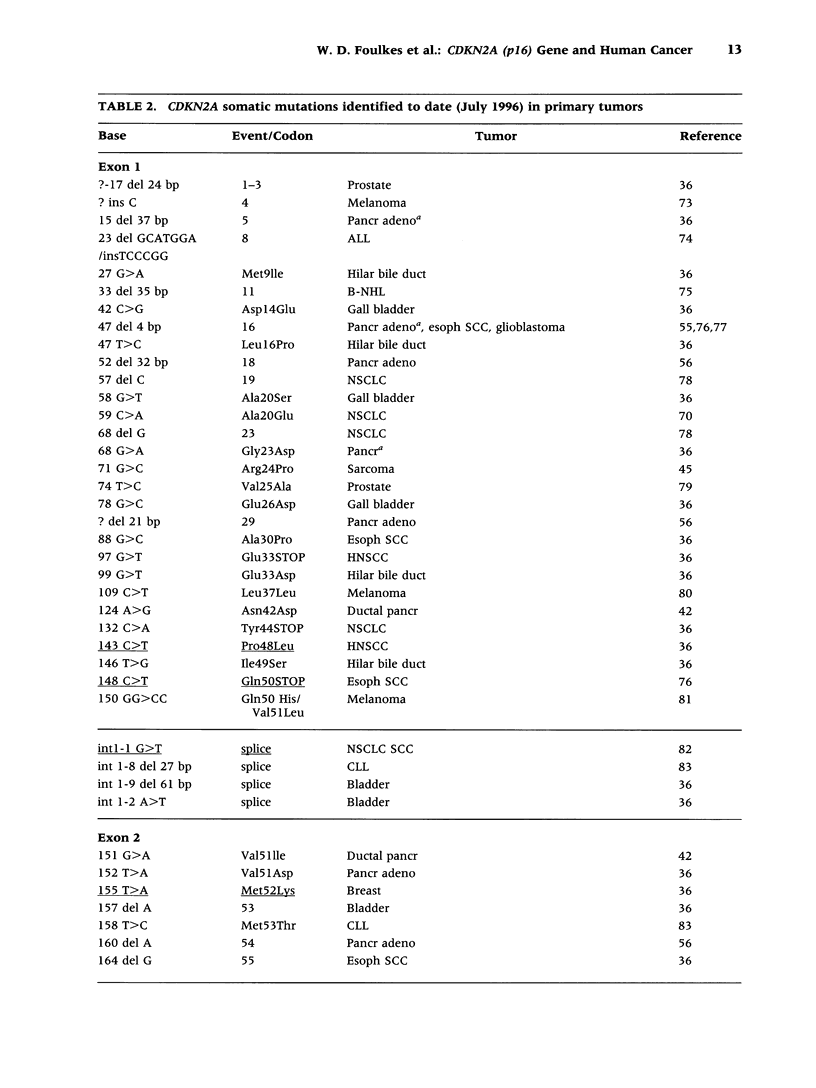




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bartsch D., Shevlin D. W., Callery M. P., Norton J. A., Wells S. A., Jr, Goodfellow P. J. Reduced survival in patients with ductal pancreatic adenocarcinoma associated with CDKN2 mutation. J Natl Cancer Inst. 1996 May 15;88(10):680–682. doi: 10.1093/jnci/88.10.680. [DOI] [PubMed] [Google Scholar]
- Bartsch D., Shevlin D. W., Tung W. S., Kisker O., Wells S. A., Jr, Goodfellow P. J. Frequent mutations of CDKN2 in primary pancreatic adenocarcinomas. Genes Chromosomes Cancer. 1995 Nov;14(3):189–195. doi: 10.1002/gcc.2870140306. [DOI] [PubMed] [Google Scholar]
- Bergman W., Watson P., de Jong J., Lynch H. T., Fusaro R. M. Systemic cancer and the FAMMM syndrome. Br J Cancer. 1990 Jun;61(6):932–936. doi: 10.1038/bjc.1990.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berns E. M., Klijn J. G., Smid M., van Staveren I. L., Gruis N. A., Foekens J. A. Infrequent CDKN2 (MTS1/p16) gene alterations in human primary breast cancer. Br J Cancer. 1995 Oct;72(4):964–967. doi: 10.1038/bjc.1995.442. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borg A., Johannsson U., Johannsson O., Häkansson S., Westerdahl J., Mäsbäck A., Olsson H., Ingvar C. Novel germline p16 mutation in familial malignant melanoma in southern Sweden. Cancer Res. 1996 Jun 1;56(11):2497–2500. [PubMed] [Google Scholar]
- Brenner A. J., Aldaz C. M. Chromosome 9p allelic loss and p16/CDKN2 in breast cancer and evidence of p16 inactivation in immortal breast epithelial cells. Cancer Res. 1995 Jul 1;55(13):2892–2895. [PubMed] [Google Scholar]
- Cairns P., Mao L., Merlo A., Lee D. J., Schwab D., Eby Y., Tokino K., van der Riet P., Blaugrund J. E., Sidransky D. Rates of p16 (MTS1) mutations in primary tumors with 9p loss. Science. 1994 Jul 15;265(5170):415–417. doi: 10.1126/science.8023167. [DOI] [PubMed] [Google Scholar]
- Cairns P., Polascik T. J., Eby Y., Tokino K., Califano J., Merlo A., Mao L., Herath J., Jenkins R., Westra W. Frequency of homozygous deletion at p16/CDKN2 in primary human tumours. Nat Genet. 1995 Oct;11(2):210–212. doi: 10.1038/ng1095-210. [DOI] [PubMed] [Google Scholar]
- Calabrò V., Strazzullo M., La Mantia G., Fedele M., Paulin C., Fusco A., Lania L. Status and expression of the p16INK4 gene in human thyroid tumors and thyroid-tumor cell lines. Int J Cancer. 1996 Jul 3;67(1):29–34. doi: 10.1002/(SICI)1097-0215(19960703)67:1<29::AID-IJC7>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Caldas C., Hahn S. A., da Costa L. T., Redston M. S., Schutte M., Seymour A. B., Weinstein C. L., Hruban R. H., Yeo C. J., Kern S. E. Frequent somatic mutations and homozygous deletions of the p16 (MTS1) gene in pancreatic adenocarcinoma. Nat Genet. 1994 Sep;8(1):27–32. doi: 10.1038/ng0994-27. [DOI] [PubMed] [Google Scholar]
- Campbell I. G., Foulkes W. D., Beynon G., Davis M., Englefield P. LOH and mutation analysis of CDKN2 in primary human ovarian cancers. Int J Cancer. 1995 Oct 9;63(2):222–225. doi: 10.1002/ijc.2910630213. [DOI] [PubMed] [Google Scholar]
- Cannon-Albright L. A., Goldgar D. E., Meyer L. J., Lewis C. M., Anderson D. E., Fountain J. W., Hegi M. E., Wiseman R. W., Petty E. M., Bale A. E. Assignment of a locus for familial melanoma, MLM, to chromosome 9p13-p22. Science. 1992 Nov 13;258(5085):1148–1152. doi: 10.1126/science.1439824. [DOI] [PubMed] [Google Scholar]
- Cayuela J. M., Madani A., Sanhes L., Stern M. H., Sigaux F. Multiple tumor-suppressor gene 1 inactivation is the most frequent genetic alteration in T-cell acute lymphoblastic leukemia. Blood. 1996 Mar 15;87(6):2180–2186. [PubMed] [Google Scholar]
- Ciotti P., Strigini P., Bianchi-Scarrà G. Familial melanoma and pancreatic cancer. Ligurian Skin Tumor Study Group. N Engl J Med. 1996 Feb 15;334(7):469–472. doi: 10.1056/NEJM199602153340714. [DOI] [PubMed] [Google Scholar]
- Eiriksdottir G., Sigurdsson A., Jonasson J. G., Agnarsson B. A., Sigurdsson H., Gudmundsson J., Bergthorsson J. T., Barkardottir R. B., Egilsson V., Ingvarsson S. Loss of heterozygosity on chromosome 9 in human breast cancer: association with clinical variables and genetic changes at other chromosome regions. Int J Cancer. 1995 Dec 20;64(6):378–382. doi: 10.1002/ijc.2910640605. [DOI] [PubMed] [Google Scholar]
- Esteve A., Martel-Planche G., Sylla B. S., Hollstein M., Hainaut P., Montesano R. Low frequency of p16/CDKN2 gene mutations in esophageal carcinomas. Int J Cancer. 1996 May 3;66(3):301–304. doi: 10.1002/(SICI)1097-0215(19960503)66:3<301::AID-IJC5>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- Fearon E. R., Cho K. R., Nigro J. M., Kern S. E., Simons J. W., Ruppert J. M., Hamilton S. R., Preisinger A. C., Thomas G., Kinzler K. W. Identification of a chromosome 18q gene that is altered in colorectal cancers. Science. 1990 Jan 5;247(4938):49–56. doi: 10.1126/science.2294591. [DOI] [PubMed] [Google Scholar]
- Fountain J. W., Karayiorgou M., Ernstoff M. S., Kirkwood J. M., Vlock D. R., Titus-Ernstoff L., Bouchard B., Vijayasaradhi S., Houghton A. N., Lahti J. Homozygous deletions within human chromosome band 9p21 in melanoma. Proc Natl Acad Sci U S A. 1992 Nov 1;89(21):10557–10561. doi: 10.1073/pnas.89.21.10557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayther S. A., Warren W., Mazoyer S., Russell P. A., Harrington P. A., Chiano M., Seal S., Hamoudi R., van Rensburg E. J., Dunning A. M. Germline mutations of the BRCA1 gene in breast and ovarian cancer families provide evidence for a genotype-phenotype correlation. Nat Genet. 1995 Dec;11(4):428–433. doi: 10.1038/ng1295-428. [DOI] [PubMed] [Google Scholar]
- Goldstein A. M., Dracopoli N. C., Engelstein M., Fraser M. C., Clark W. H., Jr, Tucker M. A. Linkage of cutaneous malignant melanoma/dysplastic nevi to chromosome 9p, and evidence for genetic heterogeneity. Am J Hum Genet. 1994 Mar;54(3):489–496. [PMC free article] [PubMed] [Google Scholar]
- Goldstein A. M., Fraser M. C., Struewing J. P., Hussussian C. J., Ranade K., Zametkin D. P., Fontaine L. S., Organic S. M., Dracopoli N. C., Clark W. H., Jr Increased risk of pancreatic cancer in melanoma-prone kindreds with p16INK4 mutations. N Engl J Med. 1995 Oct 12;333(15):970–974. doi: 10.1056/NEJM199510123331504. [DOI] [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Bender C. M., Yang A. S., Nguyen T., Beart R. W., Van Tornout J. M., Jones P. A. Methylation of the 5' CpG island of the p16/CDKN2 tumor suppressor gene in normal and transformed human tissues correlates with gene silencing. Cancer Res. 1995 Oct 15;55(20):4531–4535. [PubMed] [Google Scholar]
- Gonzalez-Zulueta M., Shibata A., Ohneseit P. F., Spruck C. H., 3rd, Busch C., Shamaa M., El-Baz M., Nichols P. W., Gonzalgo M. L., Elbaz M [corrected to El-Baz M. ]. High frequency of chromosome 9p allelic loss and CDKN2 tumor suppressor gene alterations in squamous cell carcinoma of the bladder. J Natl Cancer Inst. 1995 Sep 20;87(18):1383–1393. doi: 10.1093/jnci/87.18.1383. [DOI] [PubMed] [Google Scholar]
- Greene M. H., Tucker M. A., Clark W. H., Jr, Kraemer K. H., Elder D. E., Fraser M. C. Hereditary melanoma and the dysplastic nevus syndrome: the risk of cancers other than melanoma. J Am Acad Dermatol. 1987 Apr;16(4):792–797. doi: 10.1016/s0190-9622(87)70103-0. [DOI] [PubMed] [Google Scholar]
- Gruis N. A., Sandkuijl L. A., Weber J. L., van der Zee A., Borgstein A. M., Bergman W., Frants R. R. Linkage analysis in Dutch familial atypical multiple mole-melanoma (FAMMM) syndrome families. Effect of naevus count. Melanoma Res. 1993 Aug;3(4):271–277. [PubMed] [Google Scholar]
- Gruis N. A., van der Velden P. A., Sandkuijl L. A., Prins D. E., Weaver-Feldhaus J., Kamb A., Bergman W., Frants R. R. Homozygotes for CDKN2 (p16) germline mutation in Dutch familial melanoma kindreds. Nat Genet. 1995 Jul;10(3):351–353. doi: 10.1038/ng0795-351. [DOI] [PubMed] [Google Scholar]
- Gutman M., Cnaan A., Inbar M., Shafir R., Chaitchik S., Rozin R. R., Klausner J. M. Are malignant melanoma patients at higher risk for a second cancer? Cancer. 1991 Aug 1;68(3):660–665. doi: 10.1002/1097-0142(19910801)68:3<660::aid-cncr2820680337>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- Hatta Y., Hirama T., Takeuchi S., Lee E., Pham E., Miller C. W., Strohmeyer T., Wilczynski S. P., Melmed S., Koeffler H. P. Alterations of the p16 (MTS1) gene in testicular, ovarian, and endometrial malignancies. J Urol. 1995 Nov;154(5):1954–1957. [PubMed] [Google Scholar]
- Herman J. G., Merlo A., Mao L., Lapidus R. G., Issa J. P., Davidson N. E., Sidransky D., Baylin S. B. Inactivation of the CDKN2/p16/MTS1 gene is frequently associated with aberrant DNA methylation in all common human cancers. Cancer Res. 1995 Oct 15;55(20):4525–4530. [PubMed] [Google Scholar]
- Holland E. A., Beaton S. C., Becker T. M., Grulet O. M., Peters B. A., Rizos H., Kefford R. F., Mann G. J. Analysis of the p16 gene, CDKN2, in 17 Australian melanoma kindreds. Oncogene. 1995 Dec 7;11(11):2289–2294. [PubMed] [Google Scholar]
- Hollstein M., Rice K., Greenblatt M. S., Soussi T., Fuchs R., Sørlie T., Hovig E., Smith-Sørensen B., Montesano R., Harris C. C. Database of p53 gene somatic mutations in human tumors and cell lines. Nucleic Acids Res. 1994 Sep;22(17):3551–3555. [PMC free article] [PubMed] [Google Scholar]
- Huang L., Goodrow T. L., Zhang S. Y., Klein-Szanto A. J., Chang H., Ruggeri B. A. Deletion and mutation analyses of the P16/MTS-1 tumor suppressor gene in human ductal pancreatic cancer reveals a higher frequency of abnormalities in tumor-derived cell lines than in primary ductal adenocarcinomas. Cancer Res. 1996 Mar 1;56(5):1137–1141. [PubMed] [Google Scholar]
- Hussussian C. J., Struewing J. P., Goldstein A. M., Higgins P. A., Ally D. S., Sheahan M. D., Clark W. H., Jr, Tucker M. A., Dracopoli N. C. Germline p16 mutations in familial melanoma. Nat Genet. 1994 Sep;8(1):15–21. doi: 10.1038/ng0994-15. [DOI] [PubMed] [Google Scholar]
- Igaki H., Sasaki H., Tachimori Y., Kato H., Watanabe H., Kimura T., Harada Y., Sugimura T., Terada M. Mutation frequency of the p16/CDKN2 gene in primary cancers in the upper digestive tract. Cancer Res. 1995 Aug 1;55(15):3421–3423. [PubMed] [Google Scholar]
- Izumoto S., Arita N., Ohnishi T., Hiraga S., Taki T., Hayakawa T. Homozygous deletions of p16INK4A/MTS1 and p15INK4B/MTS2 genes in glioma cells and primary glioma tissues. Cancer Lett. 1995 Nov 6;97(2):241–247. doi: 10.1016/0304-3835(95)03981-2. [DOI] [PubMed] [Google Scholar]
- Jen J., Harper J. W., Bigner S. H., Bigner D. D., Papadopoulos N., Markowitz S., Willson J. K., Kinzler K. W., Vogelstein B. Deletion of p16 and p15 genes in brain tumors. Cancer Res. 1994 Dec 15;54(24):6353–6358. [PubMed] [Google Scholar]
- Kamb A., Gruis N. A., Weaver-Feldhaus J., Liu Q., Harshman K., Tavtigian S. V., Stockert E., Day R. S., 3rd, Johnson B. E., Skolnick M. H. A cell cycle regulator potentially involved in genesis of many tumor types. Science. 1994 Apr 15;264(5157):436–440. doi: 10.1126/science.8153634. [DOI] [PubMed] [Google Scholar]
- Kamb A., Shattuck-Eidens D., Eeles R., Liu Q., Gruis N. A., Ding W., Hussey C., Tran T., Miki Y., Weaver-Feldhaus J. Analysis of the p16 gene (CDKN2) as a candidate for the chromosome 9p melanoma susceptibility locus. Nat Genet. 1994 Sep;8(1):23–26. doi: 10.1038/ng0994-22. [DOI] [PubMed] [Google Scholar]
- Kyritsis A. P., Zhang B., Zhang W., Xiao M., Takeshima H., Bondy M. L., Cunningham J. E., Levin V. A., Bruner J. Mutations of the p16 gene in gliomas. Oncogene. 1996 Jan 4;12(1):63–67. [PubMed] [Google Scholar]
- Li F. P., Fraumeni J. F., Jr, Mulvihill J. J., Blattner W. A., Dreyfus M. G., Tucker M. A., Miller R. W. A cancer family syndrome in twenty-four kindreds. Cancer Res. 1988 Sep 15;48(18):5358–5362. [PubMed] [Google Scholar]
- Linehan W. M., Lerman M. I., Zbar B. Identification of the von Hippel-Lindau (VHL) gene. Its role in renal cancer. JAMA. 1995 Feb 15;273(7):564–570. [PubMed] [Google Scholar]
- Liu L., Lassam N. J., Slingerland J. M., Bailey D., Cole D., Jenkins R., Hogg D. Germline p16INK4A mutation and protein dysfunction in a family with inherited melanoma. Oncogene. 1995 Jul 20;11(2):405–412. [PubMed] [Google Scholar]
- Luca M., Xie S., Gutman M., Huang S., Bar-Eli M. Abnormalities in the CDKN2 (p16INK4/MTS-1) gene in human melanoma cells: relevance to tumor growth and metastasis. Oncogene. 1995 Oct 5;11(7):1399–1402. [PubMed] [Google Scholar]
- Lynch H. T., Fusaro R. M., Pester J., Oosterhuis J. A., Went L. N., Rumke P., Neering H., Lynch J. F. Tumour spectrum in the FAMMM syndrome. Br J Cancer. 1981 Oct;44(4):553–560. doi: 10.1038/bjc.1981.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maelandsmo G. M., Flørenes V. A., Hovig E., Oyjord T., Engebraaten O., Holm R., Børresen A. L., Fodstad O. Involvement of the pRb/p16/cdk4/cyclin D1 pathway in the tumorigenesis of sporadic malignant melanomas. Br J Cancer. 1996 Apr;73(8):909–916. doi: 10.1038/bjc.1996.181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merlo A., Herman J. G., Mao L., Lee D. J., Gabrielson E., Burger P. C., Baylin S. B., Sidransky D. 5' CpG island methylation is associated with transcriptional silencing of the tumour suppressor p16/CDKN2/MTS1 in human cancers. Nat Med. 1995 Jul;1(7):686–692. doi: 10.1038/nm0795-686. [DOI] [PubMed] [Google Scholar]
- Miller C. W., Aslo A., Campbell M. J., Kawamata N., Lampkin B. C., Koeffler H. P. Alterations of the p15, p16,and p18 genes in osteosarcoma. Cancer Genet Cytogenet. 1996 Feb;86(2):136–142. doi: 10.1016/0165-4608(95)00216-2. [DOI] [PubMed] [Google Scholar]
- Musgrove E. A., Lilischkis R., Cornish A. L., Lee C. S., Setlur V., Seshadri R., Sutherland R. L. Expression of the cyclin-dependent kinase inhibitors p16INK4, p15INK4B and p21WAF1/CIP1 in human breast cancer. Int J Cancer. 1995 Nov 15;63(4):584–591. doi: 10.1002/ijc.2910630420. [DOI] [PubMed] [Google Scholar]
- Nakagawa K., Conrad N. K., Williams J. P., Johnson B. E., Kelley M. J. Mechanism of inactivation of CDKN2 and MTS2 in non-small cell lung cancer and association with advanced stage. Oncogene. 1995 Nov 2;11(9):1843–1851. [PubMed] [Google Scholar]
- Nakamaki T., Kawamata N., Schwaller J., Tobler A., Fey M., Pakkala S., Lee Y. Y., Kim B. K., Fukuchi K., Tsuruoka N. Structural integrity of the cyclin-dependent kinase inhibitor genes, p15, p16 and p18 in myeloid leukaemias. Br J Haematol. 1995 Sep;91(1):139–149. doi: 10.1111/j.1365-2141.1995.tb05259.x. [DOI] [PubMed] [Google Scholar]
- Nakao M., Yokota S., Kaneko H., Seriu T., Koizumi S., Takaue Y., Fujimoto T., Misawa S. Alterations of CDKN2 gene structure in childhood acute lymphoblastic leukemia: mutations of CDKN2 are observed preferentially in T lineage. Leukemia. 1996 Feb;10(2):249–254. [PubMed] [Google Scholar]
- Nancarrow D. J., Mann G. J., Holland E. A., Walker G. J., Beaton S. C., Walters M. K., Luxford C., Palmer J. M., Donald J. A., Weber J. L. Confirmation of chromosome 9p linkage in familial melanoma. Am J Hum Genet. 1993 Oct;53(4):936–942. [PMC free article] [PubMed] [Google Scholar]
- Naumann M., Savitskaia N., Eilert C., Schramm A., Kalthoff H., Schmiegel W. Frequent codeletion of p16/MTS1 and p15/MTS2 and genetic alterations in p16/MTS1 in pancreatic tumors. Gastroenterology. 1996 Apr;110(4):1215–1224. doi: 10.1053/gast.1996.v110.pm8613012. [DOI] [PubMed] [Google Scholar]
- Nobori T., Miura K., Wu D. J., Lois A., Takabayashi K., Carson D. A. Deletions of the cyclin-dependent kinase-4 inhibitor gene in multiple human cancers. Nature. 1994 Apr 21;368(6473):753–756. doi: 10.1038/368753a0. [DOI] [PubMed] [Google Scholar]
- Ohnishi H., Kawamura M., Ida K., Sheng X. M., Hanada R., Nobori T., Yamamori S., Hayashi Y. Homozygous deletions of p16/MTS1 gene are frequent but mutations are infrequent in childhood T-cell acute lymphoblastic leukemia. Blood. 1995 Aug 15;86(4):1269–1275. [PubMed] [Google Scholar]
- Ohta M., Berd D., Shimizu M., Nagai H., Cotticelli M-G, Mastrangelo M., Shields J. A., Shields C. L., Croce C. M., Huebner K. Deletion mapping of chromosome region 9p21-p22 surrounding the CDKN2 locus in melanoma. Int J Cancer. 1996 Mar 15;65(6):762–767. doi: 10.1002/(SICI)1097-0215(19960315)65:6<762::AID-IJC9>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- Ohta M., Nagai H., Shimizu M., Rasio D., Berd D., Mastrangelo M., Singh A. D., Shields J. A., Shields C. L., Croce C. M. Rarity of somatic and germline mutations of the cyclin-dependent kinase 4 inhibitor gene, CDK4I, in melanoma. Cancer Res. 1994 Oct 15;54(20):5269–5272. [PubMed] [Google Scholar]
- Okajima E., Fukuda T., Okita S., Tsutsumi M., Hirao Y., Okajima E., Konishi Y. Infrequent somatic alteration of p16/MTS1 in human primary superficial bladder cancers. Cancer Lett. 1996 Jun 5;103(2):227–231. doi: 10.1016/0304-3835(96)04225-5. [DOI] [PubMed] [Google Scholar]
- Okamoto A., Demetrick D. J., Spillare E. A., Hagiwara K., Hussain S. P., Bennett W. P., Forrester K., Gerwin B., Serrano M., Beach D. H. Mutations and altered expression of p16INK4 in human cancer. Proc Natl Acad Sci U S A. 1994 Nov 8;91(23):11045–11049. doi: 10.1073/pnas.91.23.11045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platz A., Ringborg U., Lagerlöf B., Lundqvist E., Sevigny P., Inganäs M. Mutational analysis of the CDKN2 gene in metastases from patients with cutaneous malignant melanoma. Br J Cancer. 1996 Feb;73(3):344–348. doi: 10.1038/bjc.1996.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pollock P. M., Pearson J. V., Hayward N. K. Compilation of somatic mutations of the CDKN2 gene in human cancers: non-random distribution of base substitutions. Genes Chromosomes Cancer. 1996 Feb;15(2):77–88. doi: 10.1002/(SICI)1098-2264(199602)15:2<77::AID-GCC1>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Ranade K., Hussussian C. J., Sikorski R. S., Varmus H. E., Goldstein A. M., Tucker M. A., Serrano M., Hannon G. J., Beach D., Dracopoli N. C. Mutations associated with familial melanoma impair p16INK4 function. Nat Genet. 1995 May;10(1):114–116. doi: 10.1038/ng0595-114. [DOI] [PubMed] [Google Scholar]
- Rasool O., Heyman M., Brandter L. B., Liu Y., Grandér D., Söderhäll S., Einhorn S. p15ink4B and p16ink4 gene inactivation in acute lymphocytic leukemia. Blood. 1995 Jun 15;85(12):3431–3436. [PubMed] [Google Scholar]
- Reed A. L., Califano J., Cairns P., Westra W. H., Jones R. M., Koch W., Ahrendt S., Eby Y., Sewell D., Nawroz H. High frequency of p16 (CDKN2/MTS-1/INK4A) inactivation in head and neck squamous cell carcinoma. Cancer Res. 1996 Aug 15;56(16):3630–3633. [PubMed] [Google Scholar]
- Reed J. A., Loganzo F., Jr, Shea C. R., Walker G. J., Flores J. F., Glendening J. M., Bogdany J. K., Shiel M. J., Haluska F. G., Fountain J. W. Loss of expression of the p16/cyclin-dependent kinase inhibitor 2 tumor suppressor gene in melanocytic lesions correlates with invasive stage of tumor progression. Cancer Res. 1995 Jul 1;55(13):2713–2718. [PubMed] [Google Scholar]
- Riggins G. J., Thiagalingam S., Rozenblum E., Weinstein C. L., Kern S. E., Hamilton S. R., Willson J. K., Markowitz S. D., Kinzler K. W., Vogelstein B. Mad-related genes in the human. Nat Genet. 1996 Jul;13(3):347–349. doi: 10.1038/ng0796-347. [DOI] [PubMed] [Google Scholar]
- Rusin M. R., Okamoto A., Chorazy M., Czyzewski K., Harasim J., Spillare E. A., Hagiwara K., Hussain S. P., Xiong Y., Demetrick D. J. Intragenic mutations of the p16(INK4), p15(INK4B) and p18 genes in primary non-small-cell lung cancers. Int J Cancer. 1996 Mar 15;65(6):734–739. doi: 10.1002/(SICI)1097-0215(19960315)65:6<734::AID-IJC4>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- Shimizu T., Sekiya T. Loss of heterozygosity at 9p21 loci and mutations of the MTS1 and MTS2 genes in human lung cancers. Int J Cancer. 1995 Nov 27;63(5):616–620. doi: 10.1002/ijc.2910630503. [DOI] [PubMed] [Google Scholar]
- Sonoda Y., Yoshimoto T., Sekiya T. Homozygous deletion of the MTS1/p16 and MTS2/p15 genes and amplification of the CDK4 gene in glioma. Oncogene. 1995 Nov 16;11(10):2145–2149. [PubMed] [Google Scholar]
- Spruck C. H., 3rd, Gonzalez-Zulueta M., Shibata A., Simoneau A. R., Lin M. F., Gonzales F., Tsai Y. C., Jones P. A. p16 gene in uncultured tumours. Nature. 1994 Jul 21;370(6486):183–184. doi: 10.1038/370183a0. [DOI] [PubMed] [Google Scholar]
- Thiagalingam S., Lengauer C., Leach F. S., Schutte M., Hahn S. A., Overhauser J., Willson J. K., Markowitz S., Hamilton S. R., Kern S. E. Evaluation of candidate tumour suppressor genes on chromosome 18 in colorectal cancers. Nat Genet. 1996 Jul;13(3):343–346. doi: 10.1038/ng0796-343. [DOI] [PubMed] [Google Scholar]
- Tung W. S., Shevlin D. W., Bartsch D., Norton J. A., Wells S. A., Jr, Goodfellow P. J. Infrequent CDKN2 mutation in human differentiated thyroid cancers. Mol Carcinog. 1996 Jan;15(1):5–10. doi: 10.1002/(SICI)1098-2744(199601)15:1<5::AID-MC2>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- Uchida T., Watanabe T., Kinoshita T., Murate T., Saito H., Hotta T. Mutational analysis of the CDKN2 (MTS1/p16ink4A) gene in primary B-cell lymphomas. Blood. 1995 Oct 1;86(7):2724–2731. [PubMed] [Google Scholar]
- Ueki K., Ono Y., Henson J. W., Efird J. T., von Deimling A., Louis D. N. CDKN2/p16 or RB alterations occur in the majority of glioblastomas and are inversely correlated. Cancer Res. 1996 Jan 1;56(1):150–153. [PubMed] [Google Scholar]
- Ueki K., Rubio M. P., Ramesh V., Correa K. M., Rutter J. L., von Deimling A., Buckler A. J., Gusella J. F., Louis D. N. MTS1/CDKN2 gene mutations are rare in primary human astrocytomas with allelic loss of chromosome 9p. Hum Mol Genet. 1994 Oct;3(10):1841–1845. doi: 10.1093/hmg/3.10.1841. [DOI] [PubMed] [Google Scholar]
- Walker G. J., Hussussian C. J., Flores J. F., Glendening J. M., Haluska F. G., Dracopoli N. C., Hayward N. K., Fountain J. W. Mutations of the CDKN2/p16INK4 gene in Australian melanoma kindreds. Hum Mol Genet. 1995 Oct;4(10):1845–1852. doi: 10.1093/hmg/4.10.1845. [DOI] [PubMed] [Google Scholar]
- Whelan A. J., Bartsch D., Goodfellow P. J. Brief report: a familial syndrome of pancreatic cancer and melanoma with a mutation in the CDKN2 tumor-suppressor gene. N Engl J Med. 1995 Oct 12;333(15):975–977. doi: 10.1056/NEJM199510123331505. [DOI] [PubMed] [Google Scholar]
- Xu L., Sgroi D., Sterner C. J., Beauchamp R. L., Pinney D. M., Keel S., Ueki K., Rutter J. L., Buckler A. J., Louis D. N. Mutational analysis of CDKN2 (MTS1/p16ink4) in human breast carcinomas. Cancer Res. 1994 Oct 15;54(20):5262–5264. [PubMed] [Google Scholar]
- Yarbrough W. G., Aprelikova O., Pei H., Olshan A. F., Liu E. T. Familial tumor syndrome associated with a germline nonfunctional p16INK4a allele. J Natl Cancer Inst. 1996 Oct 16;88(20):1489–1491. doi: 10.1093/jnci/88.20.1489. [DOI] [PubMed] [Google Scholar]
- Yoshida S., Todoroki T., Ichikawa Y., Hanai S., Suzuki H., Hori M., Fukao K., Miwa M., Uchida K. Mutations of p16Ink4/CDKN2 and p15Ink4B/MTS2 genes in biliary tract cancers. Cancer Res. 1995 Jul 1;55(13):2756–2760. [PubMed] [Google Scholar]
- Zariwala M., Liu E., Xiong Y. Mutational analysis of the p16 family cyclin-dependent kinase inhibitors p15INK4b and p18INK4c in tumor-derived cell lines and primary tumors. Oncogene. 1996 Jan 18;12(2):451–455. [PubMed] [Google Scholar]
- de Vos S., Miller C. W., Takeuchi S., Gombart A. F., Cho S. K., Koeffler H. P. Alterations of CDKN2 (p16) in non-small cell lung cancer. Genes Chromosomes Cancer. 1995 Nov;14(3):164–170. doi: 10.1002/gcc.2870140303. [DOI] [PubMed] [Google Scholar]



