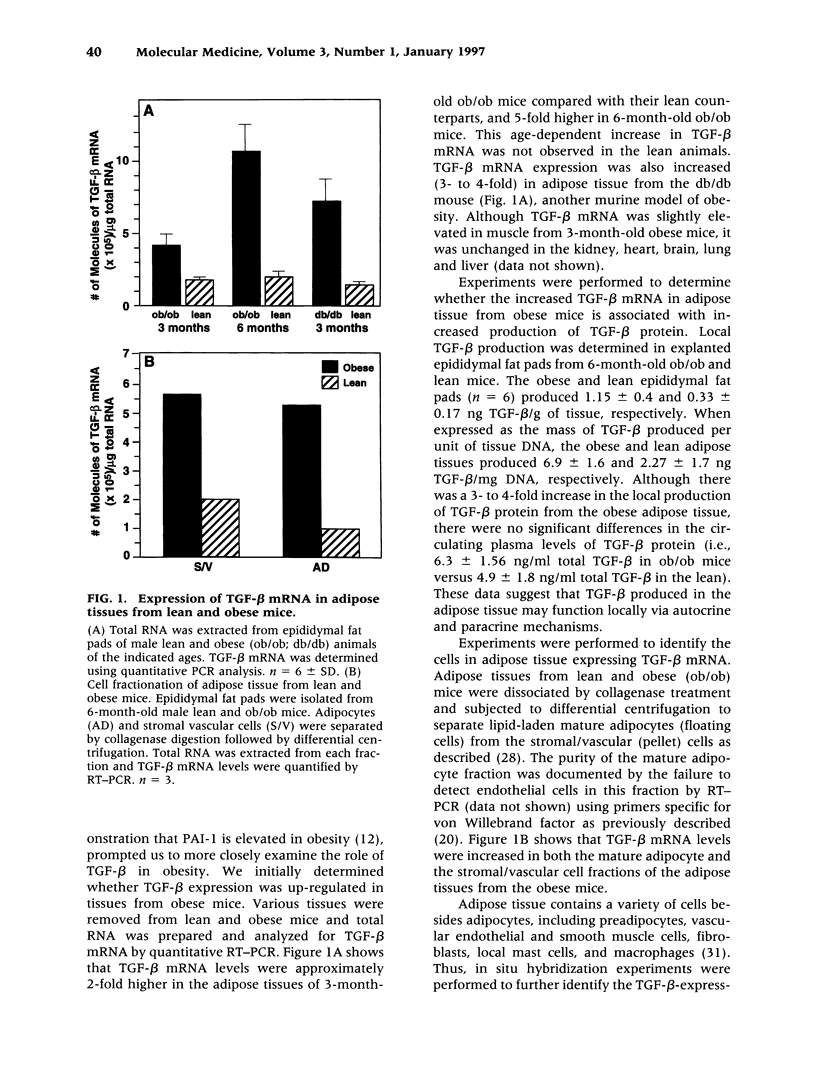
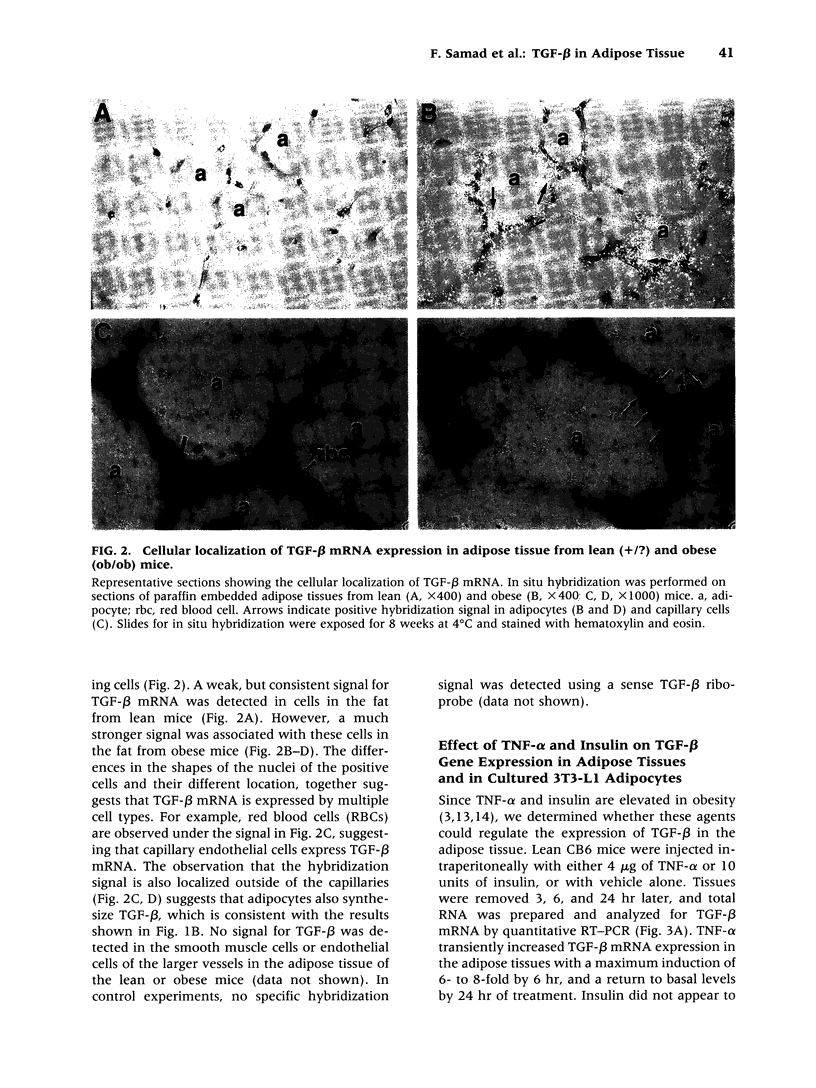
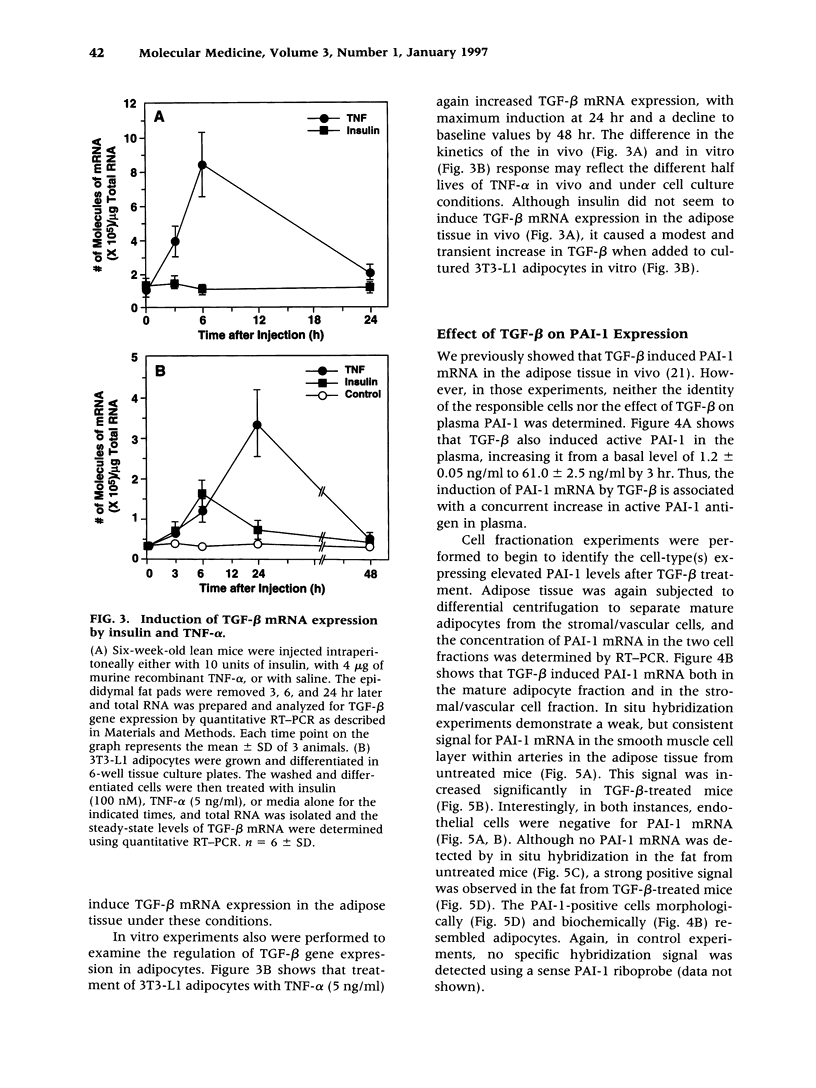
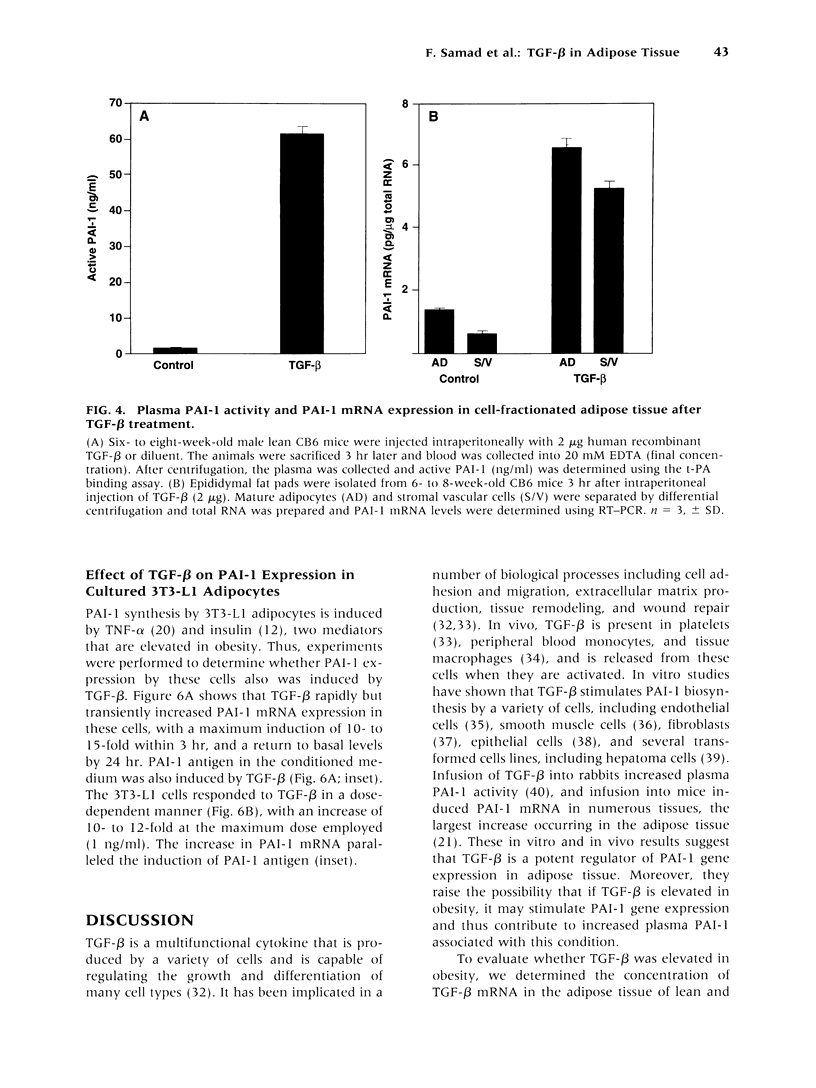
Abstract
BACKGROUND: Tumor necrosis factor-alpha (TNF-alpha) is chronically elevated in the adipose tissue from obese humans and mice. This increase in TNF-alpha contributes to the insulin resistance, elevated plasminogen activator inhibitor-1 (PAI-1) levels, and cardiovascular complications associated with obesity and noninsulin-dependent diabetes (NIDDM). PAI-1 gene expression in adipose tissue is also stimulated by transforming growth factor-beta (TGF-beta). Experiments were performed to determine whether TGF-beta is regulated by TNF-alpha and elevated in obesity. MATERIALS AND METHODS: The concentration of TGF-beta and PAI-1 mRNA in murine adipose tissue and cultured 3T3-L1 adipocytes was determined by quantitative reverse transcription polymerase chain reaction (RT-PCR), and the cellular localization of these molecules was evaluated using in situ hybridization and cell fractionation. Total TGF-beta protein was determined by employing an ELISA assay. RESULTS: TGF-beta mRNA and protein were increased in the adipose tissue from two different strains of genetically obese mice (i.e., ob/ob and db/db), compared with their lean counterparts. This increase in TGF-beta may result from TNF-alpha since TNF-alpha increased TGF-beta mRNA expression in the adipose tissue of lean mice and stimulated TGF-beta production by cultured adipocytes. Administration of TGF-beta increased PAI-1 antigen in the plasma and PAI-1 mRNA in the adipocytes of lean mice, and enhanced the rate of PAI-1 synthesis by adipocytes in vitro. CONCLUSIONS: TNF-alpha contributes to the elevated TGF-beta expression demonstrated in the adipose tissue of obese mice. A potential role for TGF-beta in the increased PAI-1 and vascular pathologies associated with obesity/NIDDM is suggested.
Full text
PDF


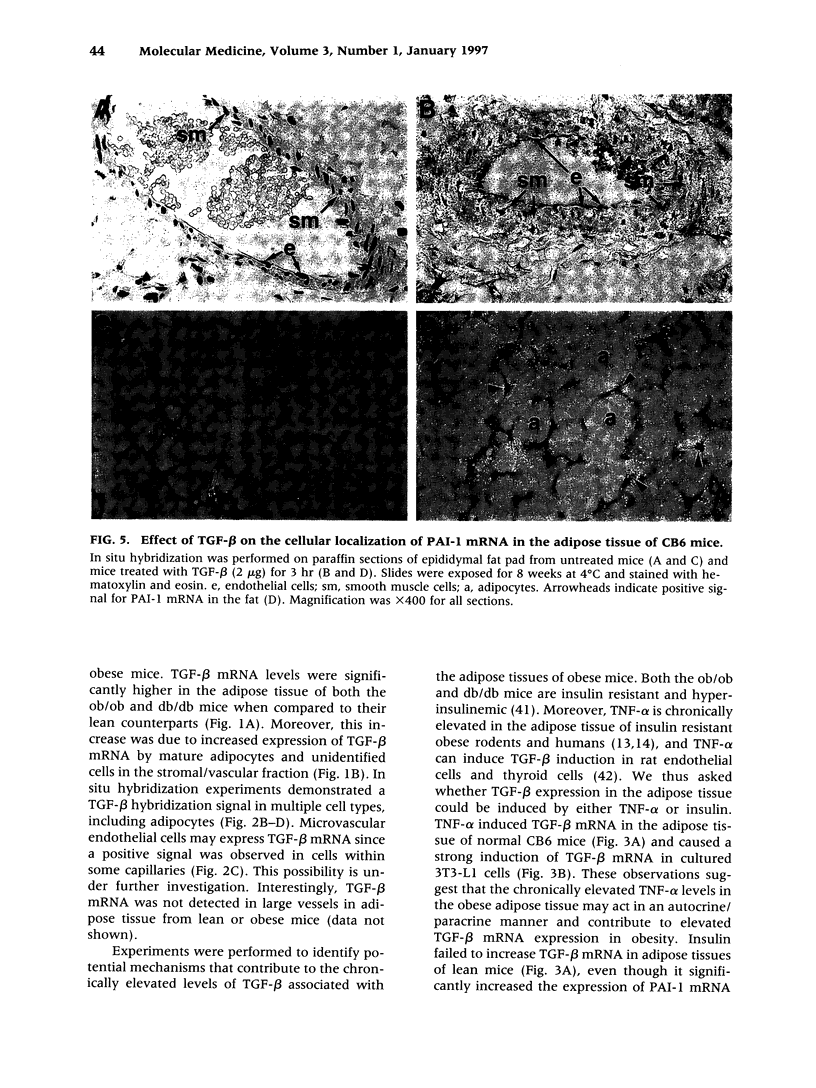
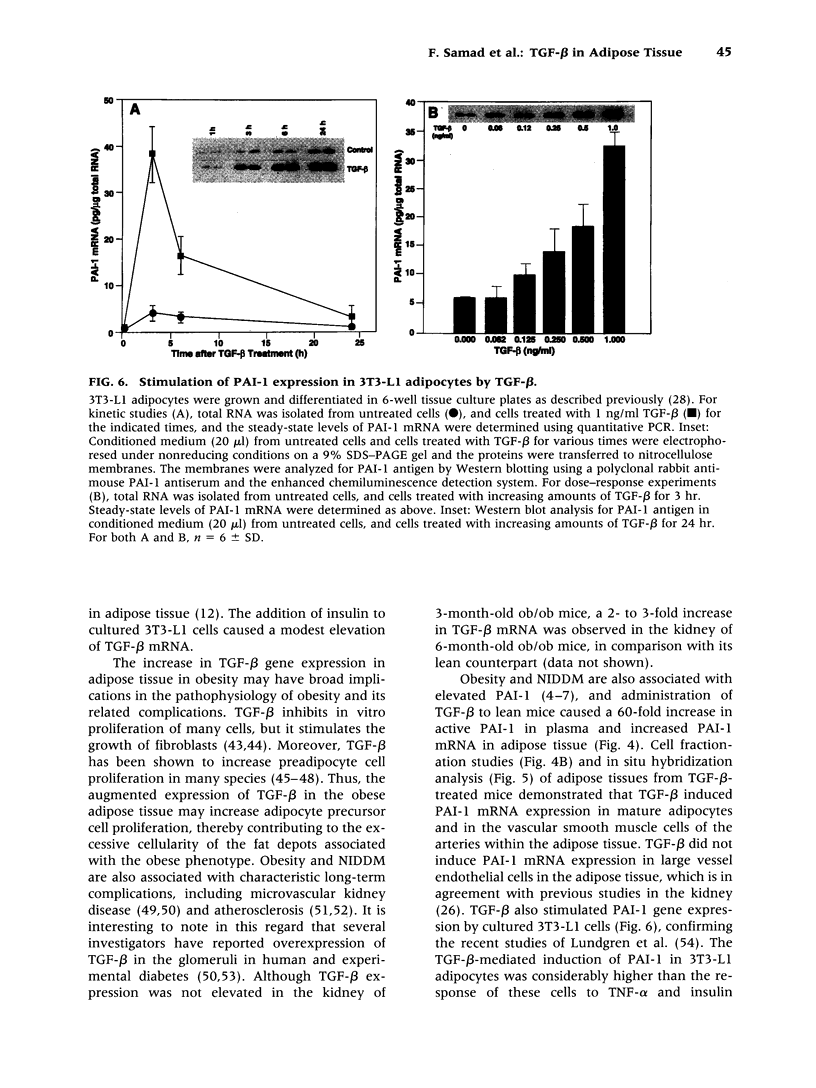



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arrants J. Hyperinsulinemia and cardiovascular risk. Heart Lung. 1994 Mar-Apr;23(2):118–124. [PubMed] [Google Scholar]
- Asplund-Carlson A., Hamsten A., Wiman B., Carlson L. A. Relationship between plasma plasminogen activator inhibitor-1 activity and VLDL triglyceride concentration, insulin levels and insulin sensitivity: studies in randomly selected normo- and hypertriglyceridaemic men. Diabetologia. 1993 Sep;36(9):817–825. doi: 10.1007/BF00400356. [DOI] [PubMed] [Google Scholar]
- Assoian R. K., Fleurdelys B. E., Stevenson H. C., Miller P. J., Madtes D. K., Raines E. W., Ross R., Sporn M. B. Expression and secretion of type beta transforming growth factor by activated human macrophages. Proc Natl Acad Sci U S A. 1987 Sep;84(17):6020–6024. doi: 10.1073/pnas.84.17.6020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Björntorp P. Abdominal fat distribution and disease: an overview of epidemiological data. Ann Med. 1992 Feb;24(1):15–18. doi: 10.3109/07853899209164140. [DOI] [PubMed] [Google Scholar]
- Border W. A. Transforming growth factor-beta and the pathogenesis of glomerular diseases. Curr Opin Nephrol Hypertens. 1994 Jan;3(1):54–58. doi: 10.1097/00041552-199401000-00007. [DOI] [PubMed] [Google Scholar]
- Bortell R., van Wijnen A. J., Ramsey-Ewing A. L., Stein G. S., Stein J. L. Differential regulation of H4 histone gene expression in 3T3-L1 pre-adipocytes during arrest of proliferation following contact inhibition or differentiation and its modulation by TGF beta 1. J Cell Biochem. 1992 Sep;50(1):62–72. doi: 10.1002/jcb.240500111. [DOI] [PubMed] [Google Scholar]
- Butterwith S. C., Goddard C. Regulation of DNA synthesis in chicken adipocyte precursor cells by insulin-like growth factors, platelet-derived growth factor and transforming growth factor-beta. J Endocrinol. 1991 Nov;131(2):203–209. doi: 10.1677/joe.0.1310203. [DOI] [PubMed] [Google Scholar]
- DeFronzo R. A., Ferrannini E. Insulin resistance. A multifaceted syndrome responsible for NIDDM, obesity, hypertension, dyslipidemia, and atherosclerotic cardiovascular disease. Diabetes Care. 1991 Mar;14(3):173–194. doi: 10.2337/diacare.14.3.173. [DOI] [PubMed] [Google Scholar]
- Fujii S., Sobel B. E. Induction of plasminogen activator inhibitor by products released from platelets. Circulation. 1990 Oct;82(4):1485–1493. doi: 10.1161/01.cir.82.4.1485. [DOI] [PubMed] [Google Scholar]
- Green H., Kehinde O. An established preadipose cell line and its differentiation in culture. II. Factors affecting the adipose conversion. Cell. 1975 May;5(1):19–27. doi: 10.1016/0092-8674(75)90087-2. [DOI] [PubMed] [Google Scholar]
- Herberg L., Coleman D. L. Laboratory animals exhibiting obesity and diabetes syndromes. Metabolism. 1977 Jan;26(1):59–99. doi: 10.1016/0026-0495(77)90128-7. [DOI] [PubMed] [Google Scholar]
- Hotamisligil G. S., Arner P., Caro J. F., Atkinson R. L., Spiegelman B. M. Increased adipose tissue expression of tumor necrosis factor-alpha in human obesity and insulin resistance. J Clin Invest. 1995 May;95(5):2409–2415. doi: 10.1172/JCI117936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hotamisligil G. S., Shargill N. S., Spiegelman B. M. Adipose expression of tumor necrosis factor-alpha: direct role in obesity-linked insulin resistance. Science. 1993 Jan 1;259(5091):87–91. doi: 10.1126/science.7678183. [DOI] [PubMed] [Google Scholar]
- Jeoung D. I., Tang B., Sonenberg M. Mitogenic response to TGF-beta in 3T3-F442A cells. Biochem Biophys Res Commun. 1995 Nov 22;216(3):964–969. doi: 10.1006/bbrc.1995.2714. [DOI] [PubMed] [Google Scholar]
- Keeton M., Eguchi Y., Sawdey M., Ahn C., Loskutoff D. J. Cellular localization of type 1 plasminogen activator inhibitor messenger RNA and protein in murine renal tissue. Am J Pathol. 1993 Jan;142(1):59–70. [PMC free article] [PubMed] [Google Scholar]
- Kehrl J. H., Wakefield L. M., Roberts A. B., Jakowlew S., Alvarez-Mon M., Derynck R., Sporn M. B., Fauci A. S. Production of transforming growth factor beta by human T lymphocytes and its potential role in the regulation of T cell growth. J Exp Med. 1986 May 1;163(5):1037–1050. doi: 10.1084/jem.163.5.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Larsson B. Obesity, fat distribution and cardiovascular disease. Int J Obes. 1991 Sep;15 (Suppl 2):53–57. [PubMed] [Google Scholar]
- Loskutoff D. J. Type 1 plasminogen activator inhibitor and its potential influence on thrombolytic therapy. Semin Thromb Hemost. 1988 Jan;14(1):100–109. doi: 10.1055/s-2007-1002762. [DOI] [PubMed] [Google Scholar]
- Lund L. R., Riccio A., Andreasen P. A., Nielsen L. S., Kristensen P., Laiho M., Saksela O., Blasi F., Danø K. Transforming growth factor-beta is a strong and fast acting positive regulator of the level of type-1 plasminogen activator inhibitor mRNA in WI-38 human lung fibroblasts. EMBO J. 1987 May;6(5):1281–1286. doi: 10.1002/j.1460-2075.1987.tb02365.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren C. H., Brown S. L., Nordt T. K., Sobel B. E., Fujii S. Elaboration of type-1 plasminogen activator inhibitor from adipocytes. A potential pathogenetic link between obesity and cardiovascular disease. Circulation. 1996 Jan 1;93(1):106–110. doi: 10.1161/01.cir.93.1.106. [DOI] [PubMed] [Google Scholar]
- Markman B. Anatomy and physiology of adipose tissue. Clin Plast Surg. 1989 Apr;16(2):235–244. [PubMed] [Google Scholar]
- McCartney-Francis N. L., Wahl S. M. Transforming growth factor beta: a matter of life and death. J Leukoc Biol. 1994 Mar;55(3):401–409. doi: 10.1002/jlb.55.3.401. [DOI] [PubMed] [Google Scholar]
- McGill J. B., Schneider D. J., Arfken C. L., Lucore C. L., Sobel B. E. Factors responsible for impaired fibrinolysis in obese subjects and NIDDM patients. Diabetes. 1994 Jan;43(1):104–109. doi: 10.2337/diab.43.1.104. [DOI] [PubMed] [Google Scholar]
- Moses H. L., Yang E. Y., Pietenpol J. A. TGF-beta stimulation and inhibition of cell proliferation: new mechanistic insights. Cell. 1990 Oct 19;63(2):245–247. doi: 10.1016/0092-8674(90)90155-8. [DOI] [PubMed] [Google Scholar]
- Mykkänen L., Rönnemaa T., Marniemi J., Haffner S. M., Bergman R., Laakso M. Insulin sensitivity is not an independent determinant of plasma plasminogen activator inhibitor-1 activity. Arterioscler Thromb. 1994 Aug;14(8):1264–1271. doi: 10.1161/01.atv.14.8.1264. [DOI] [PubMed] [Google Scholar]
- Pekary A. E., Berg L., Wang J., Lee P., Dubinett S. M., Hershman J. M. TNF-alpha, TSH, and aging regulate TGF-beta synthesis and secretion in FRTL-5 rat thyroid cells. Am J Physiol. 1995 Mar;268(3 Pt 2):R808–R815. doi: 10.1152/ajpregu.1995.268.3.R808. [DOI] [PubMed] [Google Scholar]
- Pfeiffer A., Schatz H. Diabetic microvascular complications and growth factors. Exp Clin Endocrinol Diabetes. 1995;103(1):7–14. doi: 10.1055/s-0029-1211323. [DOI] [PubMed] [Google Scholar]
- Potter van Loon B. J., Kluft C., Radder J. K., Blankenstein M. A., Meinders A. E. The cardiovascular risk factor plasminogen activator inhibitor type 1 is related to insulin resistance. Metabolism. 1993 Aug;42(8):945–949. doi: 10.1016/0026-0495(93)90005-9. [DOI] [PubMed] [Google Scholar]
- Primrose J. N., Davies J. A., Prentice C. R., Hughes R., Johnston D. Reduction in factor VII, fibrinogen and plasminogen activator inhibitor-1 activity after surgical treatment of morbid obesity. Thromb Haemost. 1992 Oct 5;68(4):396–399. [PubMed] [Google Scholar]
- RODBELL M. METABOLISM OF ISOLATED FAT CELLS. I. EFFECTS OF HORMONES ON GLUCOSE METABOLISM AND LIPOLYSIS. J Biol Chem. 1964 Feb;239:375–380. [PubMed] [Google Scholar]
- Remacle C., Grégoire F. Cellular and molecular biology in the study of the physiopathology of obesity. Acta Clin Belg Suppl. 1992;14:3–12. [PubMed] [Google Scholar]
- Samad F., Loskutoff D. J. Tissue distribution and regulation of plasminogen activator inhibitor-1 in obese mice. Mol Med. 1996 Sep;2(5):568–582. [PMC free article] [PubMed] [Google Scholar]
- Samad F., Yamamoto K., Loskutoff D. J. Distribution and regulation of plasminogen activator inhibitor-1 in murine adipose tissue in vivo. Induction by tumor necrosis factor-alpha and lipopolysaccharide. J Clin Invest. 1996 Jan 1;97(1):37–46. doi: 10.1172/JCI118404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawdey M. S., Loskutoff D. J. Regulation of murine type 1 plasminogen activator inhibitor gene expression in vivo. Tissue specificity and induction by lipopolysaccharide, tumor necrosis factor-alpha, and transforming growth factor-beta. J Clin Invest. 1991 Oct;88(4):1346–1353. doi: 10.1172/JCI115440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sawdey M., Podor T. J., Loskutoff D. J. Regulation of type 1 plasminogen activator inhibitor gene expression in cultured bovine aortic endothelial cells. Induction by transforming growth factor-beta, lipopolysaccharide, and tumor necrosis factor-alpha. J Biol Chem. 1989 Jun 25;264(18):10396–10401. [PubMed] [Google Scholar]
- Schleef R. R., Sinha M., Loskutoff D. J. Immunoradiometric assay to measure the binding of a specific inhibitor to tissue-type plasminogen activator. J Lab Clin Med. 1985 Oct;106(4):408–415. [PubMed] [Google Scholar]
- Sharma K., Ziyadeh F. N. Hyperglycemia and diabetic kidney disease. The case for transforming growth factor-beta as a key mediator. Diabetes. 1995 Oct;44(10):1139–1146. doi: 10.2337/diab.44.10.1139. [DOI] [PubMed] [Google Scholar]
- Sowers J. R. Insulin resistance, hyperinsulinemia, dyslipidemia, hypertension, and accelerated atherosclerosis. J Clin Pharmacol. 1992 Jun;32(6):529–535. doi: 10.1177/009127009203200607. [DOI] [PubMed] [Google Scholar]
- Sporn M. B., Roberts A. B., Wakefield L. M., de Crombrugghe B. Some recent advances in the chemistry and biology of transforming growth factor-beta. J Cell Biol. 1987 Sep;105(3):1039–1045. doi: 10.1083/jcb.105.3.1039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sprengers E. D., Kluft C. Plasminogen activator inhibitors. Blood. 1987 Feb;69(2):381–387. [PubMed] [Google Scholar]
- Thalacker F. W., Nilsen-Hamilton M. Specific induction of secreted proteins by transforming growth factor-beta and 12-O-tetradecanoylphorbol-13-acetate. Relationship with an inhibitor of plasminogen activator. J Biol Chem. 1987 Feb 15;262(5):2283–2290. [PubMed] [Google Scholar]
- Vague P., Juhan-Vague I., Aillaud M. F., Badier C., Viard R., Alessi M. C., Collen D. Correlation between blood fibrinolytic activity, plasminogen activator inhibitor level, plasma insulin level, and relative body weight in normal and obese subjects. Metabolism. 1986 Mar;35(3):250–253. doi: 10.1016/0026-0495(86)90209-x. [DOI] [PubMed] [Google Scholar]
- Vague P., Juhan-Vague I., Chabert V., Alessi M. C., Atlan C. Fat distribution and plasminogen activator inhibitor activity in nondiabetic obese women. Metabolism. 1989 Sep;38(9):913–915. doi: 10.1016/0026-0495(89)90241-2. [DOI] [PubMed] [Google Scholar]
- Vanden Heuvel J. P., Tyson F. L., Bell D. A. Construction of recombinant RNA templates for use as internal standards in quantitative RT-PCR. Biotechniques. 1993 Mar;14(3):395–398. [PubMed] [Google Scholar]
- Vassaux G., Négrel R., Ailhaud G., Gaillard D. Proliferation and differentiation of rat adipose precursor cells in chemically defined medium: differential action of anti-adipogenic agents. J Cell Physiol. 1994 Nov;161(2):249–256. doi: 10.1002/jcp.1041610209. [DOI] [PubMed] [Google Scholar]
- Wang A. M., Doyle M. V., Mark D. F. Quantitation of mRNA by the polymerase chain reaction. Proc Natl Acad Sci U S A. 1989 Dec;86(24):9717–9721. doi: 10.1073/pnas.86.24.9717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westerhausen D. R., Jr, Hopkins W. E., Billadello J. J. Multiple transforming growth factor-beta-inducible elements regulate expression of the plasminogen activator inhibitor type-1 gene in Hep G2 cells. J Biol Chem. 1991 Jan 15;266(2):1092–1100. [PubMed] [Google Scholar]
- Yamamoto K., Loskutoff D. J. Fibrin deposition in tissues from endotoxin-treated mice correlates with decreases in the expression of urokinase-type but not tissue-type plasminogen activator. J Clin Invest. 1996 Jun 1;97(11):2440–2451. doi: 10.1172/JCI118691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Hinsbergh V. W., Kooistra T., van den Berg E. A., Princen H. M., Fiers W., Emeis J. J. Tumor necrosis factor increases the production of plasminogen activator inhibitor in human endothelial cells in vitro and in rats in vivo. Blood. 1988 Nov;72(5):1467–1473. [PubMed] [Google Scholar]






