Abstract
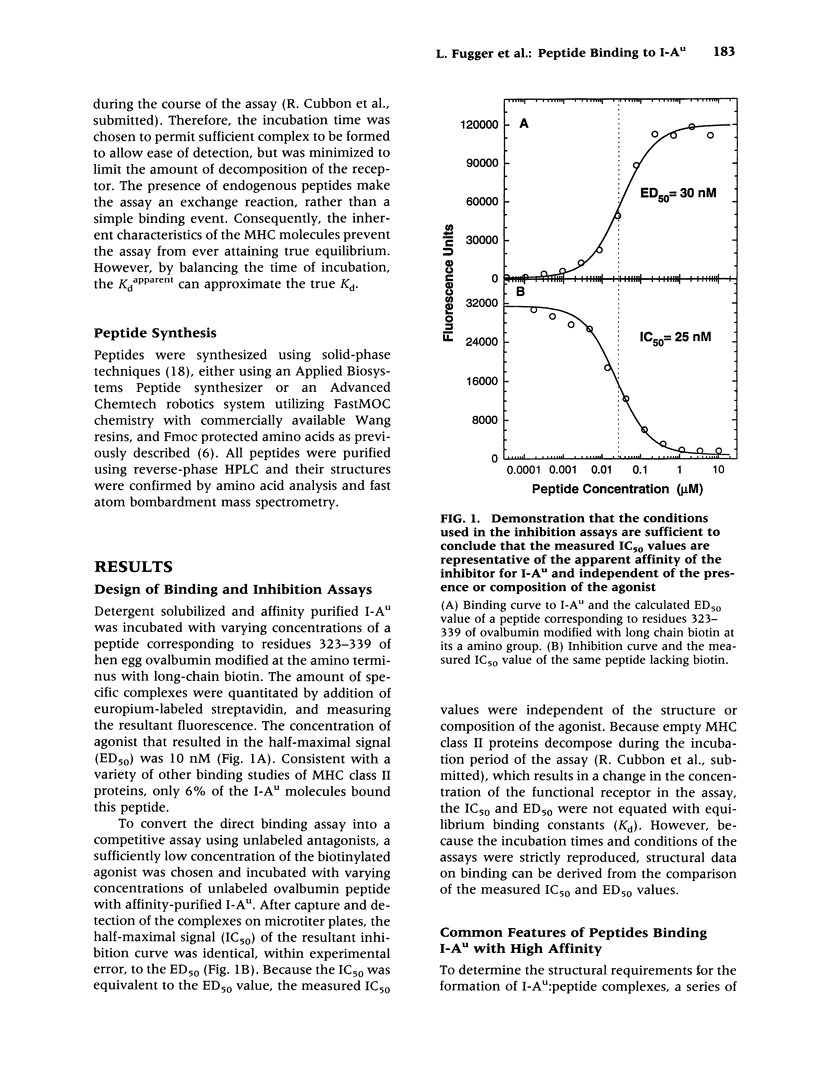
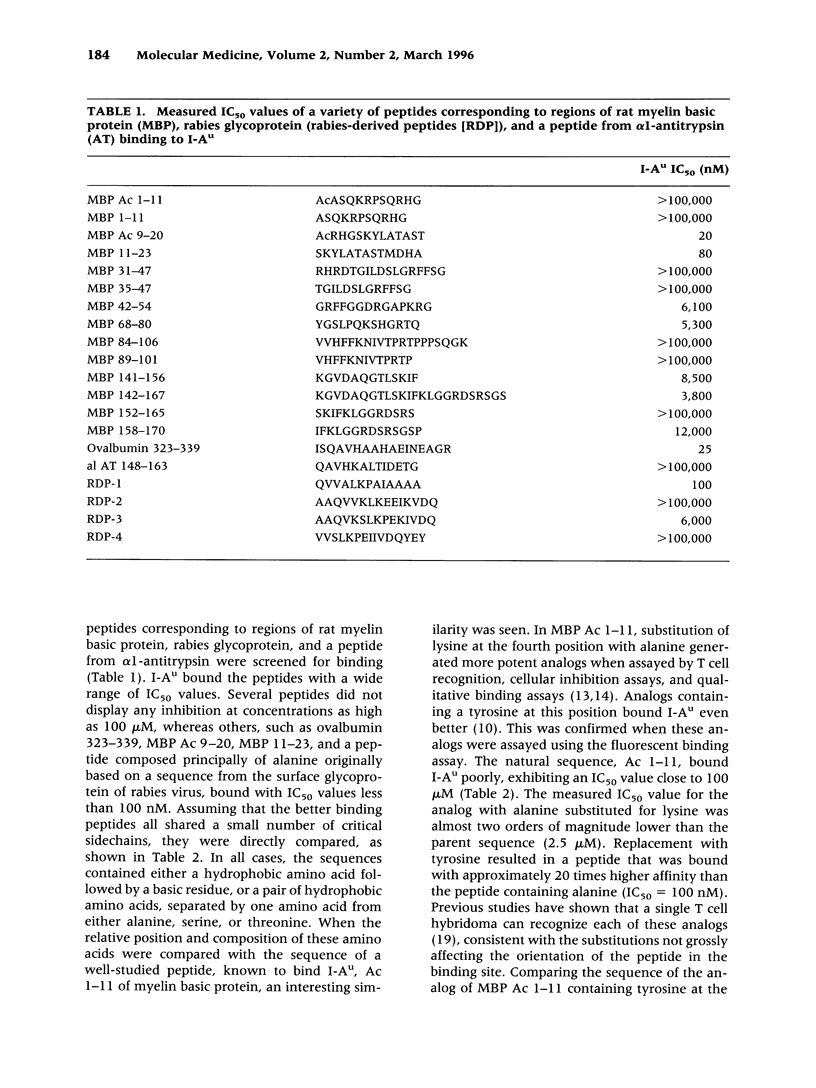
BACKGROUND: An important issue in autoimmune diseases mediated by T cells, such as experimental allergic encephalomyelitis (EAE), is the affinity of the disease-inducing determinants for MHC class II proteins. Tolerance, either due to clonal deletion or anergy induction, is thought to require high-affinity interactions between peptides and MHC molecules. Low-affinity binding is compatible with the hypothesis that breaking tolerance to self proteins does not have to occur for onset of disease. In contrast, a high-affinity interaction implies that an event leading to a breakdown of tolerance is central to the autoimmune process. MATERIALS AND METHODS: Detergent-solubilized and affinity-purified I-Au was incubated with varying concentrations of a set of peptides from myelin basic protein and a biotinylated peptide agonist. The specific complexes were separated from excess peptide by capture on antibody-coated plates, and the affinity of the peptides was measured by adding europium-labeled streptavidin and measuring the resultant fluorescence. RESULTS: The immunodominant and encephalitogenic determinant, Ac 1-11, was shown to bind to I-Au relatively poorly (IC50 = 100 microM), demonstrating that in this protein, immunodominance did not correlate with high-affinity binding. In contrast with the natural sequence, the ability of shorter analogs to induce EAE did correlate with their apparent affinity. CONCLUSIONS: The dominance of the natural determinant does not arise from a high-affinity interaction with the MHC class II molecule. This suggests that other mechanisms are operative and that the specific T cell for this peptide/MHC ligand is of high affinity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babbitt B. P., Allen P. M., Matsueda G., Haber E., Unanue E. R. Binding of immunogenic peptides to Ia histocompatibility molecules. 1985 Sep 26-Oct 2Nature. 317(6035):359–361. doi: 10.1038/317359a0. [DOI] [PubMed] [Google Scholar]
- Fairchild P. J., Wildgoose R., Atherton E., Webb S., Wraith D. C. An autoantigenic T cell epitope forms unstable complexes with class II MHC: a novel route for escape from tolerance induction. Int Immunol. 1993 Sep;5(9):1151–1158. doi: 10.1093/intimm/5.9.1151. [DOI] [PubMed] [Google Scholar]
- Gautam A. M., Lock C. B., Smilek D. E., Pearson C. I., Steinman L., McDevitt H. O. Minimum structural requirements for peptide presentation by major histocompatibility complex class II molecules: implications in induction of autoimmunity. Proc Natl Acad Sci U S A. 1994 Jan 18;91(2):767–771. doi: 10.1073/pnas.91.2.767. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gautam A. M., Pearson C. I., Smilek D. E., Steinman L., McDevitt H. O. A polyalanine peptide with only five native myelin basic protein residues induces autoimmune encephalomyelitis. J Exp Med. 1992 Aug 1;176(2):605–609. doi: 10.1084/jem.176.2.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill C. M., Liu A., Marshall K. W., Mayer J., Jorgensen B., Yuan B., Cubbon R. M., Nichols E. A., Wicker L. S., Rothbard J. B. Exploration of requirements for peptide binding to HLA DRB1*0101 and DRB1*0401. J Immunol. 1994 Mar 15;152(6):2890–2898. [PubMed] [Google Scholar]
- Jardetzky T. S., Gorga J. C., Busch R., Rothbard J., Strominger J. L., Wiley D. C. Peptide binding to HLA-DR1: a peptide with most residues substituted to alanine retains MHC binding. EMBO J. 1990 Jun;9(6):1797–1803. doi: 10.1002/j.1460-2075.1990.tb08304.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall K. W., Liu A. F., Canales J., Perahia B., Jorgensen B., Gantzos R. D., Aguilar B., Devaux B., Rothbard J. B. Role of the polymorphic residues in HLA-DR molecules in allele-specific binding of peptide ligands. J Immunol. 1994 May 15;152(10):4946–4957. [PubMed] [Google Scholar]
- O'Sullivan D., Sidney J., Appella E., Walker L., Phillips L., Colón S. M., Miles C., Chesnut R. W., Sette A. Characterization of the specificity of peptide binding to four DR haplotypes. J Immunol. 1990 Sep 15;145(6):1799–1808. [PubMed] [Google Scholar]
- Oi V. T., Jones P. P., Goding J. W., Herzenberg L. A., Herzenberg L. A. Properties of monoclonal antibodies to mouse Ig allotypes, H-2, and Ia antigens. Curr Top Microbiol Immunol. 1978;81:115–120. doi: 10.1007/978-3-642-67448-8_18. [DOI] [PubMed] [Google Scholar]
- Rothbard J. B. Antigen presentation. One size fits all. Curr Biol. 1994 Jul 1;4(7):653–655. doi: 10.1016/s0960-9822(00)00146-9. [DOI] [PubMed] [Google Scholar]
- Saper M. A., Bjorkman P. J., Wiley D. C. Refined structure of the human histocompatibility antigen HLA-A2 at 2.6 A resolution. J Mol Biol. 1991 May 20;219(2):277–319. doi: 10.1016/0022-2836(91)90567-p. [DOI] [PubMed] [Google Scholar]
- Schwartz R. H. A cell culture model for T lymphocyte clonal anergy. Science. 1990 Jun 15;248(4961):1349–1356. doi: 10.1126/science.2113314. [DOI] [PubMed] [Google Scholar]
- Sette A., Southwood S., O'Sullivan D., Gaeta F. C., Sidney J., Grey H. M. Effect of pH on MHC class II-peptide interactions. J Immunol. 1992 Feb 1;148(3):844–851. [PubMed] [Google Scholar]
- Stern L. J., Brown J. H., Jardetzky T. S., Gorga J. C., Urban R. G., Strominger J. L., Wiley D. C. Crystal structure of the human class II MHC protein HLA-DR1 complexed with an influenza virus peptide. Nature. 1994 Mar 17;368(6468):215–221. doi: 10.1038/368215a0. [DOI] [PubMed] [Google Scholar]
- Wall M., Southwood S., Sidney J., Oseroff C., del Guericio M. F., Lamont A. G., Colón S. M., Arrhenius T., Gaeta F. C., Sette A. High affinity for class II molecules as a necessary but not sufficient characteristic of encephalitogenic determinants. Int Immunol. 1992 Jul;4(7):773–777. doi: 10.1093/intimm/4.7.773. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., Bruun B., Fairchild P. J. Cross-reactive antigen recognition by an encephalitogenic T cell receptor. Implications for T cell biology and autoimmunity. J Immunol. 1992 Dec 1;149(11):3765–3770. [PubMed] [Google Scholar]
- Wraith D. C., McDevitt H. O., Steinman L., Acha-Orbea H. T cell recognition as the target for immune intervention in autoimmune disease. Cell. 1989 Jun 2;57(5):709–715. doi: 10.1016/0092-8674(89)90786-1. [DOI] [PubMed] [Google Scholar]
- Wraith D. C., Smilek D. E., Mitchell D. J., Steinman L., McDevitt H. O. Antigen recognition in autoimmune encephalomyelitis and the potential for peptide-mediated immunotherapy. Cell. 1989 Oct 20;59(2):247–255. doi: 10.1016/0092-8674(89)90287-0. [DOI] [PubMed] [Google Scholar]
- Wucherpfennig K. W., Sette A., Southwood S., Oseroff C., Matsui M., Strominger J. L., Hafler D. A. Structural requirements for binding of an immunodominant myelin basic protein peptide to DR2 isotypes and for its recognition by human T cell clones. J Exp Med. 1994 Jan 1;179(1):279–290. doi: 10.1084/jem.179.1.279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zamvil S. S., Mitchell D. J., Moore A. C., Kitamura K., Steinman L., Rothbard J. B. T-cell epitope of the autoantigen myelin basic protein that induces encephalomyelitis. Nature. 1986 Nov 20;324(6094):258–260. doi: 10.1038/324258a0. [DOI] [PubMed] [Google Scholar]
- Zamvil S. S., Steinman L. The T lymphocyte in experimental allergic encephalomyelitis. Annu Rev Immunol. 1990;8:579–621. doi: 10.1146/annurev.iy.08.040190.003051. [DOI] [PubMed] [Google Scholar]


