Abstract
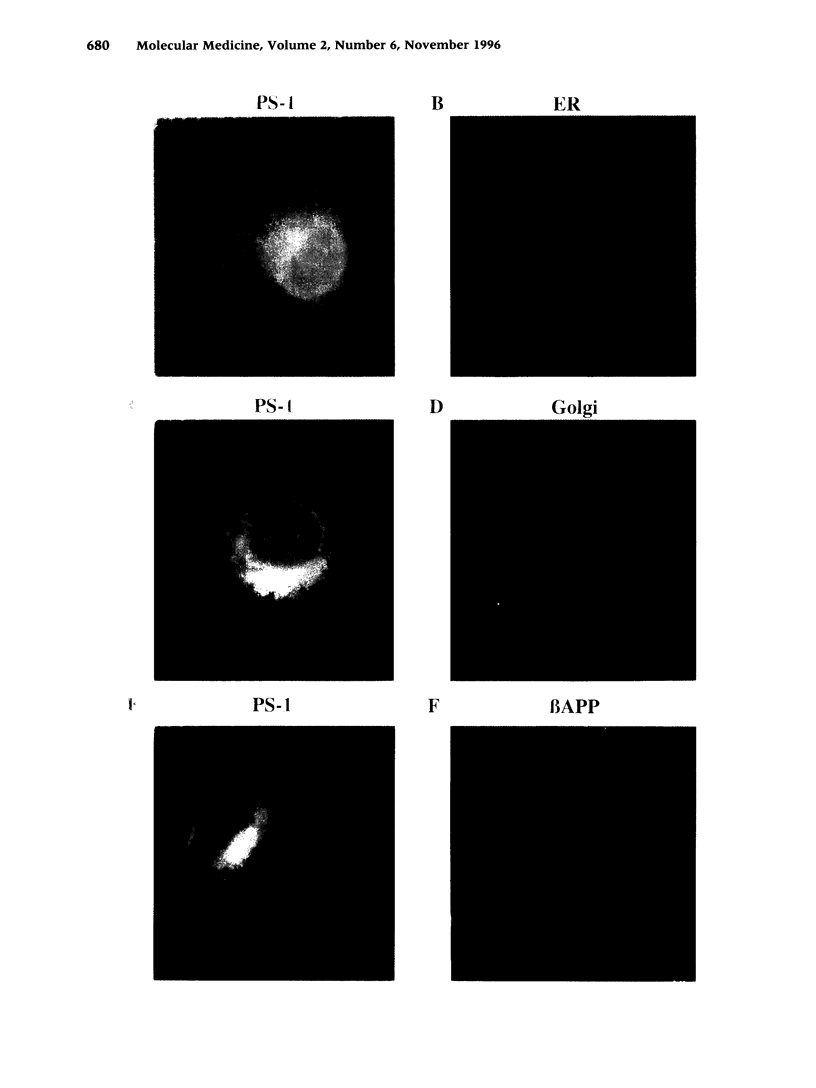
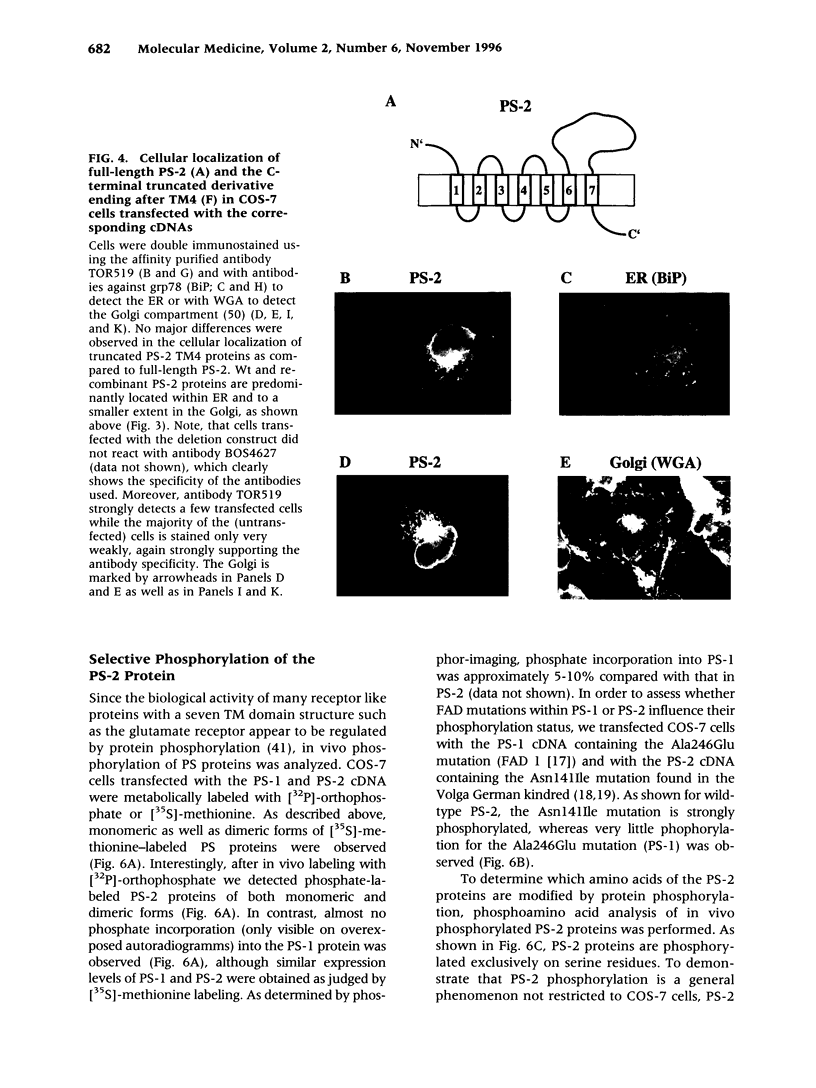
BACKGROUND: Alzheimer's disease (AD) is a progressive neurodegenerative disorder characterized by the deposition of extracellular senile plaques composed of amyloid beta-peptide (A beta). Whereas most cases of AD occur sporadically, about 10% of AD cases are inherited as a fully penetrant autosomal dominant trait. Mutations in the recently cloned Presenilin genes (PS-1 and PS-2) are by far the most common cause of early onset familial AD. MATERIALS AND METHODS: Cellular expression of endogenous and overexpressed PS proteins was analyzed by immunocytochemistry and metabolic labeling followed by immunoprecipitation. In vivo phosphorylation sites of PS proteins were analyzed by extensive mutagenesis. RESULTS: PS-1 as well as PS-2 proteins were localized predominantly within the endoplasmic reticulum (ER). However, small amounts of the PS proteins were detected within the Golgi compartment, where they colocalize with the beta-amyloid precursor protein (beta APP). The PS-2 protein was found to be highly phosphorylated, whereas very little phosphorylation was observed for PS-1. The selective phosphorylation of PS-2 occurs exclusively on serine residues. In vivo phosphorylation of PS-2 was mapped to serine residues 7, 9, and 19 within an acidic stretch at the N terminus, which is absent in PS-1. casein kinase (CK)-1 and CK-2 were shown to phosphorylate the N terminus of PS-2 in vitro. CONCLUSIONS: The majority of PS proteins were detected in the ER where little if any proteolytic processing of beta APP was reported. ER retention of PS proteins might occur by intramolecular aggregation. Small amounts of PS proteins were also detected in the Golgi where they colocalized with beta APP. This might suggest that potential interactions between PS proteins and beta APP could occur within the Golgi. Selective phosphorylation of PS-2 proteins within the acidic domain missing in PS-1 indicates differences in the biological functions and regulation of the two highly homologous proteins.
Full text
PDF












Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Bodenbach L., Fauss J., Robitzki A., Krehan A., Lorenz P., Lozeman F. J., Pyerin W. Recombinant human casein kinase II. A study with the complete set of subunits (alpha, alpha' and beta), site-directed autophosphorylation mutants and a bicistronically expressed holoenzyme. Eur J Biochem. 1994 Feb 15;220(1):263–273. doi: 10.1111/j.1432-1033.1994.tb18622.x. [DOI] [PubMed] [Google Scholar]
- Boteva K., Vitek M., Mitsuda H., de Silva H., Xu P. T., Small G., Gilbert J. R. Mutation analysis of presenillin 1 gene in Alzheimer's disease. Lancet. 1996 Jan 13;347(8994):130–131. doi: 10.1016/s0140-6736(96)90261-5. [DOI] [PubMed] [Google Scholar]
- Boyle W. J., van der Geer P., Hunter T. Phosphopeptide mapping and phosphoamino acid analysis by two-dimensional separation on thin-layer cellulose plates. Methods Enzymol. 1991;201:110–149. doi: 10.1016/0076-6879(91)01013-r. [DOI] [PubMed] [Google Scholar]
- Buxbaum J. D., Gandy S. E., Cicchetti P., Ehrlich M. E., Czernik A. J., Fracasso R. P., Ramabhadran T. V., Unterbeck A. J., Greengard P. Processing of Alzheimer beta/A4 amyloid precursor protein: modulation by agents that regulate protein phosphorylation. Proc Natl Acad Sci U S A. 1990 Aug;87(15):6003–6006. doi: 10.1073/pnas.87.15.6003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxbaum J. D., Koo E. H., Greengard P. Protein phosphorylation inhibits production of Alzheimer amyloid beta/A4 peptide. Proc Natl Acad Sci U S A. 1993 Oct 1;90(19):9195–9198. doi: 10.1073/pnas.90.19.9195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai X. D., Golde T. E., Younkin S. G. Release of excess amyloid beta protein from a mutant amyloid beta protein precursor. Science. 1993 Jan 22;259(5094):514–516. doi: 10.1126/science.8424174. [DOI] [PubMed] [Google Scholar]
- Campion D., Flaman J. M., Brice A., Hannequin D., Dubois B., Martin C., Moreau V., Charbonnier F., Didierjean O., Tardieu S. Mutations of the presenilin I gene in families with early-onset Alzheimer's disease. Hum Mol Genet. 1995 Dec;4(12):2373–2377. doi: 10.1093/hmg/4.12.2373. [DOI] [PubMed] [Google Scholar]
- Citron M., Oltersdorf T., Haass C., McConlogue L., Hung A. Y., Seubert P., Vigo-Pelfrey C., Lieberburg I., Selkoe D. J. Mutation of the beta-amyloid precursor protein in familial Alzheimer's disease increases beta-protein production. Nature. 1992 Dec 17;360(6405):672–674. doi: 10.1038/360672a0. [DOI] [PubMed] [Google Scholar]
- Cruts M., Backhovens H., Wang S. Y., Van Gassen G., Theuns J., De Jonghe C. D., Wehnert A., De Voecht J., De Winter G., Cras P. Molecular genetic analysis of familial early-onset Alzheimer's disease linked to chromosome 14q24.3. Hum Mol Genet. 1995 Dec;4(12):2363–2371. doi: 10.1093/hmg/4.12.2363. [DOI] [PubMed] [Google Scholar]
- Cruts M., Hendriks L., Van Broeckhoven C. The presenilin genes: a new gene family involved in Alzheimer disease pathology. Hum Mol Genet. 1996;5(Spec No):1449–1455. doi: 10.1093/hmg/5.supplement_1.1449. [DOI] [PubMed] [Google Scholar]
- Cummings B. J., Cotman C. W. Image analysis of beta-amyloid load in Alzheimer's disease and relation to dementia severity. Lancet. 1995 Dec 9;346(8989):1524–1528. doi: 10.1016/s0140-6736(95)92053-6. [DOI] [PubMed] [Google Scholar]
- Goate A., Chartier-Harlin M. C., Mullan M., Brown J., Crawford F., Fidani L., Giuffra L., Haynes A., Irving N., James L. Segregation of a missense mutation in the amyloid precursor protein gene with familial Alzheimer's disease. Nature. 1991 Feb 21;349(6311):704–706. doi: 10.1038/349704a0. [DOI] [PubMed] [Google Scholar]
- Haass C., Hung A. Y., Selkoe D. J. Processing of beta-amyloid precursor protein in microglia and astrocytes favors an internal localization over constitutive secretion. J Neurosci. 1991 Dec;11(12):3783–3793. doi: 10.1523/JNEUROSCI.11-12-03783.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haass C., Hung A. Y., Selkoe D. J., Teplow D. B. Mutations associated with a locus for familial Alzheimer's disease result in alternative processing of amyloid beta-protein precursor. J Biol Chem. 1994 Jul 1;269(26):17741–17748. [PubMed] [Google Scholar]
- Haass C., Lemere C. A., Capell A., Citron M., Seubert P., Schenk D., Lannfelt L., Selkoe D. J. The Swedish mutation causes early-onset Alzheimer's disease by beta-secretase cleavage within the secretory pathway. Nat Med. 1995 Dec;1(12):1291–1296. doi: 10.1038/nm1295-1291. [DOI] [PubMed] [Google Scholar]
- Haass C. Presenile because of presenilin: the presenilin genes and early onset Alzheimer's disease. Curr Opin Neurol. 1996 Aug;9(4):254–259. [PubMed] [Google Scholar]
- Haass C., Selkoe D. J. Cellular processing of beta-amyloid precursor protein and the genesis of amyloid beta-peptide. Cell. 1993 Dec 17;75(6):1039–1042. doi: 10.1016/0092-8674(93)90312-e. [DOI] [PubMed] [Google Scholar]
- Hung A. Y., Haass C., Nitsch R. M., Qiu W. Q., Citron M., Wurtman R. J., Growdon J. H., Selkoe D. J. Activation of protein kinase C inhibits cellular production of the amyloid beta-protein. J Biol Chem. 1993 Nov 5;268(31):22959–22962. [PubMed] [Google Scholar]
- Issinger O. G. Casein kinases: pleiotropic mediators of cellular regulation. Pharmacol Ther. 1993;59(1):1–30. doi: 10.1016/0163-7258(93)90039-g. [DOI] [PubMed] [Google Scholar]
- Jones B. G., Thomas L., Molloy S. S., Thulin C. D., Fry M. D., Walsh K. A., Thomas G. Intracellular trafficking of furin is modulated by the phosphorylation state of a casein kinase II site in its cytoplasmic tail. EMBO J. 1995 Dec 1;14(23):5869–5883. doi: 10.1002/j.1460-2075.1995.tb00275.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J., Lemaire H. G., Unterbeck A., Salbaum J. M., Masters C. L., Grzeschik K. H., Multhaup G., Beyreuther K., Müller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987 Feb 19;325(6106):733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- Koo E. H., Squazzo S. L. Evidence that production and release of amyloid beta-protein involves the endocytic pathway. J Biol Chem. 1994 Jul 1;269(26):17386–17389. [PubMed] [Google Scholar]
- Kovacs D. M., Fausett H. J., Page K. J., Kim T. W., Moir R. D., Merriam D. E., Hollister R. D., Hallmark O. G., Mancini R., Felsenstein K. M. Alzheimer-associated presenilins 1 and 2: neuronal expression in brain and localization to intracellular membranes in mammalian cells. Nat Med. 1996 Feb;2(2):224–229. doi: 10.1038/nm0296-224. [DOI] [PubMed] [Google Scholar]
- L'Hernault S. W., Arduengo P. M. Mutation of a putative sperm membrane protein in Caenorhabditis elegans prevents sperm differentiation but not its associated meiotic divisions. J Cell Biol. 1992 Oct;119(1):55–68. doi: 10.1083/jcb.119.1.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitan D., Greenwald I. Facilitation of lin-12-mediated signalling by sel-12, a Caenorhabditis elegans S182 Alzheimer's disease gene. Nature. 1995 Sep 28;377(6547):351–354. doi: 10.1038/377351a0. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wasco W., Poorkaj P., Romano D. M., Oshima J., Pettingell W. H., Yu C. E., Jondro P. D., Schmidt S. D., Wang K. Candidate gene for the chromosome 1 familial Alzheimer's disease locus. Science. 1995 Aug 18;269(5226):973–977. doi: 10.1126/science.7638622. [DOI] [PubMed] [Google Scholar]
- Levy-Lahad E., Wijsman E. M., Nemens E., Anderson L., Goddard K. A., Weber J. L., Bird T. D., Schellenberg G. D. A familial Alzheimer's disease locus on chromosome 1. Science. 1995 Aug 18;269(5226):970–973. doi: 10.1126/science.7638621. [DOI] [PubMed] [Google Scholar]
- Mullan M., Crawford F. Genetic and molecular advances in Alzheimer's disease. Trends Neurosci. 1993 Oct;16(10):398–403. doi: 10.1016/0166-2236(93)90007-9. [DOI] [PubMed] [Google Scholar]
- Nitsch R. M., Slack B. E., Wurtman R. J., Growdon J. H. Release of Alzheimer amyloid precursor derivatives stimulated by activation of muscarinic acetylcholine receptors. Science. 1992 Oct 9;258(5080):304–307. doi: 10.1126/science.1411529. [DOI] [PubMed] [Google Scholar]
- Podlisny M. B., Tolan D. R., Selkoe D. J. Homology of the amyloid beta protein precursor in monkey and human supports a primate model for beta amyloidosis in Alzheimer's disease. Am J Pathol. 1991 Jun;138(6):1423–1435. [PMC free article] [PubMed] [Google Scholar]
- Price D. L., Sisodia S. S., Gandy S. E. Amyloid beta amyloidosis in Alzheimer's disease. Curr Opin Neurol. 1995 Aug;8(4):268–274. doi: 10.1097/00019052-199508000-00004. [DOI] [PubMed] [Google Scholar]
- Roche K. W., Tingley W. G., Huganir R. L. Glutamate receptor phosphorylation and synaptic plasticity. Curr Opin Neurobiol. 1994 Jun;4(3):383–388. doi: 10.1016/0959-4388(94)90100-7. [DOI] [PubMed] [Google Scholar]
- Rogaev E. I., Sherrington R., Rogaeva E. A., Levesque G., Ikeda M., Liang Y., Chi H., Lin C., Holman K., Tsuda T. Familial Alzheimer's disease in kindreds with missense mutations in a gene on chromosome 1 related to the Alzheimer's disease type 3 gene. Nature. 1995 Aug 31;376(6543):775–778. doi: 10.1038/376775a0. [DOI] [PubMed] [Google Scholar]
- Selkoe D. J. Normal and abnormal biology of the beta-amyloid precursor protein. Annu Rev Neurosci. 1994;17:489–517. doi: 10.1146/annurev.ne.17.030194.002421. [DOI] [PubMed] [Google Scholar]
- Sherrington R., Rogaev E. I., Liang Y., Rogaeva E. A., Levesque G., Ikeda M., Chi H., Lin C., Li G., Holman K. Cloning of a gene bearing missense mutations in early-onset familial Alzheimer's disease. Nature. 1995 Jun 29;375(6534):754–760. doi: 10.1038/375754a0. [DOI] [PubMed] [Google Scholar]
- St George-Hyslop P., Haines J., Rogaev E., Mortilla M., Vaula G., Pericak-Vance M., Foncin J. F., Montesi M., Bruni A., Sorbi S. Genetic evidence for a novel familial Alzheimer's disease locus on chromosome 14. Nat Genet. 1992 Dec;2(4):330–334. doi: 10.1038/ng1292-330. [DOI] [PubMed] [Google Scholar]
- Strittmatter W. J., Roses A. D. Apolipoprotein E and Alzheimer disease. Proc Natl Acad Sci U S A. 1995 May 23;92(11):4725–4727. doi: 10.1073/pnas.92.11.4725. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki N., Cheung T. T., Cai X. D., Odaka A., Otvos L., Jr, Eckman C., Golde T. E., Younkin S. G. An increased percentage of long amyloid beta protein secreted by familial amyloid beta protein precursor (beta APP717) mutants. Science. 1994 May 27;264(5163):1336–1340. doi: 10.1126/science.8191290. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Nishiyama K., Murayama S., Yamamoto A., Sato S., Kanazawa I., Sakaki Y. Regional and cellular presenilin 1 gene expression in human and rat tissues. Biochem Biophys Res Commun. 1996 Feb 27;219(3):708–713. doi: 10.1006/bbrc.1996.0299. [DOI] [PubMed] [Google Scholar]
- Tartakoff A. M., Vassalli P. Lectin-binding sites as markers of Golgi subcompartments: proximal-to-distal maturation of oligosaccharides. J Cell Biol. 1983 Oct;97(4):1243–1248. doi: 10.1083/jcb.97.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Broeckhoven C., Backhovens H., Cruts M., De Winter G., Bruyland M., Cras P., Martin J. J. Mapping of a gene predisposing to early-onset Alzheimer's disease to chromosome 14q24.3. Nat Genet. 1992 Dec;2(4):335–339. doi: 10.1038/ng1292-335. [DOI] [PubMed] [Google Scholar]
- Wasco W., Pettingell W. P., Jondro P. D., Schmidt S. D., Gurubhagavatula S., Rodes L., DiBlasi T., Romano D. M., Guenette S. Y., Kovacs D. M. Familial Alzheimer's chromosome 14 mutations. Nat Med. 1995 Sep;1(9):848–848. doi: 10.1038/nm0995-848a. [DOI] [PubMed] [Google Scholar]
- Weidemann A., König G., Bunke D., Fischer P., Salbaum J. M., Masters C. L., Beyreuther K. Identification, biogenesis, and localization of precursors of Alzheimer's disease A4 amyloid protein. Cell. 1989 Apr 7;57(1):115–126. doi: 10.1016/0092-8674(89)90177-3. [DOI] [PubMed] [Google Scholar]